Abstract
Purpose
To evaluate the clinical safety and natural history of active surveillance (AS) for incidentally diagnosed small renal mass (SRM).
Materials and Methods
We analyzed prospective data for patients who underwent AS for SRM. From 2010 to 2016, 37 SRMs of less than 3 cm were registered. Computed tomography (CT) and magnetic resonance imaging were used for initial diagnosis and CT, ultrasonography, and chest CT were performed at 6-month intervals. If there was no change in size during 2 years, follow-ups were performed annually. If the growth rate was more than 0.5 cm/y, if the diameter was more than 4 cm, or if clinical progression was observed, we regarded it as progression of SRM and recommended active treatment. We compared the growth rate and clinical course of SRM between patients who remained on surveillance and those who had progressed disease.
Results
The mean age was 63 years (range, 30–86 years) and the mean diameter was 1.8 cm (range, 0.6–2.8 cm) at diagnosis. The mean follow-up period was 27.3 months (range, 6–80 months) and the average growth rate was 0.2 cm/y (range, 0–1.9 cm/y). Six patients (16.2%) showed progression of SRM. Three patients wanted continuous observation, and partial nephrectomy was performed on 3 other patients. None of the patients had clinical progression, including metastasis.
Conclusions
We could delay active treatment for patients with an SRM with scheduled surveillance if the SRM grew relatively slowly. If more long-term AS results are documented for more patients, AS could be an alternative treatment modality for SRM.
Keywords: Kidney, Neoplasms, Surveillance
INTRODUCTION
A small renal mass (SRM) has a slow growth rate, and clinical progression, such as metastasis, is rare [1,2,3,4]. In addition, the accuracy of an imaging diagnosis is lower and the likelihood of benign tumors is relatively high when their size is small [5,6,7,8]. Several studies reported that 7%–30% of them are benign after surgery [5,6,7,8]. However, SRMs still need cautious decisions for treatment, because a few SRMs grow rapidly or progress to metastasis in the early phase [9].
Preoperative histological examination using percutaneous biopsy has helped to distinguish malignant diseases, but the problem is low accuracy for distinguishing grade [10,11,12]. Several studies recommended delayed management for low-grade SRMs, while an aggressive treatment is required for high-grade SRMs [11]. Biopsy alone could not adequately determine an appropriate treatment in all cases. There is no acceptable strategy for diagnosis or treatment of SRMs, although several studies using percutaneous biopsy have been conducted [11].
Considering treatment of SRMs, immediate aggressive treatment after diagnosis is not regarded as the only standard treatment, because of the uncertainty of diagnosis and relatively mild malignant behavior. However, active surveillance (AS) for SRM is still demanded for clinical safety in addition to these rationales. Several oncological outcomes and safety have been reported in AS cohort for SRMs [1,4,9,13]. The Delayed Intervention and Surveillance for Small Renal Masses Registry, the largest prospective AS cohort for SRMs, reported that the 5-year overall survival and cancer-specific survival were 75% and 100%, respectively [13]. Although a few studies have reported early metastasis, most have demonstrated the stability of AS [4,9]. Furthermore, Shuch et al. [14] reported that patients with radical or partial nephrectomy may have more frequent secondary cancers, cardiovascular events, and deterioration of renal function than those who did not have that surgery.
We analyzed prospective outcomes of a single-center SRMs registry in the Korean population. The aim of this study is to evaluate the differences between patients who have progressive SRMs and those who remained on surveillance, and to select the appropriate AS candidates, as well as growth kinetics of SRMs.
MATERIALS AND METHODS
After the Institutional Review Board of Pusan National University Yangsan Hospital approved this prospective study, we found that, from 2010 to 2016, 37 incidentally diagnosed SRMs of less than 3 cm were registered (approval number: 04-2012-008). Overall cases that got the written informed consent, were diagnosed using abdominal computed tomography (CT) or magnetic resonance imaging and nonenhanced chest CT for enrollment. For radiological assessment during follow-up, abdominal CT or ultrasonography and chest CT were performed at 6-month intervals. If there was no change in size for 2 years, follow-ups were performed annually. For clinical assessment during follow-up, physical examination and blood tests, including a complete blood count, creatinine, electrolytes, and urinalysis, were also evaluated. The Charlson comorbidity index was collected for all patients before enrollment.
