Abstract
A major research emphasis has been focused on defining the molecular changes that occur from acute to chronic pain in order to identify potential therapeutic targets for chronic pain. As the endocannabinoid system is dynamically involved in pain signaling, a plausible mechanism that may contribute to chronic pain vulnerability involves alterations in the amount of circulating endocannabinoids. Therefore, this study sought to examine cannabinoid type-1 (CNR1), type-2 (CNR2) receptors, fatty acid amide hydrolase (FAAH) and the vanilloid receptor (TRPV1) gene expression profiles among individuals with acute and chronic LBP at their baseline visit. We also assessed associations among selected SNPs of FAAH and CNR2 and measures of somatosensory function and self-report pain measures.
Using a previously established quantitative sensory testing protocol, we comprehensively assessed somatosensory parameters among 42 acute LBP, 42 cLBP and 20 healthy participants. Samples of whole blood were drawn to examine mRNA expression and isolate genomic DNA for genotyping.
CNR2mRNA was significantly upregulated in all LBP patients compared to controls. However, FAAH mRNA and TRPV1 mRNA were significantly upregulated in cLBP compared with healthy controls. A significant association was observed between FAAH SNP genotype and self-report pain measures, mechanical and cold pain sensitivity among LBP subjects.
CLBP participants showed increased FAAH and TRPV1 mRNA expression compared to acute LBP participants and healthy controls. Further research to characterize pain-associated somatosensory changes in the context of altered mRNA expression levels and SNP associations may provide insight on the molecular underpinnings of maladaptive chronic pain.
INTRODUCTION
Chronic low back pain (cLBP) is the second most frequent chronic pain disorder in the United States [1]. Since a majority of patients with acute and persistent LBP have a nonspecific etiology without an identifiable cause of pain [2, 3], investigators have sought to identify other molecular targets that may modulate pain without the deleterious side effects of non-steroidal anti-inflammatories and opioids. Alterations of the endocannabinoid system are well documented in preclinical models of enhanced peripheral and central sensitivity, which increases the risk of chronic pain. In particular, pain sensitivity depends on the function of fatty acid amide hydrolase (FAAH), an enzyme that regulates levels of endocannabinoid anandamide (AEA); transient receptor potential cation channel subfamily V member 1 (TRPV1), a known receptor for anandamide; and cannabinoid type 1 (CNR1) and type-2 (CNR2) receptors. The CNR1 and CNR2 receptors have a critical role in the modulation of nociceptive processing in models of chronic pain [4].
FAAH is an enzyme that catabolizes a large class of fatty acid amides including the endogenous signaling molecule, anandamide (AEA). It is well established that inhibition of FAAH either by pharmacological inhibitors or genetic knockdown results in elevated levels of AEA, which produces moderate analgesia in preclinical studies [5]. Mice lacking Faah, the gene encoding the FAAH enzyme, are less sensitive to noxious thermal stimuli [6]. Prior findings suggest reduced expression of the FAAH enzyme in human subjects homozygous for FAAH SNP Pro129Thr [7] increases pain sensitivity and, possibly, susceptibility to chronic pain disorders [8].
AEA has been extensively studied, and apart from its action on cannabinoid receptors, multiple new targets including the TRPV1 receptor have been identified [9, 10]. Notably, AEA appears to interact with TRPV1 at the same intracellular binding site as capsaicin [11], a known ligand for the TRPV1 receptor, suggesting interplay between the endogenous vanilloid and cannabinoid systems. Rodent studies have found that increased expression of TRPV1 receptors, evidenced by increased TRPVI mRNA expression in the DRG and A fibers, mediate analgesic properties of AEA in chronic pain conditions such as neuropathic pain [12–14]. TRPV1 receptors are co-localized with CNR1 receptors in the CNS [15, 16] and are involved in co-modulating several centrally controlled functions including pain, hyperalgesia and allodynia [4]. In line with these prior studies showing that alterations of TRPV1 mRNA can alter pain processing, a phenomenon mediated via AEA [12–14], we recently reported increased levels of TRPV1 receptor mRNA in individuals with acute LBP [17].
