Abstract
Strain SYK-6 of the bacterium Sphingobium sp. catabolizes lignin-derived biphenyl via a meta-cleavage pathway. In this pathway, LigY is proposed to catalyze the hydrolysis of the meta-cleavage product (MCP) 4,11-dicarboxy-8-hydroxy-9-methoxy-2-hydroxy-6-oxo-6-phenyl-hexa-2,4-dienoate. Here, we validated this reaction by identifying 5-carboxyvanillate and 4-carboxy-2-hydroxypenta-2,4-dienoate as the products and determined the kcat and kcat/Km values as 9.3 ± 0.6 s−1 and 2.5 ± 0.2 × 107 m−1 s−1, respectively. Sequence analyses and a 1.9 Å resolution crystal structure established that LigY belongs to the amidohydrolase superfamily, unlike previously characterized MCP hydrolases, which are serine-dependent enzymes of the α/β-hydrolase superfamily. The active-site architecture of LigY resembled that of α-amino-β-carboxymuconic-ϵ-semialdehyde decarboxylase, a class III amidohydrolase, with a single zinc ion coordinated by His-6, His-8, His-179, and Glu-282. Interestingly, we found that LigY lacks the acidic residue proposed to activate water for hydrolysis in other class III amidohydrolases. Moreover, substitution of His-223, a conserved residue proposed to activate water in other amidohydrolases, reduced the kcat to a much lesser extent than what has been reported for other amidohydrolases, suggesting that His-223 has a different role in LigY. Substitution of Arg-72, Tyr-190, Arg-234, or Glu-282 reduced LigY activity over 100-fold. On the basis of these results, we propose a catalytic mechanism involving substrate tautomerization, substrate-assisted activation of water for hydrolysis, and formation of a gem-diol intermediate. This last step diverges from what occurs in serine-dependent MCP hydrolases. This study provides insight into C–C–hydrolyzing enzymes and expands the known range of reactions catalyzed by the amidohydrolase superfamily.
Keywords: bacterial metabolism, hydrolase, lignin degradation, metalloenzyme, zinc, C-C hydrolase, amidohydrolase superfamily, aromatic compound, meta-cleavage
Introduction
Lignin is a heterogeneous aromatic polymer that contributes to the recalcitrance of plant cell walls and is an underutilized component of biomass. Deconstructing lignin is of great interest due to its application in transforming lignocellulose to biofuels and commodity chemicals (1). The discovery that bacteria are able to at least partially deconstruct lignin has accelerated the study of enzymes and pathways potentially involved in lignin depolymerization and the catabolism of the resulting products (2, 3). Among bacterial strains able to grow on lignin-derived aromatic compounds, Sphingobium sp. strain SYK-6 (hereafter referred to as SYK-6) has emerged as one of the best characterized, with pathways having been identified for the catabolism of β-aryl ethers, pinoresinol, diaryl propane, phenylcoumarane, and 2,2′-dihydroxy-3,3′-dimethoxy-5,5′-dicarboxybiphenyl (DDVA)3 (4, 5). DDVA is thought to be derived from the biphenyl unit present in lignin.
The catabolism of DDVA by SYK-6 has been genetically elucidated, and several of the enzymes have been characterized (Fig. 1). The demethylation of DDVA by a ligX-encoded oxygenase yields 2,2′,3-trihydroxy-3′-methoxy-5,5′-dicarboxybiphenyl (OH-DDVA) (6). meta-Cleavage of OH-DDVA by LigZ, an extradiol dioxygenase (7), yields 4,11-dicarboxy-8-hydroxy-9-methoxy-2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate (DCHM-HOPDA) (8). DCHM-HOPDA is then transformed by LigY, a proposed meta-cleavage product (MCP) hydrolase, to yield 5-carboxyvanillate (5CVA) and a second product that was presumed to be 4-carboxy-2-hydroxypenta-2,4-dienoate (CHPD) but was not identified (9). Finally, 5CVA is converted to vanillate by LigW, a decarboxylase that is a member of the amidohydrolase superfamily (10).
Figure 1.
Catabolic pathway of DDVA in SYK-6. CHPD (gray) has not been previously experimentally validated.
MCP hydrolases (EC 3.7.1.-) catalyze the hydrolysis of vinylogous 1,5-diketones formed by the dioxygenative meta-ring cleavage of arenes (11). The MCP hydrolases described to date belong to the α/β-hydrolase superfamily and utilize a Ser-His-Asp triad to catalyze C–C bond cleavage (12). As first proposed by Bugg and co-workers (13, 14), the reaction mechanism involves two half-reactions: an initial enol-keto tautomerization to form a discrete keto intermediate followed by a stereospecific C–C fragmentation reaction (Fig. 2). In the first half-reaction, the enzyme induces strain in the dienoate moiety of the substrate (15), which is then thought to deprotonate the catalytic serine, simultaneously completing the ketonization and activating the serinate nucleophile (16). The second half-reaction was originally proposed to occur via a gem-diol in which the catalytic serine acts as a general base to deprotonate water (17). This was subsequently disproven with the observation of a catalytically relevant acyl-enzyme intermediate (11). Interestingly, LigY shares no significant amino acid sequence identity with α/β-hydrolases (9). Although only one reaction product was identified, 5CVA, this product was isotopically labeled when the reaction was performed in H218O, consistent with a hydrolytic reaction.
Figure 2.

Possible mechanisms for MCP hydrolases. In both mechanisms, substrate ketonization is followed by C–C bond hydrolysis. The latter step proceeds via gem-diol or acyl-enzyme intermediates. Adapted from Ruzzini et al. (11).
The amidohydrolase superfamily comprises metal-dependent enzymes that, like the α/β-hydrolases, catalyze a wide variety of hydrolytic reactions, typically of ester and amide bonds (18, 19). In further similarity to the α/β-hydrolases, some families of amidohydrolases catalyze the more thermodynamically challenging C–C fission. The first such described enzyme was α-amino-β-carboxymuconate-ϵ-semialdehyde decarboxylase (ACMSD; EC 4.1.1.45) (20). ACMSD is a Class III amidohydrolase based on the presence of a single zinc ion in the active site and the identity of the coordinating residues: three histidines and a carboxylate (18, 21). Many of the prototypical Class III amidohydrolases are hydrolytic deaminases, such as CDA and ADA, acting on cytosine and adenosine, respectively, and belong to COG0402 (18, 22). However, ACMSD belongs to COG2159 along with other decarboxylases, such as LigW (10), as well as LigJ, a 4-oxalomesoconate hydratase (23), and CouO, proposed to catalyze the hydrolysis of 4-hydroxy-3-methoxyphenyl-β-ketopropionate-CoA to vanillate and acetyl-CoA (24).
Herein we report the characterization of LigY. The phylogeny of this enzyme was established based on sequence analyses. The reaction products were characterized using mass spectrometry. A fluorescence-based assay was developed for steady-state kinetic characterization, and a crystal structure was obtained to reveal the catalytic machinery of LigY. The roles of key residues were investigated using directed mutagenesis. A molecular mechanism of catalysis is proposed based on precedents with related enzymes as well as the presented data. These results are discussed with respect to MCP hydrolases and the amidohydrolase superfamily.
Results
LigY is an amidohydrolase
LigY was previously reported to have low amino acid sequence identity with previously characterized MCP hydrolases (9). A BLASTp search against the non-redundant protein sequences of the NCBI database identified LigY as a member of the amidohydrolase superfamily. Sequence alignments using LigY as a search query against COG (25) and Pfam (26) databases placed LigY in COG2159 and Pfam04909, respectively. Among characterized COG2159 enzymes, LigY has the greatest amino acid sequence identity with LigJ (38%, T-Coffee) (23) and ∼20% identity with a number of decarboxylases: ACMSD (20), 2,6-dihydroxybenzoate decarboxylase (27), uracil-5-carboxylate decarboxylase (IDCase) (28), and LigW (10). Alignment of LigY with these homologs indicated that LigY has the motif that binds Zn2+ in these enzymes (29).
