Abstract
Immunoglobulin IgD might play an important role in autoimmune diseases, but the function of IgD has remained elusive, despite multiple attempts to define its biological function. Fibroblast-like synoviocytes (FLSs) are specialized cells of the synovium that play a key role in the pathogenesis of rheumatoid arthritis (RA). In this study we explored the possible roles of excessive IgD expression on the function of FLSs from RA patients (RA-FLSs). We showed that IgD Fc receptor (IgDR) was constitutively expressed on FLSs, and was significantly elevated in RA-FLSs compared with FLSs prepared from synovial tissues of healthy controls (HC-FLSs). Furthermore, IgDR was mainly detected on the cell surface and in the cytoplasm. We further detected the intrinsic binding affinity of IgD to IgDR on HC-FLSs with an equilibrium dissociation constant (KD) of 0.067 nmol/L. Incubation of RA-FLSs with IgD (1–10 μg/mL) for 48 h dose-dependently promoted the expression of IgDR, and stimulated the production of inflammatory cytokines and chemokines, such as IL-1β, IL-6, monocyte chemotactic protein (MCP)-1, TNF-α and receptor activator of nuclear factor-κB ligand (RANKL), thus potentially contributing to IgD-IgDR crosslinking. Moreover, incubation with IgD (0.1–10 μg/mL) for 48 h dose-dependently enhanced viability for both HC-FLSs and RA-FLSs. Our results demonstrate that IgDR is expressed on RA-FLSs and contributes to the activation of FLSs, and suggest that IgD-IgDR is a potential novel immunotherapeutic target for the management of RA.
Keywords: rheumatoid arthritis, immunoglobulin D, immunoglobulin D Fc receptor, fibroblast-like synoviocytes, fluorescence based receptor binding assay, inflammatory cytokines
Introduction
The immunoglobulin IgD can be expressed as membrane IgD (mIgD) representing one of two B cell receptors with an attached signaling machinery or can be secreted as an antibody (sIgD)1. IgD has been found to be increased in several diseases, such as IgD myeloma, tuberculosis, chronic obstructive pulmonary diseases, Hodgkin's disease, hyper IgE and hyper IgD syndromes2. Furthermore, sIgD levels are increased in autoimmune disorders such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)1,2,3,4. However, high expression of sIgD is associated with increased protein-like sediments and cell necrosis in the kidney, spleen and liver in transgenic mice5. Recent studies have suggested that IgD might play an important role in autoimmune diseases, and the function of IgD has remained elusive since its discovery, despite multiple attempts to define its biological function.
Many lymphoid and non-lymphoid cells express surface membrane receptors, and many studies have focused on specific structures located in the constant region of immunoglobulin molecules (FcR)6, such as immunoglobulin A (IgA), IgE, IgG, and IgM. Surprisingly, an Fc receptor specific for IgD (FcδR or IgDR) has been suggested to exist on T cells7; however, early attempts to detect its expression have produced variable and conflicting results8. Through quantitative immunofluorescence techniques, binding of IgD to T cells and basophilic cell lines has been demonstrated, and an increase in δ+ T cells in vivo has also been observed, thus suggesting that a putative IgDR on T cells may play a role regulating IgD2,4,9. Recently, our group has reported further evidence of the existence of an IgDR, demonstrating that human IgD antibody induces cell proliferation in the human Burkitt lymphoma B cell line, Daudi, potentially by activating the putative IgDR and consequently initiating a tyrosine phosphorylation signaling cascade that accelerates the G1/S transition10. Furthermore, we have reported that in RA patients, the levels of sIgD, mIgD and IgDR are higher than those in healthy controls, and after treatment with IgD antibodies, the expression of IgDR on peripheral T and B cells significantly increases, thereby possibly contributing to RA pathogenesis11.