Both CNR1 and CNR2 receptors are involved in mediating analgesic properties of cannabinoids and alterations in protein and gene expression have been shown to occur in chronic pain states in rodent models. CNR1 and CNR2 mRNA expression is increased in spinal cord (dorsal horn) of rodent models of neuropathic pain [18–21]. The increased expression has functional relevance as cannabinoid agonists lose their efficacy when CNR1 and CNR2 upregulation is blocked [19]. Research from our team characterized gene expression in individuals presenting with acute LBP and healthy volunteers that led to some intriguing findings that suggest a direct link between altered activity of the endocannabinoid system and acute LBP. Along with increased peripheral and central sensitivity, which leads to pain facilitation in cLBP, we have documented significantly increased levels of CNR2 mRNA in these individuals [17]. These findings are consistent with results from rodent models [20, 21], suggesting that CNR2 receptors are involved in modulating pain mechanisms in cLBP. Thus, examining differences in CNR2 expression that upregulate the expression of CNR2 between individuals whose pain resolves compared to those who develop cLBP may give insight into chronic pain vulnerability. In other chronic pain conditions, such as multiple sclerosis (MS), changes in levels of endocannabinoid mRNA levels have been linked to MS-related disability in some subtypes [22], however this link has not been explored in depth in other pain conditions.
Based on preliminary data and evidence from rodent models, we sought to compare levels of FAAH, CNR1, CNR2 and TRPV1 mRNA expression among subjects presenting with either an acute LBP episode that resolved in the expected time frame of 4–6 weeks or those who developed cLBP. Based on the differential expression of FAAH and CNR2 we then assessed relationships among SNPs of these genes and somatosensory function (quantitative sensory testing) as well as self-report pain outcomes measures.
METHODS
Participants
Men and women between the ages of 18–50 years of age diagnosed with an acute nonspecific LBP episode and able to read and write in English were invited to participate from primary healthcare clinics, college campuses and the general community through advertisements. This age range was selected to provide a more homogeneous sample in terms of general health, work status and contributing factors of cLBP. We previously reported a preliminary analysis of baseline demographic, psychological, and somatosensory measures and mRNA expression of candidate genes in a subsample of the participants presented here [17]. An acute nonspecific LBP episode was defined as pain anywhere in the region of the low back bound superiorly by the thoraco-lumbar junction and inferiorly by the lumbo-sacral junction, which had been present for >24 hours but <4 weeks duration and was preceded by at least 1 pain-free month [23]. Additionally, healthy pain-free volunteers were included to serve as a control group. Recruitment took place at two urban university health systems after approval from the Institution Review Board. All participants provided written consent prior to study participation.
Low back pain patients were excluded for the following conditions: (a) pain at another site or associated with a painful condition (eg., degenerative disc disease, herniated lumbar disc, fibromyalgia, neuropathy, rheumatoid arthritis, sciatica); (b) previous spinal surgery; (c) presence of neurological deficits; (d) history of comorbidities that affect sensorimotor function (eg., multiple sclerosis, spinal cord injury); (e) pregnant or within 3-months postpartum; (f) taking opioid, or anticonvulsant medication; and, (g) history of psychological disorders (bipolar disorder, schizophrenia) because of a possible associations with biological markers [24–26]. Eligibility for the healthy no-pain control group included men and women (a) between 18–50 years of age; (b) could read and write in English; (c) with no known medical, psychological problems or prescribed medication; (d) not pregnant or breastfeeding; and, (e) no recent history of pain at any location.
After collection of data at the baseline visit, participants were followed up every 6 weeks if they continued to have LBP up to 24 weeks. Participants whose pain had resolved as indicated by a VAS rating of 1 or below by the 6-week time-point were classified as acute LBP. Participants who had persistent low back pain at the 24 week visit were classified as cLBP [23]. Healthy controls and acute LBP participants only completed the baseline visit while the chronic LBP group completed both the baseline and 24 week visits.
Procedures
After obtaining informed consent, participants were scheduled to undergo baseline data collection as soon as possible but no longer than one week from the time of consent. Data collection took place in a private research suite at the study site. During the baseline visit, participants were asked to complete questions about their age, gender, socioeconomic status, educational attainment, lifestyle behaviors (smoking, exercise), comorbidities, and past episodes of LBP. Participants with low back pain also completed pain questionnaires including the Brief Pain Inventory (BPI) and the McGill Pain Questionnaire. Following completion of the questionnaires, participants underwent venipuncture for collection of blood samples and quantitative sensory testing (QST). The sequence of data collection was followed for all participants. The same sequence of data collection (questionnaires, venipuncture and QST) was carried out at the 24 week visit.
Pain Measures
The Brief Pain Inventory (BPI) is a pain assessment tool that has well-established reliability and validity for adult patients with LBP, and is sensitive to change over time[27]. The BPI assesses the severity of pain, location of pain, pain medications, amount of pain relief in the past 24 hours and the past week, and the impact of pain on daily functions.