Purified LigY contains zinc
Heterologously produced LigY was purified to >99% apparent homogeneity as judged by SDS-PAGE analysis at yields of 5–10 mg of protein/liter of cell culture. Mass spectrometric analysis of the purified protein (37,278.2 Da) agreed with the predicted molecular mass of LigY (37,278 Da), indicating the presence of the N-terminal methionine. Inductively coupled plasma mass spectrometry (ICP-MS) analysis revealed that the purified LigY contained 0.93 ± 0.07 eq of zinc per protomer and insignificant amounts of cadmium, cobalt, copper, iron, manganese, and nickel. Consistent with this result, a colorimetric assay based on 4-(2-pyridylazo)-resorcinol (PAR) yielded a value of 1.02 ± 0.09 eq of zinc per protomer. Size exclusion chromatography-multiangle light scattering (SEC-MALS) analysis indicated that LigY exists in solution as a monodispersed species with a mass of 208 ± 2 kDa, consistent with a hexamer (supplemental Fig. S1). Analysis of the protein absorbance at 280 nm (ϵ = 36,900 mm−1 cm−1) was in good agreement with protein concentrations determined using a BCA assay. Preparations of LigY had no significant absorbance bands in the visible region. The far-UV CD spectra minima at 222 and 212 nm indicate a high content of α-helical secondary structure (supplemental Fig. S2). When the growth medium was not supplemented with Zn2+, purified LigY contained ∼0.5 eq of zinc. Enzyme preparations containing different amounts of zinc showed no differences in their oligomeric states or overall secondary structures.
LigY hydrolyzes DCHM-HOPDA to 5CVA and CHPD
To identify the LigY reaction products, OH-DDVA was incubated with a mixture of LigZ and LigY (potassium phosphate (I = 0.1 m), pH 7.5). This approach minimized the non-enzymatic transformation of DCHM-HOPDA, the LigZ-produced MCP (8). HPLC analysis of the reaction products revealed the presence of 5CVA, consistent with an earlier report (9), together with another major peak (retention time (tR) = 8 min), the amount of which was proportional to 5CVA (Fig. 3). Mass spectrometric analysis of this compound revealed a parent ion with an m/z value of 157.01, consistent with the predicted mass of singly deprotonated CHPD (157.01). Furthermore, this compound could be derivatized with dinitrophenylhydrazine (DNPH), consistent with the presence of an α-keto acid (30). On reverse phase HPLC, the derivatized compound eluted with an absorbance (λmax = 470 nm) and polarity consistent with DNPH-CHPD (i.e. its tR = 31 min was between those of derivatives of α-ketoglutaric acid (tR = 28 min) and 2-hydroxypenta-2,4-dienoic acid (tR = 41 min)) (supplemental Fig. S3). The identities of the DNPH-derivatized α-keto acids were confirmed with mass spectrometry. Overall, DNPH derivatization confirmed that CHPD is the second product of the LigY-catalyzed reaction. Together with the previous identification of 5CVA (9), these data establish that LigY catalyzes the hydrolysis of DCHM-HOPDA.
Figure 3.
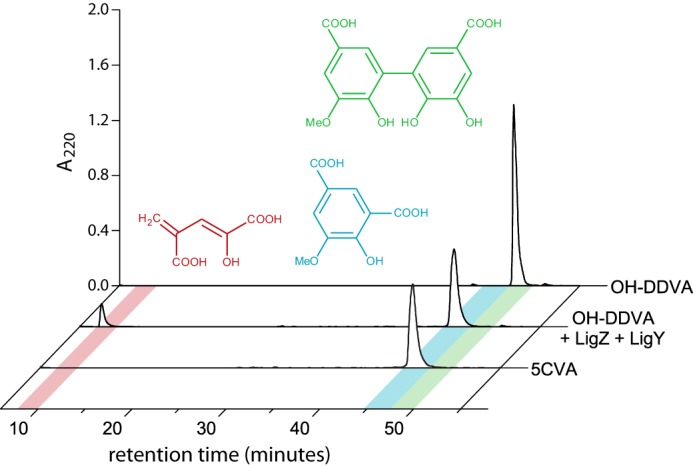
HPLC resolution of DCHM-HOPDA hydrolysis products. Traces are of OH-DDVA, OH-DDVA incubated with LigZ and LigY, and 5CVA. Peaks corresponding to OH-DDVA, 5CVA, and CHPD are highlighted with green, blue, and red, respectively.
Steady-state kinetic analyses of LigY
To evaluate the steady-state kinetic parameters of LigY, we developed an assay based on the fluorescence of 5CVA. The dependence of the fluorescence signal on 5CVA was investigated using LigW, which decarboxylates 5CVA (10) to the significantly less fluorogenic vanillate. In the presence of excess LigW, no fluorescence was detected from the activity assay. Importantly, at pH 7.5, neither OH-DDVA, DCHM-HOPDA, nor CHPD fluoresced significantly. However, DCHM-HOPDA fluoresced significantly at pH values above 7.5, particularly in the presence of Good's buffers. By contrast, the fluorescent quantum yield of 5CVA did not change significantly from pH 6 to 9. Correcting for the background fluorescence of DCHM-HOPDA, LigY had maximal activity between pH 7.5 and 8.0 (supplemental Fig. S4). Considering the pH dependence of LigZ activity and to minimize the background fluorescent observed at elevated pH, subsequent kinetic assays were performed at pH 7.5 using potassium phosphate (I = 0.1 m).
The steady-state hydrolysis of DCHM-HOPDA by LigY displayed Michaelis–Menten behavior (Fig. 4). At pH 7.5, LigY had a turnover number of 9.3 ± 0.6 s−1 and a kcat/Km value of 2.5 ± 0.2 × 107 m−1 s−1 calculated based on the metal ion content of the preparation. As reported previously (9), LigY did not detectably hydrolyze 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate, the MCP from biphenyl degradation.
Figure 4.

Steady-state kinetic hydrolysis of DCHM-HOPDA by LigY. Shown is the dependence of the initial velocity of 5CVA production on DCHM-HOPDA concentration (potassium phosphate (I = 0.1 m), pH 7.5). The solid line represents a best fit of the Michaelis–Menten equation to the data using the least squares dynamic weighting options of LEONORA (Km = 0.38 ± 0.02 μm and kcat = 9.3 ± 0.6 s−1).
Metal dependence of LigY activity
To evaluate the importance of the metal cofactor, it was removed from LigY by chelation. Apo-LigY was generated by dialyzing the enzyme for 48 h against o-phenanthroline and EDTA at pH 6.0. At pH 7–8, the metal ion was not detectably removed, and below pH 5.5, LigY was irreversibly denatured. Apo-LigY had <5% of the specific activity of WT LigY. Incubation of apo-LigY with ZnCl2 partially restored the enzymatic activity. However, we were unable to restore apo-LigY to the same specific activity as the purified enzyme. Nevertheless, the specific activity of purified LigY with ∼0.5 zinc occupancy was ∼50% that of purified LigY with full zinc occupancy.
Structure of LigY
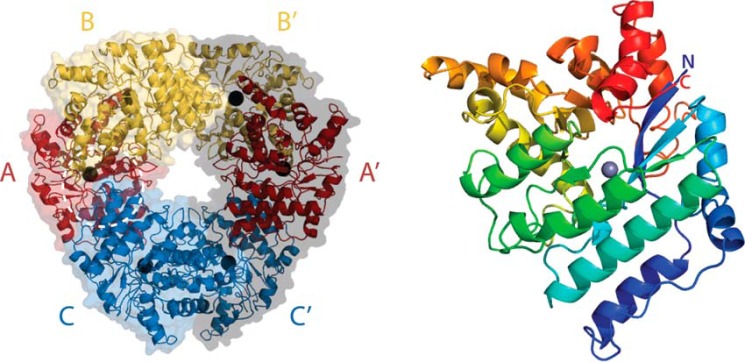
The crystal structure of LigY was solved to a resolution of 1.9 Å (Table 1). The asymmetric unit has three complete protomers (A, B, and C). The model for each contains all 332 residues of the encoded gene product with additional residues at the C-terminal end from TEV protease recognition sequences. The protomers share highly similar structures, with an average RMSD over all Cα of ∼0.33 Å as determined using the least-squared superposition tool from COOT. Protomers A and B are related by non-crystallographic 2-fold symmetry and have an interface of 2200 Å2. Protomer A is also bound to Protomer C with a smaller interface of 900 Å2 (Fig. 5A). Protomers sharing the larger interface (i.e. BA, CC′, and A′B′) are considered to be functional dimers because Arg-234, which is part of this interface, contributes to the active site of its respective dimeric partner as described below. Accordingly, the interface between protomers of a functional dimer is defined as the intradimer interface. The hexamer observed in solution can be reconstructed through crystallographic 2-fold rotational symmetry of the asymmetric unit and characterized as a trimer of dimers based on the non-crystallographic 3-fold symmetry of the hexamer. The functional dimers are related through 2-fold rotational symmetry (e.g. the RMSD value of AB and CC′ is 0.37 Å over all Cα atoms, close to that of the individual protomers). Phylogenetic analysis of closely related sequences mapped to the structure of LigY revealed highly conserved regions at the intradimer interface (supplemental Fig. S5A). In contrast, the residues at other interfaces are poorly conserved. Surface electrostatic analysis further indicated that the intradimer interactions consist of several patches of polar interactions (supplemental Fig. S5B), whereas other interfaces are dominated by hydrophobic interactions.