If the growth rate was more than 0.5 cm/y, if the diameter was more than 4 cm, or if clinical progression was observed, we regarded it as progression of SRM and recommended active intervention. In addition, patients could receive active treatment whenever they wanted. In 12 patients (32.4%), percutaneous ultrasound-guided biopsy was performed at presentation or during follow-up. Histopathological examination was not included in the initial protocol and was applied to patients mainly in the late phase of the study. Biopsy was performed mainly in patients with rapid growing SRM (>5 mm/y) before active treatment.
We compared the growth rate and clinical course of SRMs between patients who remained on surveillance and those who had progressed disease. Categorical and continuous data were evaluated by the chi-square and the Mann-Whitney U-test, respectively; p<0.05 was considered statistically significant. SPSS ver. 17 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
Demographics and tumor characteristics are summarized in Table 1. The mean age was 63 years (range, 30–86 years) and the mean follow-up period was 27.3 months (range, 6–80 months). The mean diameter was 1.8 cm (range, 0.6–2.8 cm) at diagnosis, and the average growth rate was 0.2 cm/y (range, 0–1.9 cm/y).
Table 1. Patient demographics and tumor characteristics.
| Variable | Overall | Not progressed | Progressed | p-value |
|---|---|---|---|---|
| No. of cases | 37 (100) | 31 (83.8) | 6 (16.2) | - |
| Age at presentation (y) | 64 (30–86) | 65 (37–86) | 59 (30–82) | 0.967 |
| Male sex | 26 (70.3) | 22 (71.0) | 4 (66.7) | 0.833 |
| No. of diabetes | 7 (18.9) | 5 (16.1) | 2 (33.3) | 0.315 |
| No. of hypertensions | 14 (37.8) | 14 (45.2) | 2 (33.3) | 0.680 |
| Charlson comorbidity index | 0.964 | |||
| 0 | 5 (13.5) | 4 (12.9) | 1 (16.7) | |
| 1–2 | 25 (67.6) | 21 (67.7) | 4 (66.7) | |
| ≥3 | 7 (18.9) | 6 (19.4) | 1 (16.7) | |
| Initial size (mm) | 18 (6–28) | 17 (6–28) | 21 (17–26) | 0.091 |
| No. of solid masses | 31 (83.8) | 25 (80.6) | 6 (100) | 0.239 |
| Growth rate (mm/y) | 2.3 (0–19.0) | 0.9 (0–3.0) | 9.6 (5.3–19.0) | <0.001 |
| No. of biopsies | 12 (32.4) | 7 (22.6) | 5 (83.3) | <0.001 |
| No. of active interventions | 3 (8.1) | 0 (0) | 3 (50.0) | <0.001 |
| Follow-up duration (mo) | 28 (6–80) | 29 (6–80) | 23 (12–41) | 0.773 |
Values are presented as number (%) or mean (range).
Of the 6 patients (16.2%) who had progression of SRMs, all had more than 0.5 cm/y growth rate, and only 2 patients had a diameter larger than 4 cm. Three patients underwent partial nephrectomy; 3 patients with progressed SRMs wanted only observation. Final pathology after partial nephrectomy was clear-cell carcinoma with high grade. Two renal masses over 4 cm were treated by partial nephrectomy. And they were diagnosed high grade, clear cell type, renal cell carcinoma, finally. None of the patients had clinical progression, including metastasis or cancer-specific death.
