Abstract
Background: Osteomyelitis is a complex disease. Treatment involves a combination of bone resection, antimicrobials and soft-tissue coverage. There is a difficulty in unifying a classification system for long bone osteomyelitis that is generally accepted.
Objectives: In this systematic review, we aim to investigate the classification systems for long bone osteomyelitis that have been presented within the literature. By doing this, we hope to elucidate the important variables that are required when classifying osteomyelitis.
Methods: A complete search of the Medline, EMBASE, Cochrane and Ovid databases was undertaken. Following exclusion criteria, 13 classification systems for long-bone osteomyelitis were included for review.
Results: The 13 classification systems that were included for review presented seven different variables that were used for classification. Ten of them used only one main variable, two used two variables and one used seven variables. The variables included bone involvement (used in 7 classification systems), acute versus chronic infection (used in 6), aetiopathogenesis (used in 3), host status (used in 3), soft tissue (used in 2), microbiology (used in 1) and location of infected bone (used in 1). The purpose of each classification system could be grouped as either descriptive (3 classification systems), prognostic (4) or for management (4). Two of the 13 classification systems were for both prognostic and management purposes.
Conclusions: This systematic review has demonstrated a variety of variables used for classification of long bone osteomyelitis. While some variables are used to guide management and rehabilitation after surgery (e.g., bone defect, soft tissue coverage), others were postulated to provide prognostic information (e.g., host status). Finally, some variables were used for descriptive purposes only (aetiopathogenesis). In our view and from today's perspective, bone involvement, antimicrobial resistance patterns of causative micro-organisms, the need for soft-tissue coverage and host status are important variables to include in a classification system.
Keywords: Long-bone osteomyelitis, Classification, Bone and Joint Infection.
Introduction
Osteomyelitis describes infection and inflammation of bone.1,2 Treatment strategies includes the combination of antibiotics,3 surgical resection4,5 and soft-tissue coverage.6 Ultimately, this has resulted in a specialist multi-disciplinary approach to diagnosis and treatment.7-9 Despite these advances, osteomyelitis is still a clinical challenge from diagnosis to long-term management. The challenges and inherent complexities of osteomyelitis have resulted in difficulties defining a classification system or offering a distinction between chronic and acute disease.10-12
The two most well-known classification systems, the Cierny-Mader and the Waldvogel,13-16 are over 30 years old. Since these were presented, there have been considerable advances in osteomyelitis treatment strategies and management options. In recent years, new classification systems have been introduced. However, they are not widely used owing to complexity and lack of evidence supporting their clinical effectiveness.17,18 Subsequently, there is no uniformly accepted classification system for long bone osteomyelitis.
The aim of this systematic review is to investigate existing classification systems for long bone osteomyelitis within the literature. The variables used within these classification systems and outcome data in association with the corresponding classification will be assessed. Finally, we will discuss the significance of identified variables for the management of the long bone osteomyelitis from today's perspective.
Methods
Electronic resources
The MEDLINE, EMBASE, Ovid and Cochrane databases were searched from their date of development. The study was registered on the PROSPERO database and the protocol can be accessed at the following web address.19
Classification Systems Included
Data was collected on classification systems of long-bone osteomyelitis in both adults and children. Classification systems were included if they used new approaches of classifying or categorising osteomyelitis. Those that only reported a scoring system for indicating the probability of a positive diagnosis of osteomyelitis were excluded from the review.