Table 1.
LigY data collection and refinement statistics
| SeMet-LigY peak | LigY | |
|---|---|---|
| Data collection | ||
| Wavelength (Å) | 0.97591 | 0.97950 |
| Resolution range (Å) | 37.81–2.22 (2.34–2.22)a | 59.42–1.90 (1.94–1.90) |
| Space group | C2221 | C2221 |
| Unit cell dimensions (Å) | ||
| a | 60.24 | 60.02 |
| b | 195.91 | 195.36 |
| c | 178.42 | 178.25 |
| Unique reflections | 52,630 | 82,888 |
| Completeness (%) | 99.4 (96.3) | 100.0 (100.0) |
| Redundancy | 10.9 (10.6) | 6.2 (6.1) |
| Mean I/σI | 17.8 (2.4) | 13.2 (3.2) |
| CC1/2 | 0.999 (0.779) | 0.998 (0.825) |
| Wilson B-factor (Å2) | 38.3 | 22.9 |
| Refinement | ||
| Rwork (Rfree)b | 16.2 (18.9) | |
| No. of water molecules | 535 | |
| RMSD bond length (Å) | 0.008 | |
| Average B-values (Å2) | ||
| Overall | 34.4 | |
| Protein | 34.5 | |
| Ligands-zinc | 23.2 | |
| Ligands-chlorine | 30.0 | |
| Water | 34.3 | |
| Ramachandran plot (%) | ||
| Most favorable | 97.2 | |
| Disallowed | 0.9 | |
| PDB code | 5VN5 | |
a Values in parentheses are for highest-resolution shell.
b Rfree is the Rwork value for 5% of the reflections excluded from the refinement.
Figure 5.
Structures of the LigY hexamer and protomer. A, the hexameric assembly of LigY. The different LigY protomers of the asymmetric unit are red, yellow, and blue, respectively. The protomers are depicted with their surfaces and underlying secondary structure. The LigY hexamer comprises two trimers of adjacent asymmetric units related by a rotational 2-fold symmetry. The symmetry-related trimer is depicted with a gray surface representation. The white dotted circle marks the entrance to the active-site channel. B, ribbon diagram of LigY rainbow-colored from blue (N terminus) to red (C terminus). Bound zinc ions are shown as gray spheres.
A channel leading to the active site is formed at the interface between three protomers. Residues derived from the functional dimer construct the majority of the channel, and a third protomer contributes to the mouth of the channel (Fig. 5A, white dotted circle). Due to 3-fold symmetry of LigY, three active-site channels are orientated toward each of the two faces of the hexameric ring.
The LigY protomer has a skewed (β/α)8 barrel fold with the α-helices encapsulating the parallel β-sheets of the inner core (Fig. 5B). This fold, reminiscent of the TIM-barrel, is typical of the amidohydrolase superfamily (18). Of the characterized amidohydrolases, LigY is most structurally similar to LigJ from Rhodopseudomonas palustris (PDB entry 2GWG), consistent with the amino acid sequence identity. Using Dali-Lite to perform a pairwise analysis, an RMSD of 1.6 Å was calculated over 309 Cα atoms (z-score 40.9).4 LigY retains the core (β/α)8 secondary structure elements of LigJ but has five additional α-helical elements (supplemental Fig. S6). The key secondary structure elements, eight pairs of alternating β-strand and α-helix, were numbered as in ACMSD (PDB entry 2HBV) to simplify comparison. The first, helix α1′ is a 13-residue insertion between strand β1 and helix α1. The next three insertions are of ∼3 residues each and occur as small helices α2′, α5′, and α6′. Finally, the last insertion, the C-terminal α9A and α9B, also occurs in ACMSD (21).
LigY metal-binding site
LigY's active site is at the center of the β-sheet core as observed in other Class III amidohydrolases (18), such as ACMSD (21). The anomalous map corroborates that LigY is mononuclear with a single anomalous peak observed in the active site of each protomer (supplemental Fig. S7). The metal fluorescence scan and an anomalous map density peak calculation were also consistent with Zn2+ being the active-site ion (data not shown). This metal ion is coordinated by His-6, His-8, and His-179 and is partially coordinated by Glu-282. These four residues correspond to the metal-binding ligands in other members of the amidohydrolase superfamily, although the acidic ligand is often aspartate.
The electron density indicates conformational heterogeneity in the active sites of the three LigY protomers. These are highlighted by Glu-282, which is observed in two conformations. In one conformation, the side chain is orientated toward the Zn2+ and coordinates the metal (Fig. 6A). In the second conformation, the carboxylate is orientated away from the metal ion (Fig. 6A). In Protomer C, Glu-282 is in a single conformation, orientated away from the metal ion (Fig. 6A). In Protomers A and B, Glu-282 appears to adopt both conformations, with ∼60 and 30% occupancies, respectively, for the metal-coordinating conformation as estimated from the refinement by Phenix.refine (Fig. 6A). A second difference, which correlated with the conformation of Glu-282, concerned a large spherical electron density located adjacent to the Zn2+ in Protomer C (Fig. 6A and supplemental Fig. S7). This density was too large for a water molecule and was modeled as a Cl− ion, which is present in the mother liquor. No peak was observed in an anomalous map, indicating that it is not a transition metal ion. The interatomic Zn2+–Cl− distance is 1.9 Å, as compared with a value of 2.2 Å reported for an inorganic zinc complex (32). Electron density corresponding to Cl− at a lower occupancy is present in Protomers A and B. However, the anion was not included in the models of these chains due to steric clash. Overall, two active-site metal coordination geometries were modeled in LigY. In the pentacoordinate geometry (Fig. 6B), which predominates in Protomer A, the Zn2+ is coordinated by the three histidines, Glu-282, and a solvent species. In the tetracoordinate geometry, modeled in Protomer C (Fig. 6C), the Zn2+ is coordinated by the three histidines and a Cl− ion. The state observed in Protomer B appears to be a mixture of the two geometries (Fig. 6A).
Figure 6.
The Zn2+-binding site of LigY. A, the Zn2+ coordination sphere of Protomer A (red), Protomer B (yellow), and Protomer C (blue). The green and red mesh represents a positive and negative Fo − Fc map of features within 3 Å of select residues contoured at 3.5 σ. The Zn2+, Cl−, and solvent species are represented as gray, green, and red spheres, respectively. Stick representations of the Zn2+ coordination spheres in “Glu-on” (B) and “Glu-off” (C) configurations. Zn2+–ligand and hydrogen bonds are depicted as black dotted lines with distances indicated in Å. Distances exceeding hydrogen bond lengths (3.2 Å) are denoted as gray dotted lines.
The LigY active site contains several other notable residues in addition to the metal ligands (Fig. 6). First, His-223 is conserved in Class III amidohydrolases and, as in these enzymes, acts as a second shell metal ligand via a water molecule. His-223 is not within hydrogen-bonding distance of the metal-bound solvent species in any of the LigY protomers but may form a hydrogen bond with Glu-282 when the latter is coordinated to the Zn2+. As noted above, the active site also contains Arg-234 from its dimeric partner, an arrangement that is conserved in COG2159 enzymes of known structure. In ACSMD, this arginine has been proposed to bind the C1 carboxylate of α-amino-β-carboxymuconate-ϵ-semialdehyde (33). In LigW, this arginine interacts with the nitro group of the substrate analog 5-nitrovanillate (10). The LigY active site also contains Ser-222. This residue is not conserved in amidohydrolases but lies in close proximity with His-223 and Glu-198 (Fig. 6). Finally, LigY does not contain the glutamate that is conserved in COG0402 Class III amidohydrolases. This residue is exemplified by Glu-217 in CDA (22) and is proposed to act as an acid-base catalyst deprotonating the metal-bound hydrolytic water in COG0402 enzymes.