The McGill Pain Questionnaire short form is a reliable self-report measure of pain perception [28, 29]. It entails 15 verbal descriptors of sensory and affective dimensions of pain and is scored on a 4-point scale (0-none to 3-severe) by adding the numeric value of each pain dimension. Higher scores indicate higher levels of sensory and affective components of pain (range 0–45).
Quantitative Sensory Testing (QST)
QST was used to evaluate responses to experimental pain and uses standardized stimuli to test both nociceptive and non-nociceptive systems [30]. Quantitative sensory testing was performed on the lumbar region and the dominant forearm (remote area). A standardized protocol of administration, including examination room conditions and instructions provided for the participant, were strictly followed from the same protocol described in the preliminary analysis reported by our group [17, 31]. Participants were given a practice run on the non-dominant forearm in order to verify the participant’s understanding of the protocol.
Gene Expression Profiles
Whole blood was collected by venipuncture into one 5-mL EDTA vacutainer and one 10-mL Paxgene blood RNA tubes (PreAnalytix, Qiagen USA), labeled with a unique study identification label, and transported directly to the laboratory for processing. RNA isolation was performed using the PAXgene™ total RNA isolation system (Qiagen, Valencia, CA) according to the manufacturer’s protocol and was reverse transcribed using RT2 cDNA kit (Qiagen USA). The mRNA expression of 84 genes involved in the transduction, maintenance, and modulation of pain was determined (Neuropathic & Inflammatory RT2 Profiler PCR Array; Sabio Sciences, Valencia, CA;) using qPCR performed on the ABI Step One Plus PCR machine. After an initial incubation step, 40 cycles (95°C for 15 seconds and 1 minute at 60°C) of PCR were performed. Expression levels were quantified using the 2−ΔΔCT method which normalizes data of the genes of interest to the average of three housekeeping genes β-actin (ACTB), GAPDH and Beta-microtubulin (B2M) (housekeeping genes are included in the array). The methodology used is described in detail in a previous study from our group [17]
Genotyping
Genomic DNA (gDNA) was extracted from the white blood cell layer (buffy coat) using standard protocols (Qiagen, DNA mini kit). All samples were genotyped for 3 SNPs of FAAH (rs324420, rs932816, rs4141964,) and one CNR2 SNP (rs2501431) using predesigned TaqMan primers and universal genotyping master mix (Life Technologies). Genotyping assays were performed according to the manufacturer’s protocol using an Applied Biosystems (ABI) StepOne Plus PCR machine and ABI allelic discrimination software.
Statistical Analysis
The results from the current sample are part of larger funded NIH study (NCT01981382, clinicaltrails.gov). The estimated sample size to detect a change in pain sensitivity between the control group and incident cases and in incident cases over time, was extrapolated from the OPPERA study [32]. Student t-tests were used to test for group differences in demographic and QST variables that were normally distributed, whereas the Kruskal-Wallis test followed by Bonferroni-Holm adjusted Mann-Whitney U test for post hoc analyses were used for variables that were not normally distributed. Categorical variables were compared using χ 2 tests. Post-hoc analyses were conducted as necessary to account for multiple testing.
Differences in endocannabinoid gene expression have been detected in incident groups compared to controls in smaller sample sizes [22]. For gene expression analyses, delta Ct values for each gene for each LBP participant were normalized to the average expression of that gene of the resolved LBP group. Results are based on normalization using the average of the three most stable HKGs (GAPDH, ACTB, and B2M). For each of the 4 non-housekeeping genes considered “genes of interest” (GOI), the ΔCq value were calculated as ΔCq = Cq,GOI − Cq,HKG. Thereafter, for each subject, the relative fold change in expression was calculated as 2−Δ(ΔCq) where Δ(ΔCq) = ΔCq,LBP − ΔCq,Healthy Control average. For each comparison, a linear regression analysis was carried out to control for the influence of demographic factors (Age, BMI, Gender, Race) on mRNA expression levels with respect to subject type. Then, a Univariate Analysis of Variance (ANOVA) was used to compare the fold change in the cLBP group to the acute LBP group, with p values <0.05 considered as statistically significant. Paired sample t-test was used to examine differences in fold change between baseline and the 24 week time point in the cLBP group.
Step-wise linear regressions were conducted to examine associations between genotype and pain outcomes. Demographic variables previously shown to be associated with increased incidence of chronic LBP (Age, BMI, Gender, Race) were loaded into all regressions as factor 1. SNP genotype was added as factor 2. Finally, interaction terms between the genotype and outcome variables were entered into each model in order to explore the potential associations between genotype and pain burden and/or sensitivity.