Selection Strategy and Criteria
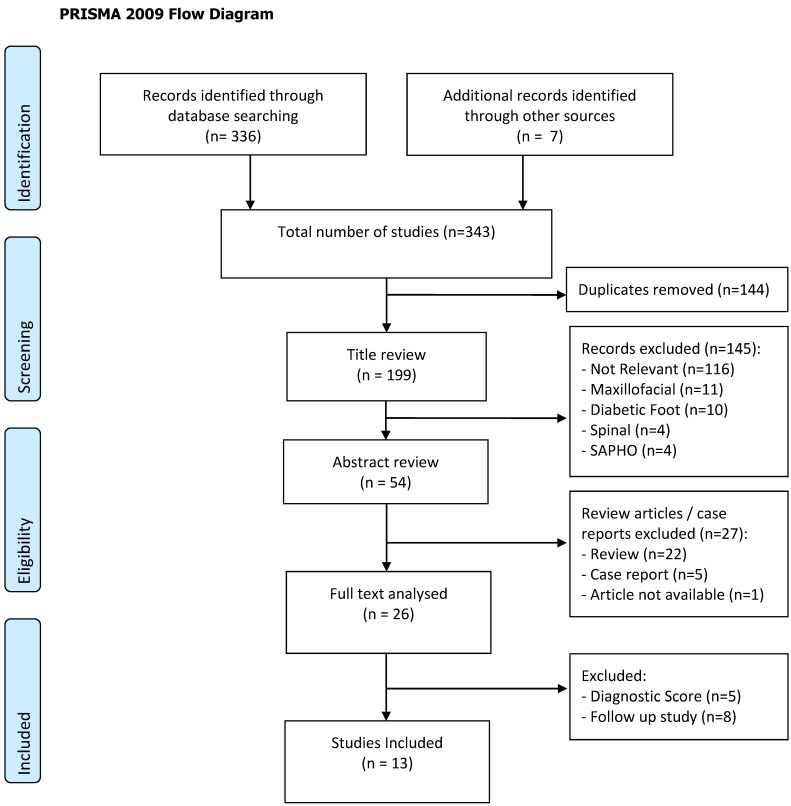
Search terms were chosen by performing preliminary literature searches. Search terms included “bone infection”, “osteomyelitis”, “classification”, “staging” and “scoring”. A full search strategy is shown in figure 1. Duplicate studies and those not published in English were then excluded. Following this, records that were not relevant to long-bone osteomyelitis (predominantly those regarding maxillofacial osteomyelitis, diabetic foot, spinal osteomyelitis or chronic recurrent multifocal osteomyelitis) were excluded. Any animal in vivo or in vitro studies were excluded. There was no minimum follow up period for the included studies. All levels of evidence except case reports were included for review. The references from the included studies were screened and any relevant studies were included for review.
Figure 1.
PRISMA 2009 Flow Diagram
Data Extraction
Demographic data were extracted from the studies which included number of patients in the validation, follow up details and patient age ranges. Each of the specific variables used for classification was reviewed. Definitions of acute and chronic osteomyelitis were also noted.
Study Selection
A total of 336 studies were returned from database searches. Through further reading and references, additional 7 studies were also included (i.e., n = 343). There were 144 duplicate studies that were removed from the results. Then, 145 publications that did not meet the inclusion criteria were excluded based on their title (i.e., n = 54). The reviews and case reports were then excluded based on the title and the abstract. One conference abstract proved difficult to obtain and was therefore excluded.20 Full text articles were retrieved for 26 publications. Of these, 13 were excluded because 8 were studies using previous classification systems, and 5 were for diagnostic purposes. This process is shown in figure 1. Thus, 13 studies met the inclusion criteria and were reviewed.
Results
1. Study Demographics
A summary of the studies included are shown in table 1. All included studies had their classification systems described in patient cohorts, either prospectively or retrospectively. The mean number of patients used to validate the classification systems was 158 (range 14 - 425). Of the 13 studies, 4 were intended for prognostic purposes,21-24 4 for guiding management strategy,25-28 and 2 were for prognosis and guiding management.16,17 Three classifications were not intended for clinical use and did not report outcomes (i.e., for descriptive purposes only).10,13,29 Seven studies reported time scales of patient follow-up (between 6 and 41 months).16,17,21-23,25,28
Table 1.
The 13 included classification systems and the variables each that were described in each.