Inhibition of LigY activity by chloride
The observation of a Zn2+-bound Cl− in one of the protomers suggests two hypotheses: (a) Glu-282 is readily displaceable, and (b) Cl− inhibits LigY's activity. To test the latter hypothesis, we measured the activity of LigY at increasing ionic strength, where ionic strength was increased using either KCl or potassium phosphate. In these experiments, the specific activity of LigY decayed exponentially with increasing concentration of Cl− but not phosphate (Fig. 7), indicating that this effect is specific. Importantly, high concentrations of KCl had negligible effect on the activity of LigZ used in the assay. Finally, similar inhibition was observed whether NaCl or KCl was used (data not shown), indicating that the chloride anion is the inhibitory species.
Figure 7.

The effect of chloride on LigY activity. Activity was measured under standard assay conditions following 5CVA fluorescence (pH 7.5, 25 °C). Ionic strength was increased using either KCl (filled squares) or potassium phosphate (open squares). Error bars, S.D.
Modeling substrate binding
To date, attempts to obtain a structure of a LigY·DCHM-HOPDA complex have not succeeded. In the absence of such data, we modeled DCHM-HOPDA into the Zn2+-containing pocket to evaluate how LigY might bind its substrate. For this experiment, we based the structure of DCHM-HOPDA on that of 2-hydroxy-6-oxo-6-phenyl-hexa-2,4-dienoate as found in complex with the S112A variant of BphD (34). DCHM-HOPDA was modeled in its enol form, (2Z,4Z)-2-hydroxy-6-oxo-4-carboxylato-6-(5-carboxylato-2-hydroxy-3-methoxyphenyl)-hex-2,4-dienoate, to account for the substrate's tautomeric state at neutral pH. We further reasoned that the substrate probably coordinates the metal ion through one of its carboxylates based on (a) the precedence for this binding mode in the COG2159 enzymes LigW and IDCase (10, 28) and (b) the ability of Cl− to coordinate the Zn2+ in LigY. Accordingly, we modeled DCHM-HOPDA into the active site of Protomer C, positioning a carboxylate oxygen in place of the Cl−. We considered three orientations of the substrate: tail-in, side-on, and head-in, in which DCHM-HOPDA was coordinated to Zn2+ by the C1-, C4-, and C11-carboxylates, respectively. Of these three, the tail-in orientation was the only one that presented no steric clashes (Fig. 8). In this configuration, DCHM-HOPDA is stabilized by a number of interactions with polar residues that would help neutralize the charged groups of the substrate. More specifically, the C1-carboxylate is stabilized by Arg-234 and His-223; the C4-carboxylate is positioned to interact with Arg-72; and finally, the solvent-exposed C11-carboxylate can interact with His-29. By contrast, coordination of the C11-carboxylate to the Zn2+ (head-in) results in clashes between the C9-methoxyl group of the substrate and Arg-72/Phe-74 (supplemental Fig. S8). Additionally, the C4-carboxylate is not positioned to interact with Arg-72 or any other charged residue, whereas the surface-exposed C1-carboxylate cannot interact with His-29 or other residues without clashing with the C4-carboxylate. Last, the side-on orientation cannot be modeled due to numerous steric clashes with the residues lining the elongated substrate-binding pocket.
Figure 8.
A tail-in model of the LigY·DCHM-HOPDA complex. The model was constructed using Protomer C with the Cl− removed and DCHM-HOPDA (green) with its dienoate moiety orientated toward the metal center. Left, putative substrate-binding residues are shown in red, and residues from neighboring subunits are shown in yellow and blue. Potential hydrogen bonds are denoted with a dashed line. Right, surface representation of the active-site channel from Fig. 5 with substrate modeled in.
Active-site variants
We evaluated the catalytic roles of six LigY residues by substituting each of them. Briefly, the H223Q, H223A, R234K, R234Q, E282Q, and E282D variants were designed to evaluate the roles of three conserved residues with respect to other members of the amidohydrolase superfamily. The S222A variant was generated to evaluate the possible role of Ser-222 in a nucleophilic mechanism. Finally, R72Q and Y190F were constructed to evaluate the substrate docking studies. None of the substitutions significantly perturbed the secondary structure or oligomeric state of LigY as determined using CD spectroscopy (supplemental Fig. S2) and SEC-MALS (data not shown), respectively. As summarized in Table 2, all of the variants contained at least 0.7 eq of Zn2+ as determined by the PAR-based assay; significant levels of other metal ions were not detected using ICP-MS. For those preparations containing less than a full equivalent of Zn2+, the activities and kinetic parameters were adjusted according to the metal ion content.
Table 2.
Relative activity of LigY variants
Experiments were performed using 50 μm DCHM-HOPDA in potassium phosphate (I = 0.1 m), pH 7.5, at 25 °C. Reported values are mean ± S.D. based on a minimum of three replicates.
| Enzyme | Zinc contenta | Relative activity |
|---|---|---|
| % | ||
| WT | 1.0 ± 0.1 | 100 ± 10 |
| R72Q | 1.0 ± 0.1 | NDb |
| Y190F | 0.7 ± 0.1 | 0.8 ± 0.1 |
| S222A | 0.9 ± 0.1 | 80 ± 10 |
| H223Q | 0.8 ± 0.1 | 46 ± 2 |
| H223A | 1.3 ± 0.2 | 1.7 ± 0.2 |
| E282Q | 1.0 ± 0.1 | ND |
| E282D | 0.8 ± 0.1 | ND |
| R234K | 1.2 ± 0.3 | 16 ± 4 |
| R234Q | 1.1 ± 0.1 | 0.2 ± 0.0 |
a Zinc content represents the ratio of Zn2+ versus protein concentrations. Relative activity was normalized to zinc content when this content was <1 eq (e.g. the relative activity of the H223Q variant was corrected for the lower Zn2+ content of this variant versus WT).
b ND, not detected.
Among the conserved residues tested, Glu-282 was most sensitive to substitution; neither E282Q nor E282D had detectable activity (Table 2). By contrast, the H223Q and R234K variants had significant activity, whereas the H223A and R234Q variants had <2% that of WT LigY. Interestingly, both the R72Q and Y190F variants also had very little activity. Finally, the activity of S222A was similar to that of WT. Variants with significant activity (>2% that of WT LigY) were further subjected to steady-state kinetic analyses (Table 3). The steady-state kinetic parameters of S222A were perturbed <3-fold versus WT LigY, indicating that Ser-222 does not play a significant catalytic role. The substrate specificity (kcat/Km value) of H223Q was ∼6-fold lower than that of WT LigY, although both the kcat and Km values were affected to a lesser extent. By contrast, the substrate specificity of R234K was <1% that of WT LigY with significant perturbation of both the kcat and Km values.
Table 3.
Steady-state kinetic parameters of LigY and select variants
Experiments were performed using potassium phosphate (I = 0.1 m), pH 7.5, at 25 °C. Parameters were calculated using a minimum of three replicates at each of seven substrate concentrations. Values are mean ± S.E.
| Enzyme | kcat | Km | kcat/Km |
|---|---|---|---|
| s−1 | μm | s−1·μm−1 | |
| WT | 9.3 ± 0.6 | 0.38 ± 0.02 | 25 ± 2 |
| S222A | 7.9 ± 0.9 | 1.01 ± 0.06 | 7.8 ± 0.9 |
| H223Q | 5.3 ± 0.7 | 1.30 ± 0.07 | 4.1 ± 0.6 |
| R234K | 1.4 ± 0.4 | 11 ± 1 | 0.12 ± 0.03 |
Discussion
The identification of LigY as an MCP hydrolase expands the known range of reactions catalyzed by amidohydrolases. More specifically, the definitive identifications of LigY's substrate as DCHM-HOPDA (8) and CHPD as the second reaction product establish that LigY catalyzes the hydrolysis of a vinylogous 1,5-diketone typical of MCP hydrolases. These results are consistent with a previous report that LigY catalyzes a hydrolytic reaction (9). This is the first report of an MCP hydrolase that is not a serine-dependent α/β-hydrolase. Nevertheless, LigY belongs to COG2159, which includes superfamily members that catalyze other C–C cleavage reactions, such as ACSMD (20), LigW (10), and CouO (24).