RESULTS
a. Study Participant Characteristics
Demographic and clinical data collected from 42 acute and 42 cLBP participants is presented in Table 1. Additionally, a subset of pain-free controls (n=20) were included in the analysis as a control group. Of note, there were more African-American participants in the cLBP group compared to the acute LBP group (p<0.01). A significantly higher number of participants in the cLBP group were current smokers which is consistent with previous studies [27, 33]. Interestingly, the acute and cLBP groups did not differ in the number of prior LBP episodes. Among both acute and cLBP groups; approximately 50% were not taking any medications, 30% were taking NSAIDS (acetaminophen, ibuprofen, naproxen, aspirin) as needed, 7% were taking a muscle relaxant as needed, 10% were using heat packs or balms and the remaining were using non-pharmacological interventions such as exercise or physical therapy. Chronic LBP participants also reported higher pain severity scores on the Brief Pain Inventory and McGill Pain Questionnaire (Table 1), and had increased sensitivity to mechanical stimuli (data not shown). The results from the somatosensory measures have been discussed in detail in Starkweather et al. (under review).
Table 1.
Demographic characteristics of study participants
| cLBP (N=42) | Acute LBP (N=42) | Healthy Controls (N=20) | ||
|---|---|---|---|---|
|
| ||||
| Race | African American | 27 (64.3%) * | 7 (16.7%) | 6 (30%) |
| Asian | 1 (2.4%)* | 8 (19.0%) | 0 (0%) | |
| White | 11 (26.2%)* | 25 (59.5%) | 13 (65%) | |
| Other | 3 (7.2%)* | 2 (4.8%) | 0 (0%) | |
|
| ||||
| Gender | Female | 22 (52.4%) | 22 (52.4%) | 6 (13%) |
| Male | 20 (47.6%) | 20 (47.6%) | 14 (70%) | |
|
| ||||
| Age | 38.5 (9.6)# | 29.02 (9.8)* | 37 (15.6) | |
|
| ||||
| BMI | 31.01 (1.24) | 27.47 (1.02) | 27.95 (6.20) | |
|
| ||||
| Smoking | Current smoker (N, %) | 25 (59.5%)*# | 8 (19.0%) | 3 (15%) |
|
| ||||
| Prior LBP episodes | Yes (N, %) | 33 (78.6%) | 34 (81.0%) | - |
|
| ||||
| BPI | Average Pain | 5.2 (1.87) # | 3.57 (1.59) | - |
|
| ||||
| Pain Interference | 4.50 (0.35) # | 2.54 (1.73) | - | |
|
| ||||
| McGill Pain questionnaire | VAS | 50.87 (3.99) # | 25.72 (18.22) | - |
Data are presented as mean ± S.D. or percentage. Statistical analysis was carried out with Chi-square or ANOVA
p<0.05 v/s healthy controls;
p<0.05v/s acute low back pain group; (LBP= low back pain; BMI= body mass index; BPI= Brief Pain Inventory; VAS= Visual Analog Scale)
c. Comparison of Gene Expression profiles of cannabinoid genes (CNR1, CNR2, FAAH, TRPV1)
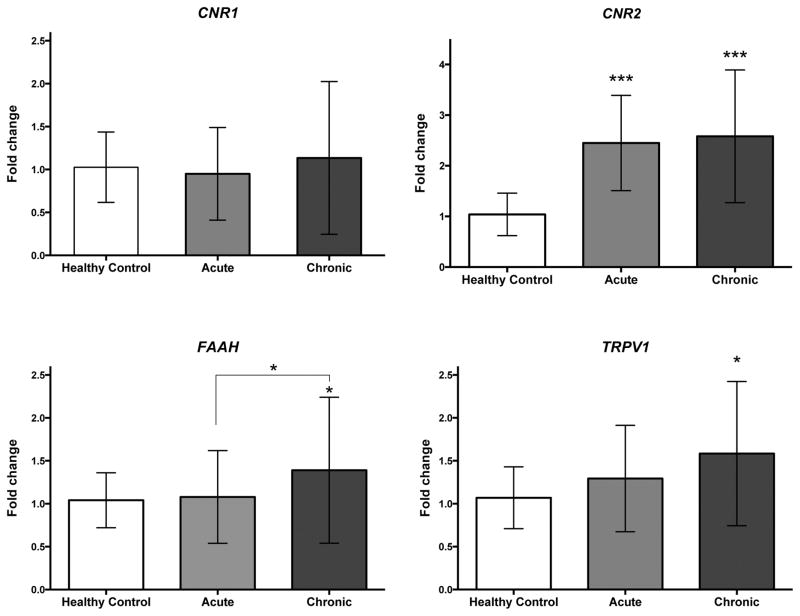
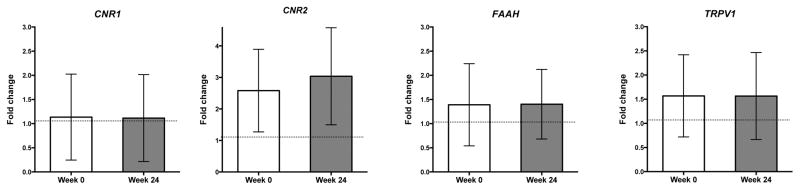
Linear regression did not reveal a significant effect of relevant demographics (Age, Gender, BMI, Race) on mRNA expression levels with respect to subject type (data not shown). CNR1 mRNA expression was not significantly different at baseline across all three groups (F(2, 95) = 0.714, p = 0.49; Fig. 1A). Findings from ANOVA confirmed a significant main effect of group on CNR2 expression (F(2, 95) = 1.091, p < 0.001; Fig. 1B). Post-hoc comparisons (Fisher’s LSD) revealed elevated CNR2 expression in both the acute and cLBP subjects compared with healthy volunteers (p<0.001). Results from ANOVA showed a main effect of group on FAAH expression that