| Year, author | Cases described | No. of Variables | Aetio-pathogenesis | Bone involvement | Soft-tissue | Host Co-morbidity | Anatomical location | Micro-organism | Acute vs chronic | Purpose |
|---|---|---|---|---|---|---|---|---|---|---|
| 1970, Waldvogel13-15 | 248 | 1 | ● (all) | - | - | - | - | - | + | Des |
| 1982, May21 | 22 | 1 | PT | - | +/- | - | - | - | ● | Pro |
| 1984, Weiland22 | 33 | 1 | PT | ● | +/- | - | - | - | - | Pro |
| 1984, Ceirny & Mader16 | 189 | 2 | All | ● | - | ● | - | - | - | Man / Pro |
| 1988, Gordon23 | 14 | 1 | PT | ● | +/- | - | - | - | + | Pro |
| 1989, May and Jupiter24 | 250 | 1 | PTT | ● | - | - | - | - | * | Pro |
| 1990, Kelly29 | 425 | 1 | ● (all) | - | - | - | - | - | * | Des |
| 1994, Lauschke25 | 55 | 1 | H | - | - | - | - | - | ● | Man |
| 2003, Solagberu26 | 271 | 1 | All | - | - | - | - | - | * | Man |
| 2009, Jones27 | 87 | 1 | H | ● | - | - | - | - | + | Man |
| 2011, Romano10 | 300 | 7 | ● (all) | ● | ● | ● | ● | ● | ● | Des |
| 2013, Yang28 | 51 | 1 | PT | +/- | ● | - | - | - | - | Man |
| 2015, Marais17 | 109 | 2 | All | ● | - | ● | - | - | - | Man / Pro |
● = used as a true / primary variable. This is defined as a variable that the classification is based upon. PT = post-traumatic osteomyelitis. PTT = post-traumatic tibial osteomyelitis only. H=Haematogenous osteomyelitis. All = all types of osteomyelitis. +/- = partially used. Des = descriptive. Pro = prognostic. Man = Management. +=defined, not used. * =used, not defined.
2. Variables
In the 13 classification systems that were reviewed, the variables used for classification included bone involvement (2.2.1), acute or chronic infection (2.2.2), aetiopathogenesis (2.2.3), host status (2.2.4) soft-tissue coverage (2.2.5), anatomical location (2.2.6.) and micro-organisms (2.2.6) (Table 1).
2.1. Number of variables
One of the 13 classification system used 7 variables and two systems used two variables for classification. Ten classification systems used only one variable. Seven of these 10 used a selection criterion to determine eligibility for classification (for example specifying that the classification was for post-traumatic osteomyelitis only or involving free flap soft tissue reconstruction).
2.2. Purpose and sub-classification of variables
2.2.1. Bone Involvement
Seven classification systems used the bone involvement as the main variable for classification, with two of these classification systems being dedicated solely to post-traumatic osteomyelitis in patients who needed free flap coverage. The sub-classification of the variable was either the pattern of bone involvement (medullary, superficial, diffuse, localised),16,17 stability of the bone,17 the extent of bone involvement10,22-24 or radiological presence of abscesses, sequestrum and sclerosis.27 One sub-classification used the extent of bone involvement, which specified that all patients needed a bone defect larger than 3 cm to be classified.28
2.2.2. Acute versus chronic osteomyelitis
Six classification systems used this variable and gave a definition for acute and chronic osteomyelitis. In three further classification systems the descriptive terms 'acute' or 'chronic' were used but not defined. In non-haematogenous osteomyelitis, the transition from acute and chronic disease ranged from 2 weeks to 6 months. One classification system defined chronic osteomyelitis as being present when there was a previous admission for the same disease.13 Two classification systems, both of which were classifications of the soft-tissue status, used the duration of a draining sinus as a marker for acute and chronic disease.21,23 Two classification systems for haematogenous osteomyelitis differed in their time frame for acute and chronic disease, varying from using days25 to months.27
2.2.3. Aetiopathogenesis
Three classification systems defined aetiopathogenesis as one of the primary variables and were for descriptive purposes in all types of osteomyelitis. Waldvogel classified aetiopathogenesis into three categories (haematogenous, contiguous and secondary to vascular insufficiency).14 Kelly used four categories which were haematogenous, secondary to a united fracture, secondary to a non-united fracture and post-orthopaedic procedure.29 Romano used five categories which were haematogenous, secondary to vascular disease, secondary to trauma, with a temporary implant (further classified as type I, II or III) or with permanent implant. Seven classification systems used aetiopathogenesis to determine eligibility for classification and not as a primary variable. Of these, four were for post-traumatic osteomyelitis21-24,28 and one was specifically for infected tibial non-union.23 Two classification systems were designed for haematogenous osteomyelitis in children.25,27 Finally, in the three classification systems for all types of osteomyelitis, the term aetiopathogenesis was used but not defined.16,17,26
2.2.4. Classification of Host Status
Three classification systems classified the host status.10,16,17 Cierny and Mader classified the host into three broad categories, (A) patients who were fit for surgery, (B) patients who were fit for surgery but with either systemic or local compromise and (C) those who were not fit for surgery. The Marais classification built on this method by specifying a points system for entry into each category, with major and minor criteria.17 The Romano classification system used a similar system to the Cierny and Madder but specified whether the patient was an adult, child (<14 years) or infant (<2 years).10
2.2.5. Classification of the Soft-tissues
Two classification systems used the soft tissue status as the primary variable for classification and subclassified clinical cases further by the size of the soft tissue defect.10,28 They related the size of the soft tissue defect in centimeters to prognosis. Three classification systems which were all for post-traumatic osteomyelitis specified the soft tissue defect in their classification.21-23 May et al.21 specified the presence of a draining sinus. Both Weiland et al.20 and Gordon et al.21 specified that the defect should be large enough to need free flap tissue transfer for reconstruction.