Our data indicate that LigY's cognate metal ion is Zn2+, typical of class III amidohydrolases. First, zinc was the predominant metal found in LigY when the growth medium used for protein production was not supplemented with metals. Second, LigY's specific activity was proportional to its zinc content. Unfortunately, LigY does not appear to take up or release its metal ion as readily as other amidohydrolases. For example, neither preincubation with Zn2+ nor its presence in the assay buffer improved the activity of LigY preparations with partial zinc occupancy. In other amidohydrolases, titration of the apoprotein with the metal ion resulted in rapid binding and a linear relationship between enzymatic activity and the amount of metal added (20, 22, 35). In this respect, LigY appears to be similar to ADA in that both are resistant to metal chelation at neutral pH (35). However, the inclusion of a reducing agent enabled rapid and complete reconstitution of ADA (35) but not LigY. Optimization for metal substitution in LigY is currently under way to facilitate further study of the enzyme.
Based on the mechanism of serine-dependent MCP hydrolases, the precedents in related amidohydrolases, and the presented data, we propose a mechanism for LigY that proceeds via an enol-keto tautomerization followed by a gem-diol intermediate and C–C bond fission (Fig. 9). Tautomerization occurs in serine-dependent MCP hydrolases (13, 14), acting as an electron sink to facilitate the subsequent C–C fragmentation reaction. The docking studies suggest that the Zn2+ and Arg-234 coordinate the C1-carboxylate, which would enable them to play a role in tautomerization by inducing strain on the dienoate, as has been proposed for Arg-190 in BphD (15). The respective activities of the R234K and R234Q variants suggest that Arg-234 has such a catalytic role, as opposed to simply binding the substrate as would occur if DCHM-HOPDA bound in the head-in mode. Formation of a gem-diol intermediate is consistent with the absence of an active-site nucleophile. The modeled LigY·DCHM-HOPDA complex predicts that Arg-72 plays a major role by (a) interacting with the 4-carboxylate of the substrate and (b) contributing to an oxyanion hole to stabilize the tetrahedral intermediate. The lack of activity of the R72Q variant is consistent with such a major role, as is the inability of LigY to hydrolyze HOPDA, which lacks the 4-carboxylate. Finally, neither the proposed mechanism nor the docking models explain the lack of activity of the Y190F variant.
Figure 9.
Proposed mechanism of LigY. The suggested roles of particular residues are discussed under “Discussion.”
The proposed gem-diol intermediate differs from the acyl-enzyme intermediate of serine-dependent MCP hydrolases (11). However, the substrate-assisted activation of the nucleophile is analogous (16). Thus, a notable feature of the proposed mechanism is the requisite protonation at C5 of DCHM-HOPDA to complete ketonization and the concomitant activation of water for nucleophilic attack of the C6-carbonyl. In serine-dependent MCP hydrolases, ketonization involves an intermediate, ESred, which possesses a remarkable bathochromically shifted absorption spectrum (15). ESred has been proposed to be a dianionic intermediate that acts as a general base to activate the serine nucleophile (16). Further work is required to substantiate aspects of this mechanism, including the involvement of an ESred species in tautomerization and water activation.
The proposed mechanism postulates roles for Glu-282 in each half-reaction that are consistent with the in crystallo and mutagenesis data. In other words, the structural data suggest that Glu-282 is easily displaced, whereas the mutagenesis data indicate that the residue is essential for catalysis but not metal ion binding. Mechanistically, the displacement of Glu-282 by DCHM-HOPDA leaves it well-positioned to assist substrate tautomerization in the first half-reaction by deprotonating the C2-hydroxyl of DCHM-HOPDA. This role as a base catalyst is similar to what has been proposed for the equivalent residue in LigW, where an aspartate deprotonates the C4-hydroxyl of 5CVA, allowing 5CVA to bind the metallocenter (10). Importantly, the pKa of the C2-hydroxyl of DCHM-HOPDA is ∼11.3 (8) and thus requires a base catalyst for tautomerization to occur. By contrast, BphD contains no such base, but none is required; 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate probably binds as an enolate because the pKa of its C2-hydroxyl is 7.3 (36). In the second half-reaction, Glu-282 donates a proton to the dienoate leaving group. Overall, the role of Glu-282 as a proton shuttle is similar to what has been proposed in other amidohydrolases (18). Finally, like Glu-282 in LigY, the aspartyl ligand in LigW also adopts two conformations (10). However, the difference between the two conformations of the carboxylate is not as dramatic as in LigY. The glutamate in LigY, which also occurs in LigJ, may afford the greater conformation flexibility required for its catalytic role.
The proposed mechanism diverges from that of other hydrolytic amidohydrolases, which exclusively favor a nucleophilic attack by a hydroxide to an electrophilic carbonyl or phosphoryl center (18). However, our docking studies suggest that in the absence of a major conformational change in the enzyme, the C6-carbonyl cannot approach the metal ion, precluding the conventional role of the metallocenter in amidohydrolases (18). Most proposed amidohydrolase mechanisms favor the formation of metal-hydroxo species as a means of generating the hydrolytic nucleophile (18) because the binding of water to Zn2+ effectively lowers its pKa to near 7 (37). LigY probably uses a different mechanism to activate the solvent species for hydrolysis because LigY lacks a metal-bound solvent species that is within hydrogen bonding distance to His-223, substrate binding probably displaces the metal-bound solvent species, and LigY lacks an acidic residue equivalent to Glu-217 in CDA. The relative activities of the H223Q and H223A variants suggest that His-223 may help stabilize a catalytic intermediate via hydrogen bonding. Nevertheless, substitution of this conserved His is less deleterious than in other amidohydrolases, such as CDA, LigW, ACMSD, and IDCase (10, 22, 28, 29).
In conclusion, the proposed mechanism for LigY suggests specific roles for catalytic residues in the proposed two half-reactions of MCP hydrolysis that are consistent with the available data as well as the modeled LigY·DCHM-HOPDA complex (Fig. 8). Further studies are aimed at substantiating this mechanism and the proposed roles of individual residues.
Experimental procedures
Chemicals and reagents
All reagents were of analytical grade unless otherwise noted. OH-DDVA was synthesized as described previously (7). Restriction enzymes and the Phusion PCR system used for cloning were from New England Biolabs. Water for buffers was purified using a Barnstead Nanopure DiamondTM system to a resistance of at least 18 megaohms.
Cloning and mutagenesis
DNA was purified, manipulated, and propagated using standard procedures (38). A gene encoding ligY (locus tag: SLG_07750) was synthesized by back-translating the protein's amino acid sequence using codons optimized for expression in Escherichia coli (GenScript USA Inc.). The ligY gene was amplified from the synthetic gene, and the resulting amplicon was cloned into pET41b (Novagen). Two constructs were made: pET41LigY and pET41LigY_His. The latter encodes the enzyme with a C-terminal TEVpro-cleavable octahistidine tag (LigY-Ht). Variants of LigY were generated from pET41LigY using PCR-based mutagenesis and either a pair of overlapping primers or a single phosphorylated primer. The ligW gene (locus tag: SLG_07850) was amplified from genomic DNA prepared from SYK-6 (NBRC 103272). The resulting amplicon was cloned into pET41b to generate pETLigW, encoding LigW with no affinity tag. The nucleotide sequence of all constructs was confirmed by sequencing. The oligonucleotides used in this study are listed in supplemental Table S1.
