Abstract
A growing body of evidence indicates that the intake of large amounts of alcohol during one session may have structural and functional effects on the still-maturing brains of young people. These effects are particularly pronounced in prefrontal and hippocampal regions, which appear to be especially sensitive to the neurotoxic effects of alcohol. However, to date, few studies have used the event-related potentials (ERPs) technique to analyze the relationship between binge drinking (BD) and associative memory. The objective of this study was to examine brain activity during memory encoding using the Subsequent memory paradigm in subjects who have followed a BD pattern of alcohol consumption for at least 2 years. A total of 50 undergraduate students (mean age = 20.6 years), i.e., 25 controls (12 females) and 25 binge drinkers (BDs; 11 females), with no personal or family history of alcoholism or psychopathological disorders, performed a visual face–name association memory task. The task used enables assessment of the Difference due to memory effect (Dm), a measure of memory encoding based on comparison of the neural activity associated with subsequent successful and unsuccessful retrieval. In ERP studies, study items that are subsequently remembered elicit larger positive amplitudes at midline parieto-frontal sites than those items that are subsequently forgotten. The Dm effect generally appears in the latency range of about 300–800 ms. The results showed a Dm effect in posterior regions in the 350–650 ms latency range in the Control group. However, in the BD group, no significant differences were observed in the electrophysiological brain activity between remembered and forgotten items during the encoding process. No differences between groups were found in behavioral performance. These findings show that young BDs display abnormal pattern of ERP brain activity during the encoding phase of a visual face–name association task, possibly suggesting a different neural signature of successful memory encoding.
Keywords: memory encoding, difference memory effect, face–name association, binge drinking, college students
Introduction
Binge drinking (BD) is a pattern of alcohol consumption characterized by the intake of five or more drinks (four or more for females) on a single occasion within a 2-h interval, reaching blood alcohol concentration to 0.08 g/dL (1) at least once in the last month (2).
The most recent report of the World Health Organization (3) has highlighted that the highest rates of BD among young people occur in Europe (31.2%), the USA (18.4%) and the Western Pacific Region (12.5%). Furthermore, the rate reaches 41.8% in the 18–25 age range (4). BD has become a major concern for public authorities because of its adverse impact on a wide range of personal, social, and health issues and also because of the associated economic cost.
Adolescence is a critical stage of development in which the brain undergoes processes of neuromaturation and reorganization (5), which extend into the third decade of life. In accordance with animal studies, this period is particularly sensitive to the effects of BD, which causes more brain damage in adolescent than in adult rats, especially in the prefrontal cortex and the hippocampus (6, 7). It has also been shown that these alterations can lead to long-lasting changes in the adult brain (8, 9).
To date, most of the relevant research in humans has focused on the consequences of this pattern of alcohol consumption on the still-maturing brain (10). Neuropsychological studies have shown that BDs display greater difficulties in processes such as working memory (11), inhibitory control (12), decision-making (13), or declarative memory (14). Functional magnetic resonance imaging (fMRI) studies have also reported abnormal brain activity in BDs during verbal learning tasks (15, 16), affective decision-making (17), working memory (18, 19), or inhibitory control (20). Furthermore, event-related potential (ERP) studies have demonstrated anomalies in BDs in different components related to processes such as attention (21), working memory (22, 23), inhibitory control (24, 25), emotional auditory processing (26), or reactivity to alcohol-related cues (27, 28).
Despite the growing evidence from research on the neurocognitive consequences of BD, few studies have examined brain activity related to associative memory processes in BDs. One type of associative memory, which has a key role in the social context, is the association between names and faces. Neuroimaging studies have shown that the encoding of face–name associations in intramodal (29–31) and intermodal tasks (32, 33) involves a network of brain structures, including the fusiform gyrus, the hippocampal formation and the dorsolateral prefrontal cortex. Scientific evidence regarding BD has reported alterations in regions such as the hippocampus and the prefrontal cortex, and it is, therefore, possible that associative memory may be impaired in BDs.
The Subsequent memory paradigm, in which the neural activity is recorded while individuals are explicitly or implicitly memorizing specific items, is a particularly powerful approach to studying memory encoding. The stimuli are classified on the basis of whether they were remembered or not in a subsequent memory test. In general, fMRI studies have revealed that medial temporal structures and prefrontal regions show greater activity for remembered than for non-remembered items, and this increased activity is assumed to reflect successful encoding processes. This effect, referred to as difference due to memory effect (Dm) or differential neural activity based on memory (34, 35), has been observed for a variety of stimuli, including faces, words, and objects (34).
Event-related potential approaches to studying declarative memory during encoding have also demonstrated the Dm effect. The ERPs elicited by study items that are subsequently remembered show larger positive amplitudes than ERPs elicited by subsequently forgotten items in midline parieto-frontal regions (36, 37). The Dm effect generally appears in the latency range of about 300–800 ms or even later, and it has been shown to be modulated by the type of encoding material (verbal vs non-verbal), task instructions (incidental vs intentional), and the type of encoding (deep vs shallow; associative vs non-associative) (35, 38). Furthermore, the effect is stronger when memory formation is intentional, associative, and requires free-recall judgments (35, 39).
In the present study, we recorded the ERPs elicited while participants performed a face–name pairs association task with subsequent memory testing, in order to shed further light on potential memory deficits associated with BD consumption in university students. We hypothesized that BD would impair face–name memory encoding at an electrophysiological and/or behavioral level because of the role of the hippocampus and the prefrontal regions in associative memory encoding, and taking into account previous reports of the influence of alcohol intake on these regions. The associative task used in this study is characterized by intentional encoding and cued-recall judgments and is, therefore, expected to elicit a clear Dm effect.