2.2.6. Anatomical Location and Micro-organism
The only classification that has used anatomical location of the infection and involved the type of micro-organism is Romano's seven item classification system.10 The anatomical location was divided into spine, hand, long bone, foot or joint infection. The microbiology was divided into Gram-positive or Gram-negative bacteria, polymicrobial growth, presence of mycobacteria, fungal organisms or no growth. The benefit of classifying by using these variables was not reported.
Discussion
Over the past 45 years, multiple classification systems for long bone osteomyelitis have been proposed within the literature. Using a comprehensive search, 13 individual classification systems were retrieved, all of which use a different approach to the classification of osteomyelitis. As of yet, none are uniformly accepted or widely clinically used. In the next paragraphs, we will discuss the variables and their sub-classification found in this review in comparison to views from today's perspective.
Bone involvement
In the Cierny and Mader classification, the pattern of bone involvement was used to guide surgical treatment strategy.16 Interestingly, the pattern of bone involvement had little correlation with prognosis at 2-year-follow-up amongst surgical candidates.30 Although the Cierny and Mader16 anatomical classification did not have a bearing on prognosis, the location within the bone may play a role. One study demonstrated the importance of the location of the infection within the bone of children. In meta-epiphyseal disease 53% (8/15) had relapse compared to none of the 72 children who had non-meta-epiphseal involvement.31 A similar study with adults has not been reported within the literature.
It is paramount to include bone involvement in an osteomyelitis classification. The variable can be classified either by stability, type of defect (segmental versus cavitary), location of infection in the bone (relation to the physis), the anatomical pattern of infection (medullary, superficial, localised or diffuse), or by defining the extent of bone that has been or has to be resected. Patients with larger, unstable defects (more often segmental) have a poorer prognosis compared to patients who have small, stable defects (more often cavitary).9 Gordon23 and Romano10 used a 3 cm bone defect and May and Jupiter24 used a 6 cm defect for their classification. The optimal sub-classification of the variable bone involvement needs to be elucidated.
Acute versus chronic infections
Multiple definitions between acute and chronic osteomyelitis exist, although these vary between authors.10,13,21,23,25,27 These definitions are based either on time scale or the presence of necrotic bone. However, making this distinction in clinical practice can be difficult and unreliable. Knowing the exact starting point of the infection is impossible except in some cases when infection presents shortly after injury or surgery. More importantly, the terms acute and chronic do not have a strong influence on the diagnostic work-up or the principles of medical or surgical management. Necrotic bone should be removed surgically, irrespective of whether the disease is 'acute' or 'chronic'. Therefore, from today's perspective, it would be reasonable to remove these terms from a classification system for long bone osteomyelitis. Another argument is that it does not alter the duration or choice of antimicrobial treatment. Traditionally, it was thought that acute bone infections should be treated for 6 weeks or less, and chronic infections for 3 months.32,33 However, this recommendation is not based on the results of a clinical trial. Moreover, this treatment duration may not be adequate for patients who experience a curative surgical procedure. For example, the treatment duration can be short after surgery for osteomyelitis (i.e., following complete removal of necrotic bone), despite the fact that the disease was 'chronic'. In addition, the expected duration of antimicrobial treatment should not influence whether surgery should be performed. Even without surgical procedure the recommendation may be inadequate. For example, patients not fit for surgery can be treated for a long period without cure (for example, suppressive treatment). On the other hand, chronic osteomyelitis without systemic inflammatory signs may not require antimicrobials (for example, in the presence of a sinus tract, dormant bacteria or low grade clinical presentation). Thus, using the terms acute and chronic does not determine the need for or the duration of antimicrobial treatment.