Protein production and purification
For kinetic characterization, LigY was produced heterologously using E. coli BL-21 λ(DE3) containing pET41LigY. Freshly transformed cells were grown at 37 °C in LB broth supplemented with 30 mg/liter kanamycin to an A600 of ∼0.7. Expression of ligY was induced with 1 mm isopropyl β-d-thiogalactopyranoside, at which time the medium was further supplemented with 1 mm ZnSO4 and the cells were incubated at 30 °C for an additional 16 h. Cells were harvested by centrifugation and stored at −80 °C until further processing. Cells collected from 2 liters of culture were suspended in 20 ml of 20 mm HEPPS, pH 8.0, and lysed at 4 °C using an EmulsiFlex-C5 homogenizer (Avestin). Cellular debris was removed by centrifugation. Ammonium sulfate was added to the cleared lysate to a final concentration of 1.6 m, and the precipitated protein was removed by centrifugation. The supernatant was loaded onto a Source 15 phenyl column and eluted with a linear gradient from 1.6 to 0 m ammonium sulfate in 120 ml of 20 mm HEPPS, pH 8.0 (ÄKTA Purifier, GE Healthcare). Fractions containing LigY, as determined through SDS-PAGE, were pooled and dialyzed into 20 mm HEPPS, pH 8.0. LigY was purified further using a MonoQ 10/100 GL column (GE Healthcare). The protein was eluted with a linear gradient from 0.2 to 0.6 m NaCl in 120 ml of 20 mm HEPPS, pH 8.0. Fractions containing LigY were pooled, dialyzed into 20 mm HEPPS, pH 8.0, concentrated to ∼30 mg/ml, flash-frozen as beads in liquid N2, and stored at −80 °C until needed.
LigY-Ht was produced for crystallization using E. coli BL-21 λ(DE3) containing pET41LigY_His. Cells were grown, and cell extracts were produced as described above. LigY-Ht was purified from the cell extract using immobilized metal affinity chromatography (nickel-nitrilotriacetic acid, Qiagen) according to the manufacturer's instructions. The His tag was proteolytically removed by overnight incubation with TEV protease, leaving a 6-residue tail at the C terminus (ENLYFQ), and was further purified using anion exchange chromatography as described above for LigY. Selenomethionyl LigY-Ht was similarly prepared with labeling performed as described previously (39). LigY variants were prepared the same way as non-tagged LigY. The mass of purified LigY and its variants was validated using Q-TOF MS. LigW was produced heterologously using E. coli BL-21 λ(DE3) containing pET41LigW and was purified essentially as for non-tagged LigY. LigZ was purified as described previously (8).
Protein analytical methods
Protein purity was evaluated using SDS-polyacrylamide gel stained with Coomassie Blue (38). Protein concentrations were determined using the Micro BCATM protein assay kit (Pierce) using bovine serum albumin as a standard. The metal content of LigY preparations was determined both (a) colorimetrically using 4-(2-pyridylazo)-resorcinol and ZnCl2 as a standard (40) and (b) using a NexION 300d inductively coupled plasma mass spectrometer (PerkinElmer Life Sciences) calibrated using IV-Stock-4 synthetic standard (Inorganic Ventures). Protein samples for ICP-MS analysis were treated with concentrated HNO3 and H2O2 as described previously (41).
SEC-MALS
LigY was dialyzed into 20 mm HEPPS, pH 8.0, 100 mm NaCl, brought to 0.5 mg/ml, and loaded onto a Superdex 200 10/300 column (GE Healthcare) attached to a 1260 Infinity LC (Agilent Technologies) and operated at 0.2 ml/min at room temperature. Data were collected using a miniDAWN TREOS multiangle static light scatterer and an Optilab T-rEX refractive index detector (Wyatt Technologies). Data were analyzed using the ASTRA6 software (Wyatt Technologies).
CD spectroscopy
Spectra were recorded using a Jasco J-810 CD spectrometer equipped with a Peltier temperature controller and a 1-mm path length quartz cuvette. Spectra were collected at 25 °C between 300 and 195 nm at a scan rate of 100 nm/min and a response time of 2 s. Samples contained 5 μm protein in potassium phosphate (I = 100 mm, pH 7.5).
Steady-state kinetic analysis
Kinetic assays were performed by monitoring the fluorescence of 5CVA using a Cary Eclipse spectrofluorometer (Agilent) with excitation and emission wavelengths of 310 and 420 nm, respectively, and slit widths of 2.5 and 10 nm, respectively. The standard assay was performed in 0.5 ml of potassium phosphate (I = 100 mm, pH 7.5) at 25 °C containing 30 μm OH-DDVA (1% DMSO final concentration) and 10 nm LigY. Assays were initiated by adding LigZ to ∼0.5 μm to generate the MCP in situ. Rates of 5CVA production were calculated from fluorescence using a standard curve of 0–20 μm 5CVA established each day the kinetics experiments were performed. Rates were corrected by subtracting fluorescence changes observed in the absence of LigY. Steady-state kinetic parameters were evaluated by fitting the Michaelis-Menten equation to the data using least-squares fitting and the program LEONORA (42). Kinetic parameters and specific activities were calculated as a function of the zinc content of the enzyme preparation when this content was less than 100%.
The effect of pH on the rate of the LigY-catalyzed reaction was evaluated using 100 mm solutions of MES (pH 6.0), PIPES (pH 6.5), MOPS (pH 7.0), HEPES (pH 7.5), HEPPS (pH 8.0), TAPS (pH 8.5), and CHES (pH 9.0). The effect of ionic strength on the reaction rate was evaluated at pH 7.5 using buffers with varying amounts of potassium phosphate to modulate the buffer's ionic strength. The effect of Cl− on the reaction rate was evaluated at pH 7.5 using solutions of 38 mm potassium phosphate, 0.9 m potassium chloride, pH 7.5, and 370 mm potassium phosphate, pH 7.5, in different proportions.
Metal chelation and reconstitution
Apo-LigY was prepared by dialyzing LigY against 20 mm MES, pH 6.0, containing 10 mm o-phenanthroline, 5 mm EDTA, and 1 mm DTT for 2 days at 4 °C. The apoprotein was dialyzed against Chelex-treated 20 mm HEPPS, pH 8.0, to remove the chelators and concentrated using ultrafiltration. LigY was reconstituted with different metals by incubating ∼200 μm apoprotein overnight at 4 °C with 500 μm ZnCl2, CoCl2, CuCl2, CaCl2, CdCl2, MnCl2, or FeCl3. Reconstitution with 500 μm Fe(NH4)2(SO4) was carried out anaerobically. Excess metal was removed using G25-Fine resin (Superdex).
CHPD characterization
The dienoate hydrolysis product was prepared by reacting 200 μm OH-DDVA with a mixture of ∼1 μm LigZ and ∼1 μm LigY in air-saturated buffer. The reaction was quenched after 5 min with formic acid (final concentration ∼1%), and the enzymes were removed by centrifugation. CHPD was resolved using a Waters 2695 Separation HPLC module (Milford, MA) equipped with a Waters 2996 photodiode array detector and an Aqua 5-μm C18 250 × 4.6-mm column (Phenomenex). CHPD was eluted using an isocratic flow of 0.1% formic acid, and the eluate was monitored at 220 nm. CHPD was derivatized using DNPH under acidic conditions as described previously (30). The CHPD-DNPH was purified by HPLC using a Kinetex 5-μm EVO C18 100 Å 150 × 3.0-mm column (Phenomenex). CHPD-DNPH was eluted using a linear gradient of 0–100% methanol in 0.1% formic acid, and the eluate was monitored at 470 nm. Mass spectra were measured using a Waters/Micromass LCT and electrospray ionization using methanol as the solvent or Applied Biosystems Qstar mass spectrometer using methanol or acetonitrile as solvents.
Protein structure determination
LigY was crystallized aerobically at room temperature using the sitting drop vapor diffusion method. Drops consisted of 2 μl of ∼350 μm LigY and 2 μl of precipitant solution (0.1 m Tris-Cl, pH 7.5, 0.2 m lithium sulfate, ∼24% PEG 4000) equilibrating over 1.0 ml of precipitant solution. Before data collection, the crystals were soaked briefly in the mother liquor supplemented with 30% glycerol and flash-frozen in liquid nitrogen. Diffraction data of WT LigY were collected at the Canadian Light Source on beamline 08B1-1. Single-wavelength anomalous diffraction data of SeMet LigY were collected at the Stanford Synchrotron Radiation Laboratory on Beamline 7-1. Data were processed using iMOSFLM and Aimless from the CCP4 program suite (43–45). Phase determination and initial model building of SeMet LigY were done using AutoSol and Autobuild programs in Phenix (46). The structure of WT LigY was solved by molecular replacement using the preliminary coordinates for SeMet LigY as the search model using Phaser-MR (47). Manual building was performed using Coot (45), and refinement was performed using phenix.refine (48). Data collection and refinement statistics are shown in Table 1. The subsequent calculations and analyses of the crystallographic model, including the calculation of the secondary structure with DSSP (49, 50), were performed using the WHAT IF server (51). Protein surface and oligomeric assembly were calculated with PISA (52). Protein surface charge analysis was done using PDB2PQR (53, 54) and APBS (55). Amino acid conservation was performed using Consurf (56, 57) using the T-COFFEE (Expresso) (58) alignment algorithm and sequences from the RefSeq NR protein database (31) sharing 35–95% sequence identity. Graphical representation of the crystallographic model was generated using the PyMOL Molecular Graphic System version 1.3 (Schrödinger LLC, New York).