approached significance (healthy controls vs acute vs cLBP at baseline (F(2, 96) = 2.908, p = 0.059; Fig. 1C). Although the group level analysis was slightly above our predetermined threshold of significance, we were interested in identifying which groups were different, which would have been undetected if relying on a priori significance level alone. Thus, we carried out post-hoc analysis (Fisher’s LSD) which showed significantly increased FAAH expression in cLBP compared to patients with acute LBP and healthy, no-pain volunteers (all p < 0.05). Findings from ANOVA also confirmed a significant effect of group on TRPV1 expression (F(2, 96) = 3.833, p < 0.05; Fig. 1D). Post-hoc analysis (Fisher’s LSD) showed increased TRPV1 mRNA expression in cLBP subjects compared to healthy volunteers (p < 0.01). Finally, we did not detect any significant correlations between mRNA levels and QST end-points in these individuals (data not shown). Gene expression did not differ within chronic low back pain patients between baseline and 24 week follow-up for any targets examined (Fig 2, all p>0.05).
Figure 1.
Mean fold-change expression values of CNR1, CNR2, FAAH, TRPV1 from whole blood of healthy control (n=20), acute LBP (n=42) and cLBP patients (n=42). Ct values of each gene of interest was normalised to average of Ct values of 3 housekeeping genes (GAPDH, ACTB and B2M) for each sample. Fold change are presented as mean±S.D. Significant difference in fold-change between groups analyzed using ANOVA, *p<0.05 is considered statistically significant.
Figure 2.
Mean fold-change expression values of CNR1, CNR2, FAAH, TRPV1 from whole blood of of cLBP patients (n=42) at baseline (week 1) and week 24. Ct values of each gene of interest was normalised to average of Ct values of 3 housekeeping genes (GAPDH, ACTB and B2M) for each sample. Fold change are presented as mean±S.D. Significant difference in fold-change between groups analyzed paired t-test, *p<0.05 is considered statistically significant.
d. Exploratory analysis of the association between FAAH SNP genotype and pain sensitivity
The minor allelic frequencies of each SNP in the FAAH gene have been previously described in detail [8]. We selected the FAAH SNPs rs324420, rs4141964 and rs2295633 based on a significant association previously reported between two haploblocks containing these SNPs and cold pain sensitivity[8]. In the present study, the FAAH SNP rs324420 (P129T) was significantly associated with higher pain sensitivity on some of the QST measures. Patients with the minor allele (A/A and A/C genotype) at rs324420 reported lower tolerance to cold pain on the CPT at the control site (Table 2) and higher pain scores on the MPS at the lower back (Table 2) at baseline, (p < 0.05), compared to C/C carriers after correcting for demographic variables. Two SNPs within FAAH, rs932816 and rs4141964, were associated with increased pain scores on the McGill Pain Questionnaire among LBP patients and accounted for ~5% variance in pain ratings. The FAAH SNP rs932816 was significantly associated with the overall increased average pain and interference of pain among LBP patients (BPIAvg and BPIInt, see Table 2). Genotype at this SNP explains ~ 8% and ~ 6% of the variance in pain-related interference and average pain, respectively.
Table 2.