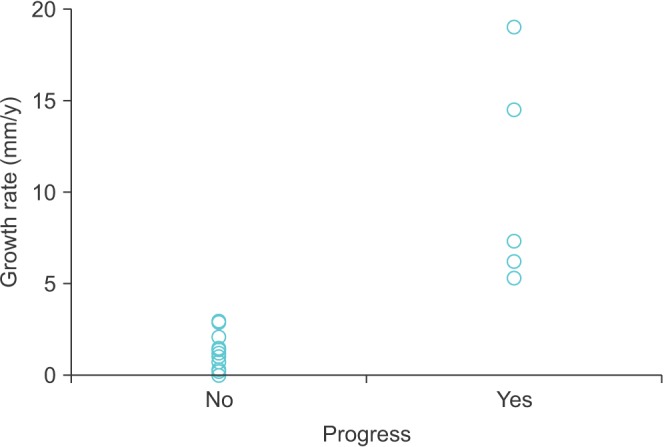
The mean growth rate for the 6 patients who had progressed SRMs were significantly higher than that observed in stable SRMs (1.0 cm/y vs. 0.1 cm/y; p<0.001) (Fig. 1). Otherwise, relative comorbidities were not different in terms of age, sex, hypertension, diabetes, or the Charlson comorbidity index (all p>0.05). There was no difference in initial size between progressed and not-progressed SRMs (2.1 cm vs. 1.7 cm, p=0.091).
Fig. 1. Growth rates of small renal masses according to progress.
Percutaneous renal biopsies were performed on 12 patients (32.3%). Oncocytoma and angiomyolipoma were diagnosed in 2 patients (Table 2). The other 10 SRMs (83.3%) were diagnosed as clear-cell carcinoma. Six cystic SRMs that were regarded as Bosniak class III or IV [15] were included in our AS registry.
Table 2. Pathological results of percutaneous biopsy (n=12).
| Case | Sex | Age (y) | Pathological diagnosis |
|---|---|---|---|
| 1 | Male | 67 | Renal cell carcinoma, clear cell, Furman grade not determined |
| 2 | Male | 45 | Renal cell carcinoma, clear cell, Furman grade not determined |
| 3 | Female | 37 | Oncocytoma |
| 4 | Male | 56 | Renal cell carcinoma, clear cell, Furman grade not determined |
| 5 | Female | 70 | Renal cell carcinoma, clear cell, Furman grade II |
| 6 | Male | 80 | Renal cell carcinoma, clear cell, Furman grade III |
| 7 | Male | 76 | Renal cell carcinoma, clear cell, Furman grade II |
| 8 | Female | 65 | Angiomyolipoma |
| 9 | Female | 59 | Renal cell carcinoma, clear cell, Furman grade II |
| 10 | Male | 60 | Renal cell carcinoma, papillary cell, Furman grade II |
| 11 | Male | 72 | Renal cell carcinoma, clear cell, Furman grade III |
| 12 | Male | 68 | Renal cell carcinoma, clear cell, Furman grade II |
DISCUISSION
Renal cell carcinoma accounts for 3%–4% of all adult cancers, and the incidence is increasing [16], especially that of asymptomatic renal mass, because abdominal ultrasonography and CT are being more frequently used [17]. Radical nephrectomy has historically been the standard treatment for SRMs, defined as less than 4 cm [18]. However, in recent years, nephron-sparing surgery has been recommended because it preserves renal function and has the same oncological outcomes [19]. Since the early 2000s, many studies have reported promising results during AS for SRM in elderly patients with medical comorbidities [1,3,9,13,20,21].
Recently, oncological outcomes of AS for SRM have been reported in large prospective studies [3,13,21]. The Delayed Intervention and Surveillance for Small Renal Masses Registry in the United States reported that overall survival for immediate intervention and AS was 98% and 96% at 2 years, and 92% and 75% at 5 years, respectively (log rank, p=0.06) [13]. At 5 years, cancer-specific survival was 99% and 100% for immediate intervention and AS, respectively (p=0.3) [13]. Canadian phase II trials reported, among 178 patients, only one patient who died from metastasis of renal cell carcinoma, and 10 patients who died from unrelated causes at 3–42 months [21].