Author contributions
E. K. and L. D. E. designed experiments and analyzed data. E. K. conducted most of the experiments. M. J. K., A. C. K. C., and M. E. P. M. assisted with the structural refinement. A. C. K. C. generated the models of ES complexes. E. K. and L. D. E. wrote the paper with input from A. C. K. C. and M. E. P. M.
Supplementary Material
Acknowledgments
Dr. Hong-Ming Chen and Prof. Stephen G. Withers synthesized OH-DDVA. Mariko Ikehata assisted with the ICP-MS. We thank the Laboratory of Molecular Biophysics for the use of the spectrofluorometer and CD spectrometer. The Laboratory of Molecular Biophysics is funded by the Canadian Foundation for Innovation. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the United States Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the Department of Energy Office of Biological and Environmental Research and by NIGMS, National Institutes of Health, Grant including P41GM103393. Research described in this paper was performed using beamline 08B1-1 at the Canadian Light Source, which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan.
This work was supported by Large Scale Applied Research Project 2108 from Genome Canada, Discovery Grant 171359 from the Natural Sciences and Engineering Research Council of Canada (NSERC) (to L. D. E.), and NSERC Discovery Grant 04802 (to M. E. P. M.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Figs. S1–S8.
The atomic coordinates and structure factors (code 5VN5) have been deposited in the Protein Data Bank (http://wwpdb.org/).
F. Forouhar, M. Abashidze, S. Jayaraman, K. Cunningham, M. Ciao, L. Ma, R. Xiao, T. B. Acton, G. T. Monteglione, J. F. Hunt, L. Tong, and the Northeast Structural Genomics Consortium, submitted for publication.
- DDVA
- 2,2′-dihydroxy-3,3′-dimethoxy-5,5′-dicarboxybiphenyl
- MCP
- meta-cleavage product
- DCHM-HOPDA
- 4,11-dicarboxy-8-hydroxy-9-methoxy-2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate
- ACMSD
- α-amino-β-carboxymuconic-ϵ-semialdehyde decarboxylase
- OH-DDVA
- 2,2′,3-trihydroxy-3′-methoxy-5,5′-dicarboxybiphenyl
- 5CVA
- 5-carboxyvanillate
- CHPD
- 4-carboxy-2-hydroxypenta-2,4-dienoate
- SEC-MALS
- size exclusion chromatography-multiangle light scattering
- ICP-MS
- inductively coupled plasma mass spectrometry
- HEPPS
- 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid
- TAPS
- [trismethylamino]propanesulfonic acid
- CHES
- N-cyclohexyl-2-aminoethanesulfonic acid
- CDA
- cytosine deaminase
- ADA
- adenosine deaminase
- IDCase
- uracil-5-carboxylate decarboxylase
- PAR
- 4-(2-pyridylazo)-resorcinol
- DNPH
- dinitrophenylhydrazine
- tR
- retention time
- TEV
- tobacco etch virus
- RMSD
- root mean square deviation
- PDB
- Protein Data Bank
- SeMet
- selenomethionine.
References
- 1. Ragauskas A. J., Beckham G. T., Biddy M. J., Chandra R., Chen F., Davis M. F., Davison B. H., Dixon R. A., Gilna P., Keller M., Langan P., Naskar A. K., Saddler J. N., Tschaplinski T. J., Tuskan G. A., and Wyman C. E. (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843. [DOI] [PubMed] [Google Scholar]
- 2. Bugg T. D., Ahmad M., Hardiman E. M., and Singh R. (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 22, 394–400 [DOI] [PubMed] [Google Scholar]
- 3. Jackson C. A., Couger M. B., Prabhakaran M., Ramachandriya K. D., Canaan P., and Fathepure B. Z. (2017) Isolation and characterization of Rhizobium sp. strain YS-1r that degrades lignin in plant biomass. J Appl. Microbiol. 122, 940–952 [DOI] [PubMed] [Google Scholar]
- 4. Masai E., Katayama Y., and Fukuda M. (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci. Biotechnol. Biochem. 71, 1–15 [DOI] [PubMed] [Google Scholar]
- 5. Varman A. M., He L., Follenfant R., Wu W., Wemmer S., Wrobel S. A., Tang Y. J., and Singh S. (2016) Decoding how a soil bacterium extracts building blocks and metabolic energy from ligninolysis provides road map for lignin valorization. Proc. Natl. Acad. Sci. U.S.A. 113, E5802–E5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshikata T., Suzuki K., Kamimura N., Namiki M., Hishiyama S., Araki T., Kasai D., Otsuka Y., Nakamura M., Fukuda M., Katayama Y., and Masai E. (2014) Three-component o-demethylase system essential for catabolism of a lignin-derived biphenyl compound in Sphingobium sp. strain SYK-6. Appl. Environ. Microbiol. 80, 7142–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng X., Egashira T., Hanashiro K., Masai E., Nishikawa S., Katayama Y., Kimbara K., and Fukuda M. (1998) Cloning of a Sphingomonas paucimobilis SYK-6 gene encoding a novel oxygenase that cleaves lignin-related biphenyl and characterization of the enzyme. Appl. Environ. Microbiol. 64, 2520–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuatsjah E., Chen H. M., Withers S. G., and Eltis L. D. (2017) Characterization of an extradiol dioxygenase involved in the catabolism of lignin-derived biphenyl. FEBS Lett. 591, 1001–1009 [DOI] [PubMed] [Google Scholar]
- 9. Peng X., Masai E., Katayama Y., and Fukuda M. (1999) Characterization of the meta-cleavage compound hydrolase gene involved in degradation of the lignin-related biphenyl structure by Sphingomonas paucimobilis SYK-6. Appl. Environ. Microbiol. 65, 2789–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vladimirova A., Patskovsky Y., Fedorov A. A., Bonanno J. B., Fedorov E. V., Toro R., Hillerich B., Seidel R. D., Richards N. G., Almo S. C., and Raushel F. M. (2016) Substrate distortion and the catalytic reaction mechanism of 5-carboxyvanillate decarboxylase. J. Am. Chem. Soc. 138, 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruzzini A. C., Ghosh S., Horsman G. P., Foster L. J., Bolin J. T., and Eltis L. D. (2012) Identification of an acyl-enzyme intermediate in a meta-cleavage product hydrolase reveals the versatility of the catalytic triad. J. Am. Chem. Soc. 134, 4615–4624 [DOI] [PubMed] [Google Scholar]
- 12. Kourist R., Jochens H., Bartsch S., Kuipers R., Padhi S. K., Gall M., Böttcher D., Joosten H. J., and Bornscheuer U. T. (2010) The α/β-hydrolase fold 3DM database (ABHDB) as a tool for protein engineering. Chembiochem 11, 1635–1643 [DOI] [PubMed] [Google Scholar]
- 13. Lam W. W., and Bugg T. D. (1997) Purification, characterization, and stereochemical analysis of a C–C hydrolase: 2-hydroxy-6-keto-nona-2,4-diene-1,9-dioic acid 5,6-hydrolase. Biochemistry 36, 12242–12251 [DOI] [PubMed] [Google Scholar]
- 14. Henderson I. M., and Bugg T. D. (1997) Pre-steady-state kinetic analysis of 2-hydroxy-6-keto-nona-2,4-diene-1,9-dioic acid 5,6-hydrolase: kinetic evidence for enol/keto tautomerization. Biochemistry 36, 12252–12258 [DOI] [PubMed] [Google Scholar]
- 15. Horsman G. P., Ke J., Dai S., Seah S. Y., Bolin J. T., and Eltis L. D. (2006) Kinetic and structural insight into the mechanism of BphD, a C–C bond hydrolase from the biphenyl degradation pathway. Biochemistry 45, 11071–11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruzzini A. C., Bhowmik S., Ghosh S., Yam K. C., Bolin J. T., and Eltis L. D. (2013) A substrate-assisted mechanism of nucleophile activation in a Ser-His-Asp containing C–C bond hydrolase. Biochemistry 52, 7428–7438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J. J., Li C., Blindauer C. A., and Bugg T. D. (2006) Evidence for a gem-diol reaction intermediate in bacterial C–C hydrolase enzymes BphD and MhpC from 13C NMR spectroscopy. Biochemistry 45, 12461–12469 [DOI] [PubMed] [Google Scholar]
- 18. Seibert C. M., and Raushel F. M. (2005) Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry 44, 6383–6391 [DOI] [PubMed] [Google Scholar]