Step-wise Linear Regression of Clinical Variables to FAAH Genotype
| Rs324420 | Rs932816 | Rs4141964 | ||||
|---|---|---|---|---|---|---|
| Self-report pain measures | ||||||
| BPI Interference | R2 change | p-value | R2 change | p-value | R2 change | p-value |
| 0.011 | 0.346 | 0.082 | < 0.001* | 0.007 | 0.433 | |
| BPI Average Pain | R2 change | p-value | R2 change | p-value | R2 change | p-value |
| 0.006 | 0.492 | 0.059 | < 0.05* | 0.036 | 0.078 | |
| McGill VAS | R2 change | p-value | R2 change | p-value | R2 change | p-value |
| 0.660 | 0.419 | 0.050 | < 0.05* | 0.049 | < 0.05* | |
| Quantitative sensory testing measures | ||||||
| Mechanical pain sensitivity (MPS)- Painsite | R2 change | p-value | R2 change | p-value | R2 change | p-value |
| 0.038 | < 0.05* | 0.019 | 0.156 | 0.019 | 0.156 | |
| Cold pain test (CPT) - Control Site | R2 change | p-value | R2 change | p-value | R2 change | p-value |
| 0.040 | < 0.05* | 0.009 | 0.348 | 0.044 | < 0.05* | |
Statistical analysis with step-wise logistical regression with final step (FAAH genotype x demographics).
p<0.05 was considered a statistically significant difference related to the SNP of interest.
CNR2 SNPs have not been examined in relation to their role in chronic pain, however, they have been studies in association with psychological disorders such as depression and schizophrenia. The presence of a G-allele at the CNR2 SNP rs2501431 has also been associated with increased risk for depression. Minor allele frequencies for this SNP range from 21–50% depending on the population[34, 35]. We did not find any significant associations with the CNR2 SNP rs2501431and any of the measures (Data not shown).
DISCUSSION
This study examined expression of endocannabinoid genes among individuals presenting with acute LBP episode, and those who went to develop cLBP. The findings suggest that those with cLBP had differential endocannabinoid gene expression compared to those whose pain resolved. Specifically, CNR2 mRNA was increased among all LBP individuals at baseline compared to healthy controls. However, only subjects who went on to develop cLBP exhibited elevated levels of FAAH and TRPV1 mRNA. Next, this study examined the relationship between SNP genotype of the differentially expressed genes (FAAH and CNR2) and somatosensory as well as self-report pain measures among all low back pain patients. The analysis revealed a modest yet significant association between FAAH SNP genotype and self-report pain measures, mechanical and cold pain sensitivity in our LBP population.
Modest yet significantly increased levels of FAAH and TRPV1 expression were observed among those who developed cLBP compared to the acute group, suggesting a potential genetic interaction that may increase vulnerability to chronic pain. Increased levels of FAAH mRNA could lead to lower AEA levels and thus dysregulation of normal pain processing [36]. Increased TRPV1 mRNA levels may reflect a mechanism underlying persistent hyperalgesia in chronic pain conditions. TRPV1 activation may contribute to alterations in the BBB that could allow for “pain relevant” signals to cross into the CNS and contribute to persistent pain [37]. It is well known that the TRPV1 receptor is activated by and mediates the transmission of pain signals resulting from noxious heat (>43 degrees C) and noxious chemical stimuli [38]. However, increased TRPV1 mRNA was not associated with heat pain threshold in the cLBP group, thus further research is needed to fully understand the role of increased gene expression and pain processing in this population.
The CNR2 receptor system is dynamically involved in pain processing with vast supporting preclinical literature [21, 39]. It is hypothesized that following induction of pain, the functional upregulation of spinal Cnr2 protein and mRNA appears to provide a crucial restraint on the development of central sensitization, as evidenced by the exacerbation of allodynia at the painful site, and the novel manifestation of allodynia in the control site in mice with genetically deleted Cnr2 (Cnr2−/−) [40]. The present data suggest that CNR2 mRNA expression is elevated in patients with an acute LBP episode and that this increase is maintained in those that develop cLBP. Taken together with findings from animal studies, CNR2 expression may be part of an endogenous analgesic mechanism initiated during painful episodes.
Overall, mRNA expression was not significantly correlated with any specific QST measures. One possible explanation is that the non-specific nature of LBP may prevent accurate comparisons between somatosensory measures and gene expression. Another explanation may be that expression in peripheral blood does not reflect expression changes that are occurring in the CNS or other pain-relevant tissues and thus, may not be directly related to alterations in pain processing.. However, expression levels of CNR2 are higher in the periphery compared to central expression and therefore we observed a robust elevation in mRNA levels in the pain populations. The overall elevation of CNR2 mRNA may thus be a protective mechanism against chronic pain and a subsequent increased receptor pool for endocannabinoids to exert their analgesic effects. On the other hand, peripheral and whole blood levels of FAAH and TRPV1 are lower than in the CNS and thus it may be difficult to link gene expression alterations in whole blood to cLBP susceptibility. [41, 42] Nonetheless, the finding that initial elevations in mRNA levels of endocannabinoid genes persist over time in the cLBP group is an important finding suggesting a role of endocannabinoids in peripheral and central sensitization that characterized the cLBP group. However, these data represent a global elevation of mRNA and do not reflect localized changes.