Materials and Methods
Participants
The sample comprised 50 undergraduate students. The participants were selected from among first-year students at the University of Santiago de Compostela (Spain) who voluntarily filled in a questionnaire administered in class. The questionnaire included the Alcohol Use Disorders Identification Test (40) and other questions about substance use [for further details, see Ref. (41)]. From this initial screening, 164 subjects were enrolled in a longitudinal neurocognitive study, after undergoing a semi-structured interview in which more detailed inclusion and exclusion criteria were verified. Those participant who (1) drank six or more standard alcoholic drinks on the same occasion, one or more times per month, or (2) during these episodes, drank at a speed of consumption of at least three drinks per hour, were classified as BDs. Those who (1) drank six standard alcoholic drinks on the same occasion less than once per month and (2) drank at a maximum speed of consumption of two drinks per hour were classified as Controls. Exclusion criteria comprised non-corrected sensory deficits, any episode of loss of consciousness for more than 20 min, history of traumatic brain injury or neurological disorder, personal or familial (first-degree) history of psychopathological disorders (according to DSM-IV criteria), use of illegal drugs except cannabis, and scores above 20 in the Alcohol Use Disorders Identification Test. Two years (mean 22 months) after the first neurocognitive evaluation, participants were called for a follow-up. They were interviewed again, and those who continued to fulfill the inclusion and exclusion criteria completed a new neurocognitive assessment in which they carried out the face–name task reported here (as well as other tests). Twenty-five (11 females) subjects from the BD group and 25 (12 females) from the Control group participated in the present study (mean age 20.6 ± 0.66 years). The main demographic data and alcohol and drug use habits of the participants in the follow-up study are summarized in Table 1.
Table 1.
Demographic and drinking characteristics of the Control and BD groups.
| Control | Binge drinkers | p-Value | |
|---|---|---|---|
| Gender (M/F) | 13/12 | 14/11 | ns |
| Age | 20.48 ± 0.58 | 20.76 ± 0.72 | ns |
| Handedness (right/left) | 23/2 | 22/3 | ns |
| Caucasian ethnicity (%) | 100 | 100 | ns |
| Regular tobacco smokers | 0 | 9 | 0.001* |
| Regular use of cannabisa | 0 | 4 | 0.070 |
| SCL-90-R: GSI percentile scores | 37.20 ± 29.93 | 38.40 ± 31.61 | 0.891 |
| Age of onset of drinking | 15.52 ± 1.03 | 14.56 ± 1.36 | 0.011* |
| Total grams of alcohol in a standard week | 39.3 ± 35.92 | 251.6 ± 136.66 | 0.001* |
| Speed of consumption: drinks per hour | 0.90 ± 0.76 | 2.56 ± 0.82 | 0.001* |
| Number of drinks in a standard drinking episode | 1.98 ± 1.28 | 5.62 ± 1.79 | 0.001* |
| Percentage of times became drunk when drinking | 14.28 ± 21.19 | 58.40 ± 26.25 | 0.001* |
| Total AUDIT score | 2.80 ± 2.35 | 11.68 ± 3.62 | 0.001* |
aOnce or more a week.
*p < 0.05.
ns, non-significant; SCL-90-R, Symptom Checklist-90-Revised; AUDIT, Alcohol Use Disorders Identification Test.
Informed consent was obtained from all subjects, who were paid for their voluntary participation in the study. The experiment study was undertaken in compliance with Spanish legislation and the Code of Ethical Principles for Medical Research Involving Humans Subjects outlined in the Declaration of Helsinki.
Procedure
Subjects were asked to abstain from consuming alcohol and other drugs for 24 h before the experiment to prevent effects of acute alcohol intake and to rule out withdrawal effects. In addition, they were instructed not to smoke or drink tea or coffee for at least 3 h before the assessments.
Participants were seated in an electrically shielded, sound attenuated, and dimly lit room at a viewing distance of 100 cm from a 21″ video CRT monitor (1,024 × 768 at 60 Hz). Each stimulus consisted of a face (2.9°× 3.4° visual angle) projected on a gray background and with a fictional first name printed underneath. The pictures, depicting 108 unfamiliar faces (half were female), were extracted from the AR Face Database (42). The images were cropped, resized, oval masked, and converted to gray level images using ImageMagick. All faces had a neutral expression.
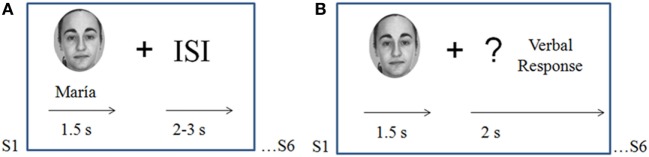
During the study phase of the experiment, participants were asked to form associations between each face and the corresponding name and to try to memorize them. Face–name pairs were arranged in 18 encoding blocks (six pairs per block) presented for 1.5 s and followed by a 2–3 s randomly varying inter-stimuli interval.
Immediately after each encoding block, participants were presented with a recall block that consisted of a cued-recall test for names in which each of the six memorized face stimuli were presented (in a different order than in the learning block) for 1.5 s, followed by a question mark that remained in the center of the screen for 2 s. Participants were instructed to verbally recall the name that matched the face presented. Responses were allowed only after the face had disappeared from the screen, during presentation of the question mark (Figure 1).
Figure 1.
Task design. (A) Study phase (encoding blocks): participants were asked to memorize novel face–name associations. Pairs were presented for 1.5 s each with a variable inter-stimulus interval of 2–3 s. (B) Recall blocks: the six faces memorized during the precedent encoding block were presented alone for 1.5 s; participants were instructed to verbally recall the name associated with each face when a question mark appeared on the screen.