- 19. Holm L., and Sander C. (1997) An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins 28, 72–82 [PubMed] [Google Scholar]
- 20. Li T., Walker A. L., Iwaki H., Hasegawa Y., and Liu A. (2005) Kinetic and spectroscopic characterization of ACMSD from Pseudomonas fluorescens reveals a pentacoordinate mononuclear metallocofactor. J. Am. Chem. Soc. 127, 12282–12290 [DOI] [PubMed] [Google Scholar]
- 21. Martynowski D., Eyobo Y., Li T., Yang K., Liu A., and Zhang H. (2006) Crystal structure of α-amino-β-carboxymuconate-ϵ-semialdehyde decarboxylase: insight into the active site and catalytic mechanism of a novel decarboxylation reaction. Biochemistry 45, 10412–10421 [DOI] [PubMed] [Google Scholar]
- 22. Hall R. S., Fedorov A. A., Xu C., Fedorov E. V., Almo S. C., and Raushel F. M. (2011) Three-dimensional structure and catalytic mechanism of cytosine deaminase. Biochemistry 50, 5077–5085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hara H., Masai E., Katayama Y., and Fukuda M. (2000) The 4-oxalomesaconate hydratase gene, involved in the protocatechuate 4,5-cleavage pathway, is essential to vanillate and syringate degradation in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 182, 6950–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otani H., Lee Y. E., Casabon I., and Eltis L. D. (2014) Characterization of p-hydroxycinnamate catabolism in a soil Actinobacterium. J. Bacteriol. 196, 4293–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tatusov R. L., Fedorova N. D., Jackson J. D., Jacobs A. R., Kiryutin B., Koonin E. V., Krylov D. M., Mazumder R., Mekhedov S. L., Nikolskaya A. N., Rao B. S., Smirnov S., Sverdlov A. V., Vasudevan S., Wolf Y. I., Yin J. J., and Natale D. A. (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bateman A., Coin L., Durbin R., Finn R. D., Hollich V., Griffiths-Jones S., Khanna A., Marshall M., Moxon S., Sonnhammer E. L., Studholme D. J., Yeats C., and Eddy S. R. (2004) The Pfam protein families database. Nucleic Acids Res. 32, D138–D141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto M., Hayashi H., Miyahara I., Hirotsu K., Yoshida M., and Oikawa T. (2006) Crystal structures of nonoxidative zinc-dependent 2,6-dihydroxybenzoate (γ-resorcylate) decarboxylase from Rhizobium sp. strain MTP-10005. J. Biol. Chem. 281, 34365–34373 [DOI] [PubMed] [Google Scholar]
- 28. Xu S., Li W., Zhu J., Wang R., Li Z., Xu G. L., and Ding J. (2013) Crystal structures of isoorotate decarboxylases reveal a novel catalytic mechanism of 5-carboxyl-uracil decarboxylation and shed light on the search for DNA decarboxylase. Cell Res. 23, 1296–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li T., Iwaki H., Fu R., Hasegawa Y., Zhang H., and Liu A. (2006) α-Amino-β-carboxymuconic-ϵ-semialdehyde decarboxylase (ACMSD) is a new member of the amidohydrolase superfamily. Biochemistry 45, 6628–6634 [DOI] [PubMed] [Google Scholar]
- 30. Fowden L., and Webb J. A. (1955) Evidence for the occurrence of γ-methylene-α-oxoglutaric acid in groundnut plants (Arachis hypogaea). Biochem. J. 59, 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pruitt K. D., Tatusova T., and Maglott D. R. (2005) NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 33, D501–D504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X. X., and Wang Y. (2015) Crystal structure and luminescent properties of [1-(biphenyl-4-yl)-1H-imidazole-κN3]di-chloridozinc. Acta Crystallogr. E Crystallogr. Commun. 71, 272–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huo L., Davis I., Chen L., and Liu A. (2013) The power of two: arginine 51 and arginine 239* from a neighboring subunit are essential for catalysis in α-amino-β-carboxymuconate-ϵ-semialdehyde decarboxylase. J. Biol. Chem. 288, 30862–30871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horsman G. P., Bhowmik S., Seah S. Y., Kumar P., Bolin J. T., and Eltis L. D. (2007) The tautomeric half-reaction of BphD, a C–C bond hydrolase: kinetic and structural evidence supporting a key role for histidine 265 of the catalytic triad. J. Biol. Chem. 282, 19894–19904 [DOI] [PubMed] [Google Scholar]
- 35. Cooper B. F., Sideraki V., Wilson D. K., Dominguez D. Y., Clark S. W., Quiocho F. A., and Rudolph F. B. (1997) The role of divalent cations in structure and function of murine adenosine deaminase. Protein Sci. 6, 1031–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seah S. Y., Labbé G., Nerdinger S., Johnson M. R., Snieckus V., and Eltis L. D. (2000) Identification of a serine hydrolase as a key determinant in the microbial degradation of polychlorinated biphenyls. J. Biol. Chem. 275, 15701–15708 [DOI] [PubMed] [Google Scholar]
- 37. Silverman D. N., and Lindskog S. (1988) The catalytic mechanism of carbonic-anhydrase: implications of a rate-limiting protolysis of water. Acc. Chem. Res. 21, 30–36 [Google Scholar]
- 38. Ausubel F. M. (2002) Short Protocols in Molecular Biology: A Compendium of Methods from Current Protocols in Molecular Biology, 5th Ed., Wiley, New York [Google Scholar]
- 39. Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., and Clardy J. (1993) Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 [DOI] [PubMed] [Google Scholar]
- 40. McCall K. A., and Fierke C. A. (2000) Colorimetric and fluorimetric assays to quantitate micromolar concentrations of transition metals. Anal. Biochem. 284, 307–315 [DOI] [PubMed] [Google Scholar]
- 41. Shiel A. E., Weis D., and Orians K. J. (2012) Tracing cadmium, zinc and lead sources in bivalves from the coasts of western Canada and the U.S.A. using isotopes. Geochim. Cosmochim. Acta 76, 175–190 [Google Scholar]
- 42. Cornish-Bowden A. (1994) Analysis of Enzyme Kinetic Data, Oxford University Press, Oxford [Google Scholar]
- 43. Battye T. G., Kontogiannis L., Johnson O., Powell H. R., and Leslie A. G. (2011) iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Afonine P. V., Grosse-Kunstleve R. W., Echols N., Headd J. J., Moriarty N. W., Mustyakimov M., Terwilliger T. C., Urzhumtsev A., Zwart P. H., and Adams P. D. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Touw W. G., Baakman C., Black J., te Beek T. A., Krieger E., Joosten R. P., and Vriend G. (2015) A series of PDB-related databanks for everyday needs. Nucleic Acids Res. 43, D364–D368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kabsch W., and Sander C. (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 [DOI] [PubMed] [Google Scholar]
- 51. Vriend G. (1990) WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 8, 52–56, 29 [DOI] [PubMed] [Google Scholar]
- 52. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 53. Dolinsky T. J., Czodrowski P., Li H., Nielsen J. E., Jensen J. H., Klebe G., and Baker N. A. (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dolinsky T. J., Nielsen J. E., McCammon J. A., and Baker N. A. (2004) PDB2PQR: an automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baker N. A., Sept D., Joseph S., Holst M. J., and McCammon J. A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Celniker G., Nimrod G., Ashkenazy H., Glaser F., Martz E., Mayrose I., Pupko T., and Ben-Tal N. (2013) ConSurf: using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 53, 199–206 [Google Scholar]
- 57. Goldenberg O., Erez E., Nimrod G., and Ben-Tal N. (2009) The ConSurf-DB: pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Res. 37, D323–D327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Notredame C., Higgins D. G., and Heringa J. (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.