RA is a chronic inflammatory autoimmune disease characterized by synovial hyperplasia and chronic inflammation. A marked increase in macrophage-like and fibroblast-like synoviocytes (FLSs) in the synovial membrane and the accompanying inflammatory infiltration of other immune cells such as synovial macrophages result in bone erosion and joints destruction. Accumulating evidence indicates that activated FLSs in RA patients contribute to joint destruction, and the possible roles of excessive IgD expression on the function of FLSs from RA patients (RA-FLSs) are discussed in this work. The expression of IgDR has been described on CD4+ and CD8+ T cells in mice and humans1,3,11. However, the role of IgDR on cells of the synovial infiltrate has not yet been studied. Therefore, we investigated the expression of IgDR in RA-FLSs and explored the effects of IgD. We identified IgD-binding IgDR as a ∼70 kDa protein complex that was detected on FLSs by using flow cytometry and confocal laser scanning microscopy. Studies of the binding properties indicated that human IgD bound to IgDR with an equilibrium dissociation constant (KD) of 0.067 nmol/L. Furthermore, we found that IgD significantly promoted the viability of FLSs, induced cytokines/chemokine secretion and increased the expression of IgDR in FLSs, thus potentially contributing to RA pathogenesis.
Materials and methods
Reagents
Human IgD was purchased from Abcam (Cambridge, MA, USA). Biotinylated IgD was prepared in our laboratory with a protein biotinylation kit from Pierce Biotechnology (Rockford, IL, USA), according to the manufacturer's instructions12. APC-Cy7 conjugated streptavidin, PE conjugated anti-IgD antibody and IgG1 isotype control were purchased from BD Pharmingen (San Diego, CA, USA).
Preparation and culture of FLSs
Human synovial membranes were obtained from patients with RA undergoing arthroscopy. The diagnosis of RA conformed to the American College of Rheumatology revised criteria from 198713. Normal knee tissues were obtained from donors undergoing traumatic surgery. Synovial tissues were minced and digested with type IA collagenase for 3 h, filtered, extensively washed and then cultured in DMEM containing 20% fetal bovine serum (FBS, Sigma-aldich, MO, USA) at 37 °C in a humidified atmosphere of 5% CO2. At confluence, adherent cells were trypsinized, split in a 1:3 ratio, and re-cultured in medium14. The spindle-shaped cells at passage 3 were used in subsequent experiments, during which time they were a homogeneous population of FLSs. The study protocol was carried out in accordance with the Declaration of Helsinki (2008) and was approved by the Ethics Committee of Anhui Medical University. Written informed consent was obtained from each participant before the start of the study.
Flow cytometry analysis
Expression of IgDR was detected by treatment of FLSs with biotinylated IgD. For detecting IgD binding receptors, a total of 1×106 cells were washed twice with PBS and incubated with 1 μg of biotinylated IgD for 30 min at room temperature (RT). After washing, APC-Cy7 conjugated streptavidin was added, and cells were incubated for an additional 30 min on ice and washed again. Cells stained with APC-Cy7 conjugated streptavidin alone were used as an isotype control. For assessment of IgD, a total of 1×106 cells were washed twice with PBS and incubated with PE conjugated anti-IgD antibodies for 30 min at RT, and the IgG1 isotype control was also matched. To determine the ability of IgD to induce the expression of IgDR, FLS were stimulated with IgD for 48 h or were left unstimulated. After incubation, expression of IgDR was detected with biotinylated IgD followed by APC-Cy7 conjugated streptavidin. Data were acquired on a FC 500 flow cytometer (Beckman Coulter Ltd, CA, USA ) and analyzed with CXP analysis software (Beckman Coulter Ltd, CA, USA).
Confocal microscopy analysis
FLSs were plated on coverslips and fixed with 4% paraformaldehyde for 20 min at RT. Then, cells were permeabilized with 0.2% Triton X-100 for 10 min at RT and washed with PBS three times. After being blocked with 1% BSA in PBS for 1 h at RT, biotinylated IgD was added, and cells were incubated overnight at 4 °C. After being washed with PBS three times, cells were stained with streptavidin labeled APC-Cy7 secondary antibody for 2 h at 37 °C. Cells were then washed with PBS buffer three times. When the coverslips were dry, a mounting solution containing DAPI (Vector Laboratories, Inc, Burlingame, CA, USA) was applied, and images were taken using a SP8 confocal laser scanning microscope (Leica, Wetzlar, Germany). The expression of IgDR was quantitatively analyzed using ImageJ software.