Faces and names were never repeated during the encoding blocks of the experiment to ensure that the brain activation during this phase only reflected encoding processes and not automatic retrieval (recognition of familiar faces).
Electroencephalogram (EEG) Recording and Processing
The electroencephalogram was recorded with Brain Vision amplifiers (BrainAmp), using an Easycap with 32 synterized Ag–AgCl electrodes placed at AF3, AFz, AF4, F7, F3, Fz, F4, F8, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4, T7, T8, P7, P3, Pz, P4, P8, PO7, PO3, POz, PO4, PO8, O1, Oz, and O2 (according to the extended 10–20 International System). All active electrodes were referred to the nose tip and grounded with an electrode placed at Fpz. Vertical electrooculogram was recorded bipolarly from above and below the left eye to control eye movements and blinks. Electrode impedances were kept below 20 kΩ. EEG signals were continuously amplified and digitized at a rate of 500 Hz, and filtered on-line with a 0.01–100 Hz band pass filter.
Electroencephalogram data were off-line processed with Brain Vision Analyzer software (Version 2.0). Ocular artifacts were corrected by the procedure developed by Gratton et al. (43). The data were then digitally filtered with a 0.1–30 Hz bandpass filter (24 dB/oct) and segmented into epochs of 1000 ms, from 100 ms pre-stimulus to 900 ms post-stimulus. Those which exceeded ±90 μV were rejected and baseline-corrected.
Epochs recorded during encoding blocks were averaged separately according to the participant’s memory judgments in the subsequent cued-recall test. Therefore, two conditions per group were computed: correctly encoded (CE) face–name pairs and incorrectly encoded (IE) pairs. There were no statistical differences in the number of averaged epochs between the groups for the CE (Controls: 51.04 ± 16.69; BD: 44.76 ± 14.36) or IE condition (Controls: 36.16 ± 15.49; BD: 39.84 ± 15.46).
Several measures were extracted for each averaged ERPs: (1) the Dm, an index of memory encoding that was the focus of this study, was quantified by comparing the mean ERP amplitudes elicited during encoding of pairs that were later remembered or forgotten in three separate latency intervals (200–350, 350–500, and 500–650 ms) at centroparietal (mean amplitude of the sites CP3, CPz, CP4, P3, Pz, and P4 at each latency interval), and parieto-occipital (mean amplitude of sites PO3, POz, PO4, O1, Oz, and O2) regions. These latency windows were selected to cover the deflection where Dm is apparent by visual inspection of the grand-averages and adjusted so that they comprised 150 ms equal length and covered all positive deflection. (2) N170 and vertex positive potential (VPP) were measured to confirm whether the task elicited the usual ERP pattern for perceptual processing of faces. N170 was identified as the mean amplitude in the 140–180 ms post-stimulus interval at P7, PO7, P8, and PO8 electrode sites; VPP was measured as the mean amplitude in the same latency interval at C3, Cz, and C4.
Data Analysis
Preliminary analysis considering Gender and Laterality (left vs right hemisphere) did not indicate any significant main effects and/or interactions of these factors, and therefore they were not included in subsequent analyses.
Behavioral performance was analyzed by using a Student’s t-test to compare (Control vs BD) the percentage of hits (subsequently recalled names associated with faces).
Regarding the ERPs, analysis of variance (ANOVA) was conducted for the Dm effect in a 2 × 2 × 2 mixed-model design, with two within-subject factors, Condition (CE vs IE) and Region (Centroparietal vs Parieto-occipital), and one between-subject factor, Group (Control vs BD) for each of the three measured latency intervals. N170 was analyzed using a 2 × 4 × 2 mixed-model design with Condition (CE vs IE) and Electrode (P7, PO7, P8, and PO8) as within-subject factors, and Group (Control vs BD) as a between-subject factor, and VPP in a 2 × 3 × 2 mixed-model design with Condition (CE vs IE) and Electrode (C3, Cz, C4) as within-subjects factors, and Group (Control vs BD) as a between-subject factor.
Alpha levels were considered at 0.05 and the degrees of freedom were corrected, when appropriate, by the Greenhouse-Geisser estimate. Post hoc paired comparisons were performed with the Bonferroni adjustment for multiple comparisons.
Results
Behavioral Performance
The percentage of correctly recalled names (Control = 58.59 ± 13.2%; BD = 55.55 ± 13.3%) was equivalent in the two groups [t(48) = 0.808, p = 0.423].