Aetiopathogenesis
Classification of osteomyelitis by aetiopathogenesis alone is unable to offer detailed information on management or prognosis. Most published series describe cases as either haematogenous, post-traumatic or secondary to co-morbidity associated soft-tissue defects (for example ulcers in diabetic foot syndrome). By using this as a basis, the most simplistic and appropriate method for describing osteomyelitis is either as exogenous (referring to post-traumatic, post-operative or following bone exposure to a non-sterile environment) or endogenous (haematogenous). Waldvogel et al.14 classified osteomyelitis irrespective of anatomic localization and Kelly et al.29 classified only tibial and femoral disease. Both authors defined haematogenous osteomyelitis as a separate entity to osteomyelitis arising from external sources. Kelly et al.29 classified the exogenous osteomyelitis into three subcategories dependent on the presence of a fracture and whether this fracture was united or not. Exogenous osteomyelitis encompasses an exposure to the surrounding environment either through a break in the skin or from direct spread from local soft tissues. This can range from an iatrogenic procedure to an ulcer that allows the entrance of micro-organisms that can cause infection in the bone.
Although classification of osteomyelitis by aetiopathogenesis alone is useful in describing the history of the disease, in our view, it is unlikely to be able to guide treatment in adults or give information on prognosis.
Host Status
Patient co-morbidity is thought to play a role in both guiding appropriate surgical strategies as well as giving an indication of prognosis to patients.30,34 Cierny and Mader first categorised host status dependent on medical co-morbidity.16 Long-term follow up showed that increasing medical co-morbidity did correlate with increased recurrence of infection and need for further treatment.30 This was supported in a 50-patient series which observed longer hospital stays for B hosts when compared to A hosts (12.3 days versus 7.1 days). However, there was no difference in recurrence at mean follow up of 3.2 years.35 This difference in longer hospital stays could be due to the subjective nature of host B and C stratification. In an attempt to remove this subjectivity, a different approach to host status classification was proposed in 2002.18 McPherson built on the Cierny and Mader classification by defining criteria for each A, B and C hosts. These criteria were designed for implant-associated infection rather than osteomyelitis, although it was thought to be applicable to osteomyelitis.10 Using this method, a series of 50 patients with implant-associated infections were followed up over an average of 23.3 months. All of the post-operative deaths were in those defined as C hosts (5/50). There was a correlation between systemic host grade and having one or more complication, amputation or exchange of the foreign body. In addition to McPherson, Marais has proposed a modification to the host status described by Cierny and Mader.17 Here, the definition for A, B and C hosts were dependent on the number of major and minor risk factors. In contrast to the Cierny and Mader classification system where host status may predict outcome and define suitability for surgery, the Marais classification was unable to predict prognosis in the cohort used.17 From today's perspective, recent advances in medical treatment have meant that previously untreatable diseases are now optimisable or curable. One specific example of this is the improvement of outcomes in prosthetic joint infection following the introduction of highly active anti-retroviral therapy in patients with Human Immunodeficiency Virus.36 To accommodate ongoing advances in the treatment of chronic diseases and treating patient's co-morbidities, the use of host status in an osteomyelitis classification system needs to be tailored to factors that adapt accordingly with medical progress. These factors therefore must assess the risk of surgery, and hence, influence the decision on management, and preferably predict the outcome.
Soft-tissue classification
The extent of soft tissue defect in osteomyelitis has been categorised by both duration of skin sinuses and size of the defect. The importance of achieving soft tissue coverage in osteomyelitis has been reported.37-39 The success of soft tissue restoration techniques relies on the state of surrounding soft tissues and this will dictate the need for direct closure, local flap or free flap cover. However, not all cases that are treated with bone debridement require complex soft tissue reconstruction.38,40 The May classification described the success of soft tissue coverage rather than the resolution of the bone infection.37 For larger soft tissue defects, the Yang classification classified three distinct sizes (less than 10x20cm, more than 10x20cm and with limb shortening of 3cm or more) and presented an option for management of each one.28 The soft tissue compromise present in osteomyelitis will therefore vary on an individual basis. If managed correctly, using appropriate soft tissue reconstruction techniques, the osteomyelitis can have a good prognosis.