In previous small retrospective studies, the possibility of metastasis during AS for SRMs has been reported as 0%–5% [1,2,3,4,9]. The Delayed Intervention and Surveillance for Small Renal Masses Registry, the largest prospective study, did not show distant metastasis in 223 patients after 5 years of follow-up [13]. An earlier Canadian prospective study reported 2 metastases during AS in 178 patients [21]. Therefore, these large prospective data suggested that the rate of metastasis was a little more than 1% during AS for SRMs [3,13,20,21]. Because the most common metastatic site was the lungs [3,9,21], chest CT was checked routinely in the present study.
Among the 37 patients diagnosed with SRMs of less than 3 cm, only 6 (16.2%) were diagnosed as progressed SRMs. The criteria for diagnosis of progressed SRMs were rapid growth rate of more than 0.5 cm/y and diameter of more than 4 cm. Of the 6 progressed cases, fast growth was observed in all 6 patients (100%), but large diameter was diagnosed in only two patients (33.3%). Rapid growth as a diagnostic criterion for aggressive SRM seems to be more sensitive than large diameter. There was no difference in initial size between progressed and not-progressed SRMs. Even though growth rate is related to the initial size of SRMs, we can suggest that growth rates represent the malignant potential of SRMs better than initial size.
The possibility of benign pathological lesions at nephrectomy for SRMs smaller than 4 cm was reported to be 10%–20% in the previous literature [22,23,24,25]. The incidence of benign SRMs according to ethnicity was various. The reported incidences of benign SRMs were 17.3%–23.4% in studies from Western countries [5,22,24,26,27,28] and 7.1%–15.0% in studies from Asian countries [6,23,25]. In Asia, the incidence of benign SRMs is relatively low, because the incidence of oncocytoma is low [6].
In particular, SRMs of less than 2 cm are more likely to be benign lesions, and the accuracy of the imaging method for diagnosis is also poor [23,29]. The accuracy of diagnosis for angiomyolipoma with a low fat component and cystic lesions classified as Bosniak class III or IV is low, making it more difficult to treat it aggressively [15,29]. Two-thirds of the patients in this study had SRMs of less than 2 cm in length. We have set up a strict inclusion criterion to perform AS, which is not yet a definitively safe treatment modality for SRMs.
There are several limitations to this study. First, only a few patients were included during a relatively short follow-up, because AS is still a challenging option for SRMs in Korea. Second, pathological confirmation was not conducted in all cases. Only 12 SRMs were pathologically diagnosed, because biopsy was not included in the initial study protocol. Third, more than half the patients in this study were over 70 years old, with many of them having significant medical comorbidity. The results of this study for specific patients may not be representative of the results for normal patients.
CONCLUSIONS
For incidentally discovered SRMs, AS may be recommended as an alternative to active treatment such as surgery or ablation. In this study, 37 patients with SRM were followed up with predefined protocols and criteria for active treatment. No clinical progression, such as metastasis, was observed. Although there is no significant baseline characteristics to predict SRMs that will progress in the future, the growth rates were significantly different (1.0 cm/y vs. 0.1 cm/y). We can suggest that the rapid-growth rate represents the malignant potential of SRMs better than other parameters, such as initial size.
This initial Korean data is not different from Western or other Asian data. We did not perform biopsy as a routine procedure. If biopsy is performed as a follow-up test or initial diagnostic method in the future, AS can be suggested as a safer and more effective treatment method for SRM.