Event-Related Potentials
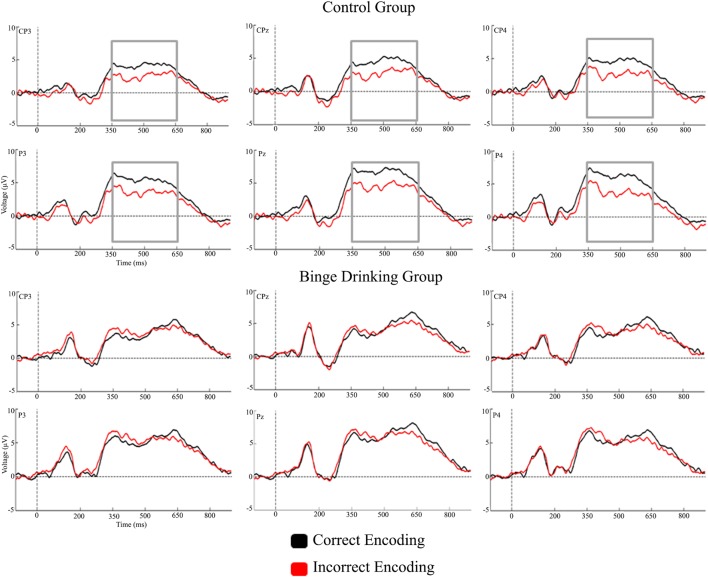
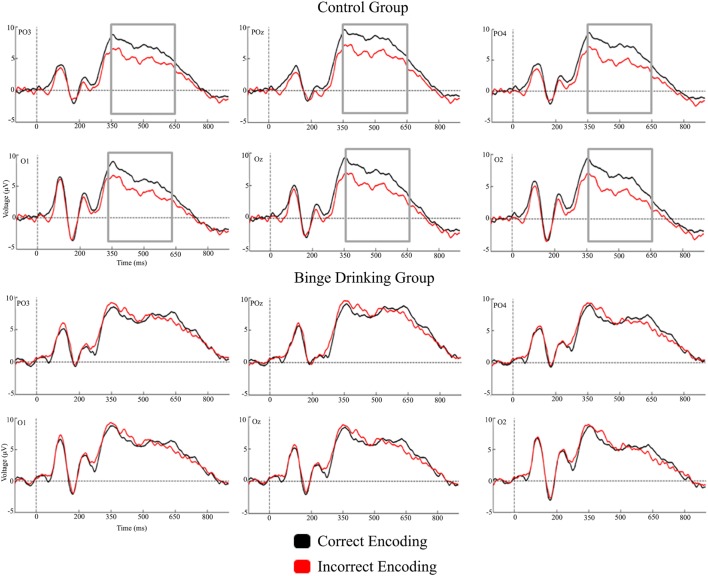
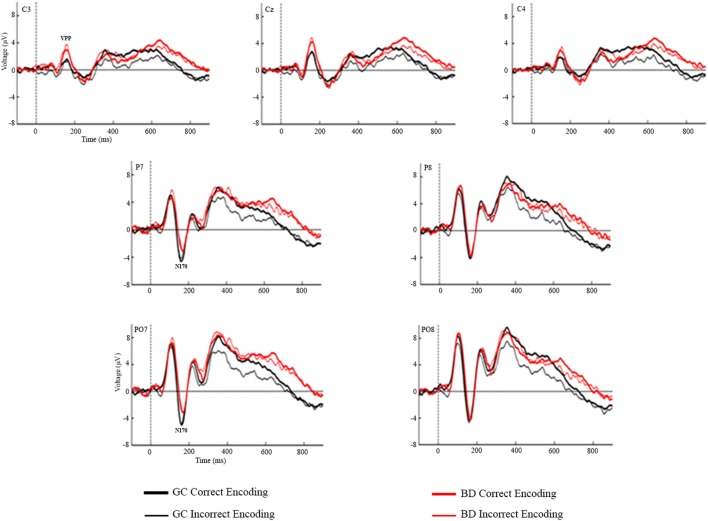
Figures 2 and 3 represent the grand-averages of the ERP at the centroparietal and parieto-occipital sites considered to evaluate the Dm. Figure 4 represents the grand-averages corresponding to the electrode sites considered to evaluate N170 and VPP.
Figure 2.
Grand-averages of event-related potentials from Control and binge drinking groups at centroparietal region.
Figure 3.
Grand-averages of event-related potentials from Control and binge drinking groups at parieto-occipital region.
Figure 4.
Grand-averages of event-related potentials from Control and binge drinking groups at C3, Cz, C4 (VPP) and at P7, P8, PO7, and PO8 electrodes (N170).
Table 2 presents the descriptive statistics of the amplitude values for the two groups and conditions at the three latency windows used to analyze the Dm.
Table 2.
Mean amplitude (μV) in the CE and IE conditions (mean ± SD) at the 12 electrodes analyzed in Control and BD groups at 200–350, 350–500, and 500–650 ms latency intervals.
| BD |
CG |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200–350 ms |
350–500 ms |
500–650 ms |
200–350 ms |
350–500 ms |
500–650 ms |
|||||||
| CE | IE | CE | IE | CE | IE | CE | IE | CE | IE | CE | IE | |
| CP3 | 0.77 ± 2.94 | 1.31 ± 2.33 | 3.34 ± 3.61 | 3.92 ± 4.32 | 4.94 ± 3.48 | 4.46 ± 4.79 | 0.72 ± 3.27 | −0.10 ± 4.23 | 4.00 ± 4.28 | 2.36 ± 5.34 | 4.29 ± 4.26 | 2.95 ± 4.72 |
| CPz | 0.58 ± 2.95 | 0.71 ± 2.48 | 3.68 ± 3.72 | 3.92 ± 4.22 | 5.83 ± 3.22 | 4.94 ± 4.57 | 0.40 ± 3.41 | −0.41 ± 4.35 | 4.29 ± 4.68 | 2.52 ± 5.60 | 4.71 ± 4.66 | 3.25 ± 4.92 |
| CP4 | 1.11 ± 2.72 | 1.35 ± 2.57 | 3.82 ± 3.58 | 4.27 ± 4.24 | 5.26 ± 3.03 | 4.48 ± 4.22 | 1.23 ± 3.33 | 0.60 ± 4.38 | 4.62 ± 4.53 | 2.90 ± 5.73 | 4.58 ± 4.33 | 2.84 ± 4.64 |
| P3 | 2.25 ± 2.90 | 2.79 ± 2.76 | 5.31 ± 3.84 | 5.81 ± 4.92 | 6.24 ± 3.45 | 5.70 ± 5.11 | 1.81 ± 3.41 | 0.82 ± 4.66 | 5.67 ± 4.42 | 3.79 ± 5.49 | 5.22 ± 4.68 | 3.66 ± 5.10 |
| Pz | 1.97 ± 3.22 | 2.29 ± 2.76 | 6.02 ± 4.01 | 6.38 ± 4.84 | 7.49 ± 3.23 | 6.70 ± 4.85 | 1.78 ± 3.52 | 0.59 ± 4.75 | 6.77 ± 4.62 | 4.59 ± 5.76 | 6.49 ± 4.93 | 4.74 ± 5.12 |
| P4 | 2.77 ± 2.87 | 3.05 ± 2.87 | 5.62 ± 3.80 | 6.01 ± 4.69 | 6.24 ± 3.04 | 5.47 ± 4.48 | 2.63 ± 3.60 | 1.58 ± 4.75 | 6.45 ± 4.55 | 4.20 ± 5.93 | 5.69 ± 4.68 | 3.56 ± 5.00 |
| PO3 | 3.92 ± 3.66 | 4.45 ± 3.62 | 7.25 ± 4.02 | 7.73 ± 5.45 | 7.46 ± 3.60 | 6.88 ± 5.45 | 3.23 ± 3.75 | 2.15 ± 5.15 | 7.54 ± 4.71 | 5.55 ± 5.83 | 6.10 ± 5.20 | 4.48 ± 5.44 |
| POz | 3.30 ± 3.92 | 3.85 ± 3.72 | 7.83 ± 4.13 | 8.38 ± 5.52 | 8.44 ± 3.31 | 7.97 ± 5.43 | 2.85 ± 4.00 | 1.64 ± 5.14 | 8.64 ± 4.57 | 6.34 ± 5.88 | 7.32 ± 5.30 | 5.49 ± 5.36 |
| PO4 | 4.43 ± 3.49 | 4.92 ± 3.52 | 7.29 ± 4.18 | 7.81 ± 5.19 | 7.13 ± 3.13 | 6.44 ± 4.79 | 3.77 ± 3.87 | 2.56 ± 5.08 | 8.00 ± 4.25 | 5.59 ± 5.95 | 6.36 ± 4.84 | 4.26 ± 5.18 |
| O1 | 4.70 ± 4.57 | 5.18 ± 4.90 | 7.07 ± 4.15 | 7.47 ± 5.74 | 6.39 ± 3.97 | 5.89 ± 5.87 | 4.03 ± 4.06 | 3.03 ± 5.40 | 7.02 ± 4.95 | 5.18 ± 5.83 | 4.99 ± 5.31 | 3.42 ± 5.56 |