Micro-organisms
There is little evidence within the literature regarding correlation between causative organism and prognosis in osteomyelitis, although there have been epidemiological changes in the most prevalent organisms in recent years.41 Romano's 2011 classification is the only classification system to incorporate causative organism.10 It is suggested that different organisms require different clinical approaches. In our experience, this is not the case except in mycobacterial osteomyelitis where samples may need specific culture mediums42 and can be treated with antibiotic therapy alone.43,44 Romano's classification may be intended for descriptive purposes only, and to the best of our knowledge, has not been validated clinically. Others reported that the number and type of organism are reported not to have a bearing on the outcome of treatment.16 Although, some micro-organisms, Staphylococcus aureus in particular, are able to reside in the bone in a dormant state after an episode of osteomyelitis. After this, they may reactivate many years later despite adequate surgical and antimicrobial treatment.45,46 In addition, the recent changes in causative organisms and increased bacterial resistance will likely have an impact on clinical outcome. However, there are currently no data proving this theory.41 It has been demonstrated that there is an improvement in outcome when using a specialist musculoskeletal infectious disease consultant, suggesting specialist input in microbial management is an important aspect of managing osteomyelitis.8
Concluding remarks
Traditional classification systems have been extremely helpful to achieve our current state of knowledge. This review highlighted both the values and the limitations of these classification systems. It may reveal why they may not commonly be used in modern clinical practice. The reasons for this observation may include complexity of classification systems, classification systems that are too broad and those that have no definitive aim for clinical use. In our view some of the variables and definitions used are not helpful for the management of the disease in the context of modern diagnostic and management approaches. In addition, this review has demonstrated the lack of consensus in defining acute and chronic osteomyelitis, and that aetiopathogenesis is useful in describing the history of the disease but not a useful variable in a classification system.
To propose a new and applicable classification system, these issues need to be addressed with a practical and clinical focus. In our view, the terms acute and chronic osteomyelitis and the variable aetiopathogenesis should be avoided in a future classification system. We suggest that the bone involvement, antimicrobial resistance patterns of causative micro-organisms, need for soft-tissue coverage and host status are important variables to include. Furthermore, they must be defined in a flexible way in order to remain valid alongside with medical progress.
References
- 1.Chassaignac E. De l'osteomyelite. Bull Mem Soc Chir; 1852. pp. 431–6. [Google Scholar]
- 2.Nelaton A. Elemens de pathologie chirurgicale. 1844.
- 3.Buchman J, Blair JE. Penicillin in the treatment of chronic osteomyelitis; a preliminary report. Arch Surg (Chicago, Ill 1920) 1945 Sep;51:81–92. doi: 10.1001/archsurg.1945.01230040086003. [DOI] [PubMed] [Google Scholar]
- 4.Orr HW. The treatment of acute osteomyelitis by drainage and rest. 1927. Clin Orthop Relat Res. 2006 Oct 1;451(4):4–9. doi: 10.1097/01.blo.0000238778.34939.66. [DOI] [PubMed] [Google Scholar]
- 5.Trueta J. Treatment of War Wounds and Fractures. Br Med J. 1942 May 16;1(4245):616–7. doi: 10.1136/bmj.1.4245.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stark WJ. The use of pedicled muscle flaps in the surgical treatment of chronic osteomyelitis resulting from compound fractures. J Bone Joint Surg Am. 1946 Apr;28:343–50. [PubMed] [Google Scholar]
- 7.Salvana J, Rodner C, Browner BD, Livingston K, Schreiber J, Pesanti E. Chronic osteomyelitis: results obtained by an integrated team approach to management. Conn Med. 2005 Apr;69(4):195–202. [PubMed] [Google Scholar]