ACKNOWLEDGMENTS
This study was supported by a Research Grant from Pusan National University Yangsan Hospital.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–745. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 2.Rosales JC, Haramis G, Moreno J, Badani K, Benson MC, McKiernan J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183:1698–1702. doi: 10.1016/j.juro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Mason RJ, Abdolell M, Trottier G, Pringle C, Lawen JG, Bell DG, et al. Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol. 2011;59:863–867. doi: 10.1016/j.eururo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Haramis G, Mues AC, Rosales JC, Okhunov Z, Lanzac AP, Badani K, et al. Natural history of renal cortical neoplasms during active surveillance with follow-up longer than 5 years. Urology. 2011;77:787–791. doi: 10.1016/j.urology.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Pahernik S, Ziegler S, Roos F, Melchior SW, Thüroff JW. Small renal tumors: correlation of clinical and pathological features with tumor size. J Urol. 2007;178:414–417. doi: 10.1016/j.juro.2007.03.129. [DOI] [PubMed] [Google Scholar]
- 6.Fujii Y, Komai Y, Saito K, Iimura Y, Yonese J, Kawakami S, et al. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology. 2008;72:598–602. doi: 10.1016/j.urology.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Heuer R, Gill IS, Guazzoni G, Kirkali Z, Marberger M, Richie JP, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol. 2010;57:223–232. doi: 10.1016/j.eururo.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Vasudevan A, Davies RJ, Shannon BA, Cohen RJ. Incidental renal tumours: the frequency of benign lesions and the role of preoperative core biopsy. BJU Int. 2006;97:946–949. doi: 10.1111/j.1464-410X.2006.06126.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunocilla E, Borghesi M, Monti C, Schiavina R, Martorana G. Surveillance for small renal masses: retrospective analysis of a cohort of 42 patients with long-term follow-up. Int Urol Nephrol. 2013;45:307–312. doi: 10.1007/s11255-013-0389-z. [DOI] [PubMed] [Google Scholar]
- 10.Richard PO, Jewett MA, Tanguay S, Saarela O, Liu ZA, Pouliot F, et al. Safety, reliability and accuracy of small renal tumour biopsies: results from a multi-institution registry. BJU Int. 2017;119:543–549. doi: 10.1111/bju.13630. [DOI] [PubMed] [Google Scholar]
- 11.Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol. 2013;189:441–446. doi: 10.1016/j.juro.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Leveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shiff DA, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578–584. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Pierorazio PM, Johnson MH, Ball MW, Gorin MA, Trock BJ, Chang P, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68:408–415. doi: 10.1016/j.eururo.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Shuch B, Hanley JM, Lai JC, Vourganti S, Setodji CM, Dick AW, et al. Adverse health outcomes associated with surgical management of the small renal mass. J Urol. 2014;191:301–308. doi: 10.1016/j.juro.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Israel GM, Bosniak MA. An update of the Bosniak renal cyst classification system. Urology. 2005;66:484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 17.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 18.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Crispen PL, Viterbo R, Fox EB, Greenberg RE, Chen DY, Uzzo RG. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112:1051–1057. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 21.Jewett MA, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Remzi M, Ozsoy M, Klingler HC, Susani M, Waldert M, Seitz C, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176:896–899. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Soga N, Nishikawa K, Takaki H, Yamada Y, Arima K, Hayashi N, et al. Low incidence of benign lesions in resected suspicious renal masses greater than 2 cm: Single-center experience from Japan. Int J Urol. 2012;19:729–734. doi: 10.1111/j.1442-2042.2012.03030.x. [DOI] [PubMed] [Google Scholar]
- 24.Snyder ME, Bach A, Kattan MW, Raj GV, Reuter VE, Russo P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. J Urol. 2006;176(6 Pt 1):2391–2395. doi: 10.1016/j.juro.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Xiong YH, Zhang ZL, Li YH, Liu ZW, Hou GL, Liu Q, et al. Benign pathological findings in 303 Chinese patients undergoing surgery for presumed localized renal cell carcinoma. Int J Urol. 2010;17:517–521. doi: 10.1111/j.1442-2042.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 26.Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology. 2003;62:827–830. doi: 10.1016/s0090-4295(03)00658-7. [DOI] [PubMed] [Google Scholar]
- 27.Schlomer B, Figenshau RS, Yan Y, Venkatesh R, Bhayani SB. Pathological features of renal neoplasms classified by size and symptomatology. J Urol. 2006;176(4 Pt 1):1317–1320. doi: 10.1016/j.juro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 29.Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN. Benign renal neoplasms in adults: cross-sectional imaging findings. AJR Am J Roentgenol. 2008;190:158–164. doi: 10.2214/AJR.07.2724. [DOI] [PubMed] [Google Scholar]