| Oz | 3.61 ± 4.37 | 4.15 ± 4.58 | 6.85 ± 4.32 | 7.30 ± 5.68 | 6.54 ± 3.94 | 5.97 ± 5.65 | 3.22 ± 4.23 | 2.00 ± 5.27 | 7.89 ± 4.90 | 5.76 ± 5.90 | 5.94 ± 5.20 | 4.18 ± 5.42 |
| O2 | 4.83 ± 3.94 | 5.32 ± 4.40 | 6.65 ± 4.48 | 7.04 ± 5.45 | 5.64 ± 3.44 | 5.06 ± 4.96 | 4.14 ± 4.35 | 2.88 ± 5.14 | 7.58 ± 4.64 | 5.25 ± 6.06 | 5.47 ± 4.83 | 3.48 ± 5.34 |
BD, binge drinking; CG, Control group; CE, correct encoding; IE, incorrect encoding.
With regard to the Dm effect, the analysis performed in the 200–350 ms interval did not reveal either a main effect or an interaction between Group and Condition factors. In the 350–500 ms latency window, the analysis revealed a significant difference between Regions [F(1,48) = 56.37, p < 0.001, ], with larger amplitudes at the parieto-occipital region. There was also a significant Condition by Group interaction [F(1,48) = 5.01, p = 0.030, ]; post hoc analysis indicated that differences between conditions (larger amplitude for CE than IE, i.e., Dm effect) were only significant in the Control group (p = 0.012). Analysis of the 500–650 ms interval revealed a significant main effect of Condition (Dm effect) [F(1,48) = 4.83, p = 0.033, ] and Region [F(1,48) = 10.14, p = 0.003, ] (larger amplitude at the parieto-occipital region). Although no significant Condition by Group interaction was detected, post hoc analysis revealed that differences between conditions were only significant in the Control group (p = 0.028).
Statistical analysis of the mean amplitudes in the N170 latency range did not reveal any significant main effects or interactions of the Group or Condition factors. Regarding the VPP component, the analysis revealed significant differences between groups [F(1,48) = 4.56, p = 0.038], with larger amplitudes in the BD (3.08 µV) than in the Control group (1.64 µV), there were also a main effects of the Electrode factor [F(2,96) = 46.89, p < 0.001, ε = 0.89, ] (larger amplitudes at the midline location) and Electrode by Group interaction [F(2,96) = 3.46, p = 0.036, ], post hoc analysis indicated significant differences between groups at C3 (p = 0.015) and Cz (p = 0.031) electrodes, with larger amplitudes in BD than Control group.
Discussion
In the present study, ERPs were used to examine the effects of alcohol BD on declarative memory encoding among undergraduate students, using a subsequent memory paradigm with a visual face–name association memory task.
The results revealed that, despite the absence of behavioral differences between the groups (percentage of associations remembered), the Control and BD groups showed electrophysiological differences during memory encoding. The Control group displayed the classic Dm effect at the 350–500 ms latency window, with larger amplitudes for subsequently remembered face–name pairs than for those that were subsequently forgotten, whereas the BD group did not show this effect, and displayed similar neural activity for successful and unsuccessful encoding. The Dm appeared in the global sample at the 500–650 interval; however, post hoc analyses showed that it was only significant in the Control group.