- 8.Ziran BH, Rao N, Hall R a. A dedicated team approach enhances outcomes of osteomyelitis treatment. Clin Orthop Relat Res. 2003 Sep;(414):31–6. doi: 10.1097/01.blo.0000087320.60612.86. [DOI] [PubMed] [Google Scholar]
- 9.Bose D, Kugan R, Stubbs D, McNally M. Management of infected nonunion of the long bones by a multidisciplinary team. Bone Joint J. 2015 Jun 1;97B(6):814–7. doi: 10.1302/0301-620X.97B6.33276. [DOI] [PubMed] [Google Scholar]
- 10.Romanò CL, Romanò D, Logoluso N, Drago L. Bone and joint infections in adults: A comprehensive classification proposal. Eur Orthop Traumatol. 2011 May;1(6):207–17. doi: 10.1007/s12570-011-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons B, Strauss E. Surgical management of chronic osteomyelitis. Am J Surg. 2004;188(1 SUPPL. 1):31–3. doi: 10.1016/S0002-9610(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 12.Levine SE, Esterhai Jr. JL, Heppenstall RB, Calhoun J, Mader JT. Diagnoses and staging. Osteomyelitis and prosthetic joint infections. Clin Orthop Relat Res. 1993 Oct;(295):77–86. [PubMed] [Google Scholar]
- 13.Waldvogel F a, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects (first of three parts) N Engl J Med. 1970 Jan 22;282(4):198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 14.Waldvogel F a, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects (Second of Three Parts) N Engl J Med. 1970 Jan 29;282(5):260–6. doi: 10.1056/NEJM197001292820507. [DOI] [PubMed] [Google Scholar]
- 15.Waldvogel F a, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects (Third of Three Parts) N Engl J Med. 1970 Feb 5;282(6):316–22. doi: 10.1056/NEJM197002052820606. [DOI] [PubMed] [Google Scholar]
- 16.Cierny G, Mader JT. Adult chronic osteomyelitis. Orthopedics. 1984 Oct 1;7(10):1557–64. doi: 10.3928/0147-7447-19841001-07. [DOI] [PubMed] [Google Scholar]
- 17.Marais LC, Ferreira N, Aldous C, Sartorius B, Le Roux T. A modified staging system for chronic osteomyelitis. J Orthop. 2015 Dec;12(4):184–92. doi: 10.1016/j.jor.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;(403):8–15. [PubMed] [Google Scholar]
- 19.Hotchen AJ, Sendi P, McNally MA. PROSPERO. http://www.crd.york.ac.uk/prospero/display_record.asp?src=trip&ID=CRD42017052263.
- 20.Ger R. Muscle transposition for the treatment of osteomyelitis. Am Acad Orthop Surg; 1982. p. 49. th Annua(Lecture 204) [Google Scholar]
- 21.May JW, Gallico GG, Lukash FN. Microvascular Transfer of Free Tissue for Closure of Bone Wounds of the Distal Lower Extremity. N Engl J Med. 1982 Feb 4;306(5):253–7. doi: 10.1056/NEJM198202043060501. [DOI] [PubMed] [Google Scholar]
- 22.Weiland AJ, Moore JR, Daniel RK. The efficacy of free tissue transfer in the treatment of osteomyelitis. J Bone Joint Surg Am. 1984;66(2):181–93. [PubMed] [Google Scholar]
- 23.Gordon L, Chiu EJ. Treatment of infected non-unions and segmental defects of the tibia with staged microvascular muscle transplantation and bone-grafting. J Bone Jt Surg Am. 1988 Mar;70(3):377–86. [PubMed] [Google Scholar]
- 24.May JW, Jupiter JB, Weiland AJ, Byrd HS. Clinical classification of post-traumatic tibial osteomyelitis. J Bone Joint Surg Am. 1989 Oct;71(9):1422–8. [PubMed] [Google Scholar]
- 25.Lauschke FH, Frey CT. Hematogenous osteomyelitis in infants and children in the northwestern region of Namibia. Management and two-year results. J Bone Jt Surg Am. 1994;76(4):502–10. doi: 10.2106/00004623-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Solagberu BA. A new classification of osteomyelitis for developing countries. East Afr Med J. 2003 Jul;80(7):373–8. doi: 10.4314/eamj.v80i7.8722. [DOI] [PubMed] [Google Scholar]
- 27.Jones HW, Harrison JW, Bates J, Evans G a, Lubega N. Radiologic classification of chronic hematogenous osteomyelitis in children. J Pediatr Orthop. 2009 Jan;29(7):822–7. doi: 10.1097/BPO.0b013e3181b76933. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Xu Z, Zhang G, Wang J, Hu S, Hou Z. et al. Modified classification and single-stage microsurgical repair of posttraumatic infected massive bone defects in lower extremities. J Reconstr Microsurg. 2013 Nov;29(9):593–600. doi: 10.1055/s-0033-1348064. [DOI] [PubMed] [Google Scholar]