We found modest yet significant associations between FAAH genotype and self-reported pain measures as well as QST end-points among low back pain patients (acute and chronic). Specifically the FAAH SNPs rs324420 and rs 4141964 (Table 1) showed a significant association with increased cold pain sensation. These findings are consistent with a previous study examining FAAH genotype and cold pain sensitivity in healthy individuals [8]. In addition, further associations were found between FAAH genotypes and self-report pain as well as mechanical pain sensitivity. Further research with larger sample sizes is necessary to determine the functional and mechanistic nature of these interactions i.e whether these SNPs are associated with risk factors for developing persistent pain. Nonetheless, these results provide strong exploratory evidence that FAAH genotypes may be important in modulating sensitivity to different types of pain.
Our definition for chronic LBP is based on established parameters from de Vet et al., 2002. These parameters conform to the recently published NIH standards for low back pain research with respect to chronicity for the chronic LBP group (pain for at least half the days in past 6 months) and location of the pain. Based on the more recent definition published by the National Institutes of Health Task Force on Research Standards for Chronic Low-Back Pain (2014) approximately 25% (10/42) of the acute LBP participants could have been classified as cLBP. Thus, one major drawback of this study is inability to compare both time points between the acute and cLBP groups. Another limitation is that a significant percentage (approx. 45%) of the low back pain participants were using analgesics (predominantly over the counter NSAIDs), which may impact circulating endocannabinoid levels. Other limitations for this study include a relatively modest sample size and small control population. Power analysis revealed the study was underpowered for examining effects of genotype and pain measures [8]. However, these findings are relevant to pathophysiology of a non-specific chronic pain condition such as LBP and reflect CNS endocannabinoid mRNA levels accurately. Future studies will examine genotype effects in a larger pain population to expand our findings in our sample.
In conclusion, we found enhanced CNR2 mRNA levels in all LBP subjects and elevated FAAH and TRPV1 levels only among those that developed cLBP. We found some exploratory associations between 3 FAAH SNPs and self-report and somatosensory pain outcomes in our population, however these results should be interpreted with caution due to the small sample size. The potential influence of the endocannabinoid system on clinical outcomes in chronic pain conditions has been indicated in many studies. The genetic deletion of FAAH as well as FAAH inhibitors have been shown to have beneficial outcomes in models of neuropathic and inflammatory pain [5], while results remain inconclusive in a single study examining FAAH inhibitors in clinical studies [43]. CNR2 agonists show robust analgesic effects in preclinical models and lack psychoactive side-effects. Although further study is needed to determine the mechanisms involved in LBP-associated changes in the endocannabinoid system and expression of its components, pharmacological targets that modulate actions of AEA (TRPV1 and FAAH) as well as CNR2 agonists could lead to novel treatments for cLBP and other chronic pain conditions.
Acknowledgments
Source of Funding: This research was funded by the National Institute of Nursing Research (Starkweather, PI; R01 NR013932). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research (NINR) or the National Institutes of Health (NIH).
The authors would like to thank the assistance of Leena Kader, Sharmeen Jaffry, Irina Milhuyak and Nicole Glidden.
Footnotes
Conflicts of Interest
For the remaining authors none were declared.