The literature about Dm effect has described significant differences in ERPs between conditions (remembered and unremembered stimuli) during the encoding of verbal and non-verbal material in young healthy people (37, 44, 45). In the present study, significant differences were observed in the Control group, whereas the BD group showed a lack of electrophysiological differences between successful and unsuccessful memory encoding. The absence of differences in neural activity would indicate anomalous processing during this memory stage in young BD subjects that seems to mask the expected Dm effect.
Studies focusing on alcoholic patients have suggested that face–name association learning is impaired in this population (46, 47). Pitel et al. (48) used magnetic resonance imaging with a face–name association task to assess different memory processes, such as face–name memory encoding with different levels of processing (i.e., shallow and deep encoding), showing poorer associative and single-item recognition in alcoholics than in controls.
Regarding BD, neuropsychological (14, 49–51), and fMRI studies (15, 16, 52) have reported impairments in declarative memory among BDs. However, to our knowledge, no previous studies have used ERPs to assess this type of memory in young BDs.
Two previous studies used fMRI to evaluate neural activity during declarative memory in BD subjects. Schweinsburg et al. (15, 16) reported that BDs exhibited increased BOLD activity in frontal and parietal regions and decreased activity in frontal and occipital cortex during memory encoding of new words; however, they did not differentiate items according to subsequent recall. Dager et al. (52) used the subsequent memory paradigm to assess the Dm effect during encoding of visual abstract stimuli. They found that heavy drinkers displayed increased BOLD activity during successful encoding in frontal, posterior parietal, and medial temporal regions, together with less left inferior frontal activation and greater precuneus deactivation during incorrect encoding. Dager et al. (52) suggested that heavy drinkers could show compensatory hyperactivation during correct encoding and greater deactivation of default mode regions during incorrect encoding, which would mean that this population would use different encoding strategies. These results are not in line with our ERP study. However, it should be noted that there were technical and experimental differences between both studies, as they analyzed BOLD activity and they used different stimuli. Moreover, in the study of Dager et al. (52), the sample characteristics were also different, as these authors did not exclude subjects with alcohol use disorder (39.1% of their Heavy Drinkers Group met criteria for this disorder). The results of the two studies are not, therefore, directly comparable.
Previous ERP and fMRI studies have also found neural hyperactivation associated with BD during different cognitive processes, such as working memory (19, 53, 54), inhibitory processes (20, 24, 25), decision-making (17, 55), or reactivity to alcohol-related cues (27, 28). These authors have suggested that the increased activity may be related to the recruitment of additional resources to compensate for underlying neurocognitive deficits in BD. On the contrary, a few other studies have reported smaller amplitudes of ERP components related to perceptual and attentional processes (56) and working memory (23). Accordingly, these authors have suggested that BDs show some neurocognitive anomalies that have been found to be similar in alcoholics, and they have proposed that the maintenance of a BD pattern could be considered a first step toward the development of alcoholism.
In the present study, it is not possible to relate the absence of the Dm effect in the BD group with neural hyper- or hypoactivation, because differences between groups were not significant for the CE or the IE ERP amplitudes; however, they point out to an anomalous activity in regions involved in memory encoding that prevents the emergence of the Dm effect observed in normal population. Further studies are necessary to replicate these results and to clarify whether the absence of Dm is due to abnormal unspecific hyperactivation when encoding fails or to decreased activity when it is successful.
Regarding the inconsistency between behavioral and neural results, most ERP and fMRI studies on BD have found anomalies in neural activity with no behavioral differences between groups (15, 16, 18, 19, 22–24, 26, 27, 53, 54, 56, 57). It has been argued that college BDs who did not develop alcohol dependency manifest subtle deficits that go unnoticed at the behavioral level, but are detected by ERP or neuroimaging techniques. It is possible that subjects who maintain the BD pattern of consumption for a long time may begin to show similar behavioral impairments to those described in patients with alcohol use disorder following after several years of BD.
Although this study focused on components associated with memory encoding, the N170 and VPP were assessed because they are specifically related to perceptual processing of faces (58, 59). Moreover, it should be noted that very few studies on BD have reported anomalies in perceptual ERP components (26, 27, 56). Regard face perception processes, only Maurage et al. (56), in an oddball task using faces as stimuli, reported lower N170 amplitude in high BDs in comparison with light BDs, daily drinkers, and controls. The VPP component was not assessed in that work, and the reported N170 effect was not replicated in the present study. Our results revealed significant differences between groups in the VPP component, with larger amplitudes in BD than Control group. However, in this task each stimulus consisted of a face with a name written below, and face–related and name-related visual ERP components may, therefore, have overlapped. In this sense, these results should be interpreted with caution because we cannot be sure whether the central positive component reflects only face perceptual processes.
In summary, the results of the present study indicate the presence of electrophysiological differences between young college student BDs and controls during the memory encoding process in a visual face–name associative memory task, with an absence of the Dm effect in the BDs. Although the neural significance of these results is not clear, it points, as neuropsychological and fMRI previous evidence, that encoding on declarative memory could be affected by BD in young population. Further studies with larger samples are required to replicate these findings and to further inquiry in its meaning.
Ethics Statement
The protocol was approved by the Bioethics Committee of the Universidade de Santiago de Compostela. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
FC and SRH designed the general study, including sample selection criteria. PP-A implemented the task and wrote a preliminary draft. AC and EL-C collected the data. PP-A, RF-A, and SRH analyzed the data. FC, PP-A, RF-A, and SRH interpreted the data. RF-A wrote the manuscript. All authors have critically revised the manuscript for intellectual content. The final version was approved for publication by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by grants from the Spanish Ministerio de Sanidad, Servicios Sociales e Igualdad—Plan Nacional sobre Drogas (2005/PN014, 2015/034), Ministerio de Economía y Competitividad (PSI2015-70525-P) co-funded by the European Regional Development Fund. RF-A is funded by a Predoctoral Fellowship (ED481A-2016/141) from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia, co-funded by FSE Galicia 2014-2020. EL-C and AC are currently supported by the SFRH/BPD/109750/2015 and the SFRH/BPD/91440/2012 Postdoctoral Fellowships of the Portuguese Foundation for Science and Technology, respectively.