- 29.Kelly PJ, Fitzgerald RH, Cabanela ME, Wood MB, Cooney WP, Arnold PG. et al. Results of treatment of tibial and femoral osteomyelitis in adults. Clin Orthop Relat Res. 1990 Oct;(259):295–303. [PubMed] [Google Scholar]
- 30.Cierny G. Surgical Treatment of Osteomyelitis. Plast Reconstr Surg. 2011;127:190S–204S. doi: 10.1097/PRS.0b013e3182025070. [DOI] [PubMed] [Google Scholar]
- 31.Mantero E, Carbone M, Calevo MG, Boero S. Diagnosis and treatment of pediatric chronic osteomyelitis in developing countries: Prospective study of 96 patients treated in Kenya. Musculoskelet Surg. 2011 Apr;95(1):13–8. doi: 10.1007/s12306-011-0104-0. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes JL, Viana SL, Mendonça JLF, Freitas FMO, Bezerra ASA, Lima G-AS. et al. Mucoid degeneration of the anterior cruciate ligament: magnetic resonance imaging findings of an underdiagnosed entity. Acta Radiol. 2008 Feb;49(1):75–9. doi: 10.1080/02841850701660497. [DOI] [PubMed] [Google Scholar]
- 33.Walter G, Kemmerer M, Kappler C, Hoffmann R. Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int. 2012;109(14):257–64. doi: 10.3238/arztebl.2012.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotchen AJ, Vonberg FW, Ironside EC, Ross-Thriepland S, Avery N, Pearce OJN. Predictors of Infective Outcomes Following Hip Fracture: A Cohort Study. Gerontol Geriatr Med; 2016. p. 2. (0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung AHC, Hawthorn BR, Simpson AHRW. The Effectiveness of Local Antibiotics in Treating Chronic Osteomyelitis in a Cohort of 50 Patients with an Average of 4 Years Follow-Up. Open Orthop J. 2015 Jan;9:372–8. doi: 10.2174/1874325001509010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falakassa J, Diaz A, Schneiderbauer M. Outcomes of total joint arthroplasty in HIV patients. Iowa Orthop J. 2014 Jan;34:102–6. [PMC free article] [PubMed] [Google Scholar]
- 37.May JW, Jupiter JB, Gallico GG, Rothkopf DM, Zingarelli P. Treatment of chronic traumatic bone wounds. Microvascular free tissue transfer: a 13-year experience in 96 patients. Ann Surg. 1991;214(3):241–50. doi: 10.1097/00000658-199109000-00007. -2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNally MA, Small JO, Tofighi HG, Mollan RA. Two-stage management of chronic osteomyelitis of the long bones. The Belfast technique. J Bone Joint Surg Br. 1993 May;75(3):375–80. doi: 10.1302/0301-620X.75B3.8496203. [DOI] [PubMed] [Google Scholar]
- 39.McNally MA, Ferguson JY, Lau ACK, Diefenbeck M, Scarborough M, Ramsden AJ. et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016 Sep 1;98B(9):1289–96. doi: 10.1302/0301-620X.98B9.38057. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson JY, Dudareva M, Riley ND, Stubbs D, Atkins BL, McNally MA. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. Bone Joint J. 2014;96B(6):829–36. doi: 10.1302/0301-620X.96B6.32756. [DOI] [PubMed] [Google Scholar]
- 41.Kremers HM, Nwojo ME, Ransom JE, Wood-wentz CM, Iii LJM, Iii PMH. Trends in the Epidemiology of Osteomyelitis. J Bone Joint Surg Am. 2015 May 20;97(10):837–45. doi: 10.2106/JBJS.N.01350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardam M, Lim S. Mycobacterial osteomyelitis and arthritis. Infect Dis Clin North Am. 2005 Dec;19(4):819–30. doi: 10.1016/j.idc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Bi S, Hu F-S, Yu H-Y, Xu K-J, Zheng B-W, Ji Z-K. et al. Nontuberculous mycobacterial osteomyelitis. Infect Dis (London, England) 2015 Jan 14;47(10):673–85. doi: 10.3109/23744235.2015.1040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sendi P, Brent A. Mycobacterium tuberculosis and prosthetic joint infection. Lancet Infect Dis. 2016 Aug;16(8):893–4. doi: 10.1016/S1473-3099(16)30142-6. [DOI] [PubMed] [Google Scholar]
- 45.Libraty DH, Patkar C, Torres B. Staphylococcus aureus Reactivation Osteomyelitis after 75 Years. N Engl J Med. 2012 Feb 2;366(5):481–2. doi: 10.1056/NEJMc1111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sendi P, Meier R, Sonderegger B, Bonel HM, Schäfer SC, Vögelin E. Reactivated Moraxella osteitis presenting as granulomatous disease. Neth J Med. 2014 Nov;72(9):491–3. [PubMed] [Google Scholar]