References
- 1.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Report of the NIH Task Force on research standards for chronic low back pain. The journal of pain: official journal of the American Pain Society. 2014;15:569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou R, Qaseem A, Snow V, Casey D, Cross JT, Jr, Shekelle P, Owens DK. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of internal medicine. 2007;147:478–91. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne JC, editor. IASP Low Back Pain. International Association for the Study of Pain; 2010. [Google Scholar]
- 4.Starowicz K, Przewlocka B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:3286–99. doi: 10.1098/rstb.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11:39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–6. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13:2113–9. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Mittal DP, Iadarola MJ, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. Journal of medical genetics. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–30. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–60. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premkumar LS, Sikand P. TRPV1: a target for next generation analgesics. Curr Neuropharmacol. 2008;6:151–63. doi: 10.2174/157015908784533888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–20. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 14.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–14. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–8. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- 16.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–15. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 17.Starkweather AR, Ramesh D, Lyon DE, Siangphoe U, Deng X, Sturgill J, Heineman A, Elswick RK, Jr, Dorsey SG, Greenspan J. Acute Low Back Pain: Differential Somatosensory Function and Gene Expression Compared With Healthy No-Pain Controls. The Clinical journal of pain. 2016;32:933–939. doi: 10.1097/AJP.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212–2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–83. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 19.Paszcuk AF, Dutra RC, da Silva KA, Quintao NL, Campos MM, Calixto JB. Cannabinoid agonists inhibit neuropathic pain induced by brachial plexus avulsion in mice by affecting glial cells and MAP kinases. PloS one. 2011;6:e24034. doi: 10.1371/journal.pone.0024034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. European Journal of Neuroscience. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 21.Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2012;367:3300–11. doi: 10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS. Plasma endocannabinoid levels in multiple sclerosis. Journal of the neurological sciences. 2009;287:212–5. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 23.de Vet HC, Heymans MW, Dunn KM, Pope DP, van der Beek AJ, Macfarlane GJ, Bouter LM, Croft PR. Episodes of low back pain: a proposal for uniform definitions to be used in research. Spine. 2002;27:2409–16. doi: 10.1097/01.BRS.0000030307.34002.BE. [DOI] [PubMed] [Google Scholar]
- 24.Gama CS, Andreazza AC, Kunz M, Berk M, Belmonte-de-Abreu PS, Kapczinski F. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neuroscience letters. 2007;420:45–8. doi: 10.1016/j.neulet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Harley J, Roberts R, Joyce P, Mulder R, Luty S, Frampton C, Kennedy M. Orosomucoid influences the response to antidepressants in major depressive disorder. Journal of psychopharmacology. 2010;24:531–5. doi: 10.1177/0269881109105101. [DOI] [PubMed] [Google Scholar]
- 26.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34:645–51. doi: 10.1016/j.pnpbp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. The Clinical journal of pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 29.Melzack R, Katz J. The McGill Pain Questionnaire: Appraisal and current status. In: Melzack DCTR, editor. Handbook of pain assessment. 2. New York, NY, US: Guilford Press; 2001. pp. 35–52. [Google Scholar]
- 30.Belfer I, Dai F. Phenotyping and genotyping neuropathic pain. Current pain and headache reports. 2010;14:203–12. doi: 10.1007/s11916-010-0110-1. [DOI] [PubMed] [Google Scholar]
- 31.Starkweather AR, Heineman A, Storey S, Rubia G, Lyon DE, Greenspan J, Dorsey SG. Methods to Measure Peripheral and Central Pain Sensitization Using Quantitative Sensory Testing: A Focus on Individuals with Low Back Pain. Applied Nursing Research. 2015 doi: 10.1016/j.apnr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. The journal of pain: official journal of the American Pain Society. 2011;12:T61–74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson H, Ejlertsson G, Leden I. Widespread musculoskeletal chronic pain associated with smoking. An epidemiological study in a general rural population. Scandinavian journal of rehabilitation medicine. 1998;30:185–91. [PubMed] [Google Scholar]
- 34.Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, Morikawa M, Inada T, Watanabe Y, Takahashi M, Someya T, Ujike H, Iwata N, Ozaki N, Onaivi ES, Kunugi H, Sasaki T, Itokawa M, Arai M, Niizato K, Iritani S, Naka I, Ohashi J, Kakita A, Takahashi H, Nawa H, Arinami T. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67:974–82. doi: 10.1016/j.biopsych.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Annals of the New York Academy of Sciences. 2008;1139:434–49. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci. 2014;17:164–74. doi: 10.1038/nn.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beggs S, Liu XJ, Kwan C, Salter MW. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Molecular pain. 2010;6:74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 39.Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain research reviews. 2009;60:255–66. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, Pintado B, Gutierrez-Adan A, Sanguino E, Manzanares J, Zimmer A, Maldonado R. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J Neurosci. 2008;28:12125–35. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witt SH, Sommer WH, Hansson AC, Sticht C, Rietschel M, Witt CC. Comparison of gene expression profiles in the blood, hippocampus and prefrontal cortex of rats. In silico pharmacology. 2013;1:15. doi: 10.1186/2193-9616-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunde DA, Crawford A, Geraghty DP. Tachykinin (NK1, NK2 and NK3) receptor, transient receptor potential vanilloid 1 (TRPV1) and early transcription factor, cFOS, mRNA expression in rat tissues following systemic capsaicin treatment. Regulatory peptides. 2013;183:35–41. doi: 10.1016/j.regpep.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]