References
- 1.National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined (2016). Available from: http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- 2.Parada M, Corral M, Caamaño-Isorna F, Mota N, Crego A, Rodríguez Holguín S, et al. Definition of adolescent binge drinking. Adicciones (2011) 23:53–63. 10.1016/j.alcohol.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; (2014). [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; (2013). [Google Scholar]
- 5.Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol (2010) 44:15–26. 10.1016/j.alcohol.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Crews F, Braun C, Hoplight B, Switzer R, III, Knapp D. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res (2000) 24:1712–23. 10.1111/j.1530-0277.2000.tb01973.x [DOI] [PubMed] [Google Scholar]
- 7.Risher ML, Fleming R, Risher C, Miller KM, Klein R, Wills T, et al. Adolescent intermittent alcohol exposure: persistence of structural and functional hippocampal abnormalities into adulthood. Alcohol Clin Exp Res (2015) 39:989–97. 10.1111/acer.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews F, Vetreno R, Broadwater M, Robinson D. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev (2016) 68:1074–109. 10.1124/pr.115.012138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetreno R, Yaxley R, Paniagua B, Johnson G, Crews F. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addict Biol (2016) 22:712–23. 10.1111/adb.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear LP. Adolescent neurodevelopment. J Adolesc Health (2013) 52:S7–13. 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parada M, Corral M, Mota N, Crego A, Rodríguez Holguín S, Cadaveira F. Executive functioning and alcohol binge drinking in university students. Addict Behav (2012) 37:167–72. 10.1016/j.addbeh.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Nederkoorn C, Baltus M, Guerrieri R, Wiers R. Heavy drinking is associated with deficient response inhibition in women but not in men. Pharmacol Biochem Behav (2009) 93:331–6. 10.1016/j.pbb.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 13.Goudriaan A, Grekin E, Sher K. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res (2007) 31:928–38. 10.1111/j.1530-0277.2007.00378.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parada M, Corral M, Caamaño-Isorna F, Mota N, Crego A, Rodríguez Holguín S, et al. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res (2011) 35:1475–84. 10.1111/j.1530-0277.2011.01484.x [DOI] [PubMed] [Google Scholar]
- 15.Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol (2010) 44:111–7. 10.1016/j.alcohol.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction (2011) 106:564–73. 10.1111/j.1360-0443.2010.03197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, et al. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol Addict Behav (2013) 27:443–54. 10.1037/a0027892 [DOI] [PubMed] [Google Scholar]
- 18.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs (2012) 73:749–60. 10.15288/jsad.2012.73.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campanella S, Peigneux P, Petit G, Lallemand F, Saeremans M, Nöel X, et al. Increased cortical activity in binge drinkers during working memory task: a preliminary assessment through a functional magnetic resonance imaging study. PLoS One (2013) 8:e62260. 10.1371/journal.pone.0062260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (2013) 230:663–71. 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol Teratol (2007) 29:153–63. 10.1016/j.ntt.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 22.Crego A, Rodríguez Holguín S, Parada M, Mota N, Corral M, Cadaveira F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol Clin Exp Res (2009) 33:1870–9. 10.1111/j.1530-0277.2009.01025.x [DOI] [PubMed] [Google Scholar]
- 23.Crego A, Rodríguez Holguín S, Parada M, Mota N, Corral M, Cadaveira F. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend (2010) 109:45–56. 10.1016/j.drugalcdep.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 24.López-Caneda E, Cadaveira F, Crego A, Gómez-Suárez A, Corral M, Parada M, et al. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction (2012) 107:1796–808. 10.1111/j.1360-0443.2012.03908.x [DOI] [PubMed] [Google Scholar]
- 25.Smith JL, Mattick RP. Evidence of deficits in behavioral inhibition and performance monitoring in young female heavy drinkers. Drug Alcohol Depend (2013) 133:398–404. 10.1016/j.drugalcdep.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 26.Maurage P, Pesenti M, Philippot P, Joassin F, Campanella S. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J Psychiatry Neurosci (2009) 34:111–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Petit G, Kornreich C, Maurage P, Noël X, Letesson C, Verbanck P, et al. Early attentional modulation by alcohol-related cues in young binge drinkers: an event-related potentials study. Neurophysiol Clin (2012) 123:925–36. 10.1016/j.clinph.2011.10.042 [DOI] [PubMed] [Google Scholar]
- 28.Petit G, Kornreich C, Verbanck P, Campanella S. Gender differences in reactivity to alcohol cues in binge drinkers: a preliminary assessment of event related potentials. Psychiatry Res (2013) 209:494–503. 10.1016/j.psychres.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 29.Kirwan CB, Stark CE. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus (2004) 14:919–30. 10.1002/hipo.20014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campanella S, Joassin F, Rossion B, De Volder A, Bruyer R, Crommelinck M. Association of the distinct visual representations of faces and names: a PET activation study. Neuroimage (2001) 14:873–82. 10.1006/nimg.2001.0877 [DOI] [PubMed] [Google Scholar]
- 31.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus (2007) 17:1071–80. 10.1002/hipo.20340 [DOI] [PubMed] [Google Scholar]
- 32.Herholz K, Ehlen P, Kessler J, Strotmann T, Kalbe E, Markowitsch HJ. Learning face-name associations and the effect of age and performance: a PET activation study. Neuropsychologia (2001) 39:643–50. 10.1016/S0028-3932(00)00144-5 [DOI] [PubMed] [Google Scholar]
- 33.Small S, Nava S, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci (2001) 4:442–9. 10.1038/86115 [DOI] [PubMed] [Google Scholar]
- 34.Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci (2002) 1:93–102. 10.1016/S1364-6613(00)01845-3 [DOI] [PubMed] [Google Scholar]
- 35.Werkle-Bergner M, Müller V, Li SC, Lindenberger U. Cortical EEG correlates of successful memory encoding: implications for lifespan comparisons. Neurosci Biobehav Rev (2006) 30:839–54. 10.1016/j.neubiorev.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 36.Sanquist TF, Rohrbaugh JW, Syndulko K, Lindley DB. Electrocortical signs of levels of processing: perceptual analysis and recognition memory. Psychophysiology (1980) 17:568–76. 10.1111/j.1469-8986.1980.tb02299.x [DOI] [PubMed] [Google Scholar]
- 37.Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol (1987) 67:360–71. 10.1016/0013-4694(87)90124-6 [DOI] [PubMed] [Google Scholar]
- 38.Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia (2009) 47:1765–79. 10.1016/j.neuropsychologia.2009.02.028 [DOI] [PubMed] [Google Scholar]
- 39.Mangels JA, Picton TW, Craik FIM. Attention and successful episodic encoding: an event-related potential study. Brain Res Cogn Brain Res (2001) 11:77–95. 10.1016/S0926-6410(00)00066-5 [DOI] [PubMed] [Google Scholar]
- 40.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care. 2nd ed Geneva: World Health Organization; (2001). [Google Scholar]
- 41.Caamaño-Isorna F, Corral M, Parada M, Cadaveira F. Factors associated with risky consumption and heavy episodic drinking among Spanish university students. J Stud Alcohol Drugs (2008) 69:308–12. 10.15288/jsad.2008.69.308 [DOI] [PubMed] [Google Scholar]
- 42.Martinez AM, Benavente R. The AR Face Database (1998). CVC Tech. Rep. No 24. [Google Scholar]
- 43.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol (1983) 55:468–84. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- 44.Guo C, Voss JL, Paller KA. Electrophysiological correlates of forming memories for faces, names, and face-name associations. Brain Res Cogn Brain Res (2005) 22:153–64. 10.1016/j.cogbrainres.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 45.Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: familiarity and recollection in a face memory task. Neuroimage (2004) 21:789–800. 10.1016/j.neuroimage.2003.09.034 [DOI] [PubMed] [Google Scholar]
- 46.Becker JT, Butters N, Hermann A, D’Angelo N. Learning to associate names and faces: impaired acquisition on an ecologically relevant memory task by male alcoholics. J Nerv Ment Dis (1983) 171:617–23. 10.1097/00005053-198310000-00005 [DOI] [PubMed] [Google Scholar]
- 47.Schaeffer KW, Parsons OA. Learning impairment in alcoholics using an ecologically relevant test. J Nerv Ment Dis (1987) 175:213–8. 10.1097/00005053-198704000-00004 [DOI] [PubMed] [Google Scholar]
- 48.Pitel AL, Chanrauda S, Rohlfing T, Pfefferbaum A, Sullivan E. Face-name association learning and brain structural substrates in alcoholism. Alcohol Clin Exp Res (2012) 36:1171–9. 10.1111/j.1530-0277.2011.01731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, Tapert SF. Learning and memory in adolescent moderate, binge and extreme binge drinkers. Alcoholism (2016) 40:1895–904. 10.1111/acer.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carbia C, Cadaveira F, Caamaño-Isorna F, Rodríguez Holguín S, Corral M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS One (2017) 2:e0171393. 10.1371/journal.pone.0171393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartley DE, Elsabagh S, File SE. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol Biochem Behav (2004) 78:611–9. 10.1016/j.pbb.2004.04.027 [DOI] [PubMed] [Google Scholar]
- 52.Dager AD, Jamadar S, Stevens MC, Rosen R, Jiantonio-Kelly RE, Sisante J-F, et al. fMRI response during figural memory task performance in college drinkers. Psychopharmacology (2014) 231:167–79. 10.1007/s00213-013-3219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crego A, Cadaveira F, Parada M, Corral M, Caamaño-Isorna F, Rodríguez Holguín S. Increased amplitude of P3 event-related potential in young binge drinkers. Alcohol (2012) 46:415–25. 10.1016/j.alcohol.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 54.López-Caneda E, Cadaveira F, Crego A, Doallo S, Corral M, Gómez-Suárez A, et al. Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol Alcohol (2013) 48:464–71. 10.1093/alcalc/agt046 [DOI] [PubMed] [Google Scholar]
- 55.Worbe Y, Irvine M, Lange I, Kundu P, Howell NA, Harrison NA, et al. Neuronal correlates of risk-seeking attitudes to anticipated losses in binge drinkers. Biol Psychiatry (2014) 76:717–24. 10.1016/j.biopsych.2013.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maurage P, Joassin F, Speth A, Modave J, Philippot P, Campanella S. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin Neurophysiol (2012) 123:892–901. 10.1016/j.clinph.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 57.López-Caneda E, Rodríguez Holguín S, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol (2014) 49:173–81. 10.1093/alcalc/agt168 [DOI] [PubMed] [Google Scholar]
- 58.Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci (1996) 8:551–65. 10.1162/jocn.1996.8.6.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeffreys DA. A face-responsive potential recorded from the human scalp. Exp Brain Res (1989) 78:193–202. 10.1007/BF00230699 [DOI] [PubMed] [Google Scholar]