Fluorescence based receptor binding assay and Scatchard analysis
Protocols were used to evaluate the intrinstic binding affinity of fluorescence labeled IgD antibody to IgD on IgDR in FLSs. Binding of IgD for detection by flow cytometry was done as described below. Various concentrations of IgD (0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, 10 μg/mL) were used. Maximal binding of IgD was observed on FLS in 6-well microtiter plates at 1×106 cells/well, incubated at 37 °C for 2 h in fresh medium (with 0.1% BSA), then cells were washed three times with PBS at RT, then with buffer appropriate to the assay. Binding of IgD was detected with PE conjugated anti-IgD antibody, and IgG1 isotype control was used to calculate non-specific binding. The binding of IgD to the anti-IgD antibody was analyzed by using flow cytometry. To measure the affinity of the fluorescent ligand IgD, the intensity values corresponding to the maximum of fluorescence emission were plotted against the total ligand concentrations. The ligand binding properties were evaluated by using the fluorescence intensities, assuming that the protein was 100% active, with a stoichiometry of 1:1 (protein:ligand) at saturation. The curves were made linear by using Scatchard Plots. The KD values for IgD protein were calculated15,16.
Western blot
FLSs were added to 6-well microtiter plates at 1×106 cells/well, stimulated with IgD (1, 3, 10 μg/mL) for 48 h and incubated at 37 °C with 5% CO2. After culture, FLSs were lysed and centrifuged at 2000×g for 20 min at 4 °C. The supernatants of cultures were recollected and kept frozen at −80 °C until use. Protein samples were fractionated through 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride (PVDF) microporous membranes (Bio-Rad, Shanghai, China). After membranes were blocked with blocking buffer (0.05% Tween 20-PBS with 5% non-fat milk) at 37 °C for 2 h, biotinylated IgD was used as a primary antibody to IgDR and incubated overnight at 4 °C. Subsequently, cells were treated with HRP-conjugated streptavidin as a secondary antibody (Beyotime Institute of Biotechnology, Beijing, China) at 37 °C for 2 h. Immunodetection was performed using enhanced chemiluminescence reagent, according to the manufacturer's instructions. Equivalent protein loading and transfer efficiency were verified by staining for β-actin. GSM-3.0 gel graph analyzing system was used to calculate the numerical value of every blot.
FLSs viability assay
FLSs of human obtained, as described above, were added to 96-well microtiter plates at 2×104 cells/well in DMEM with 10% FBS. FLSs were stimulated with IgD (0.1, 0.3, 1, 3, 10 μg/mL) for 24, 48, or 72 h in 96-well plates (each group in triplicate wells) and incubated at 37 °C under 5% CO2. For each experiment, control cells were treated with medium only. A Cell Counting Kit-8 (WST-8; Dojindo Laboratories, Kumamoto, Japan) was used to explore the effects of IgD on FLS viability. Moreover, 10 μL of WST-8 solution was added per well after co-culture. Blank controls included 100 μL of DMEM and 10 μL of WST-8 solution in triplicate wells17. The cells were incubated for 4 h at 37 °C, 5% CO2, and the absorbance at 450 nm was measured colorimetrically on an Elx-808 microplate reader (BioTek, VT, USA), according to the manufacturer's protocol. Proliferative responses were expressed as a stimulation index (SI), wherein the SI was equal to the absorbance at 450 nm in cells cultured with IgD divided by the absorbance in cells cultured with medium alone.
Cytokine and chemokine assays
FLSs were stimulated with IgD (0.1, 0.3, 1, 3, 10 μg/mL) for 48 h in 24-well plates and incubated at 37 °C under 5% CO2. The levels of inflammatory cytokines and chemokines, including (IL-1α, IL-1β, TNF-α, IL-6, IL-10, IL-8, IL-4, INF-γ, IL-13, and monocyte chemotactic protein (MCP)-1, in FLS supernatants were measured by using a quanti body human inflammation array 1 (RayBiotech, Norcross, USA). In addition, simultaneous quantification of the cytokines was performed on a GenePix 4000B microarray scanner (Molecular Devices, CA, USA). Human receptor activator of nuclear factor-κB ligand (RANKL) in supernatant samples was determined using the ELISA method (CUSABIO, Wuhan, China), according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed with analysis of variance (ANOVA) in SPSS 11.5 software (SPSS, Inc, Chicago, IL, USA). ANOVA was used exclusively for multigroup comparisons. Parametric or nonparametric tests were used according to the normality of the distribution. Data are presented as the mean±standard error of the mean (SEM) unless otherwise indicated. Differences with P values less than 0.05 were considered statistically significant.
Results
IgDR is constitutively expressed on FLSs
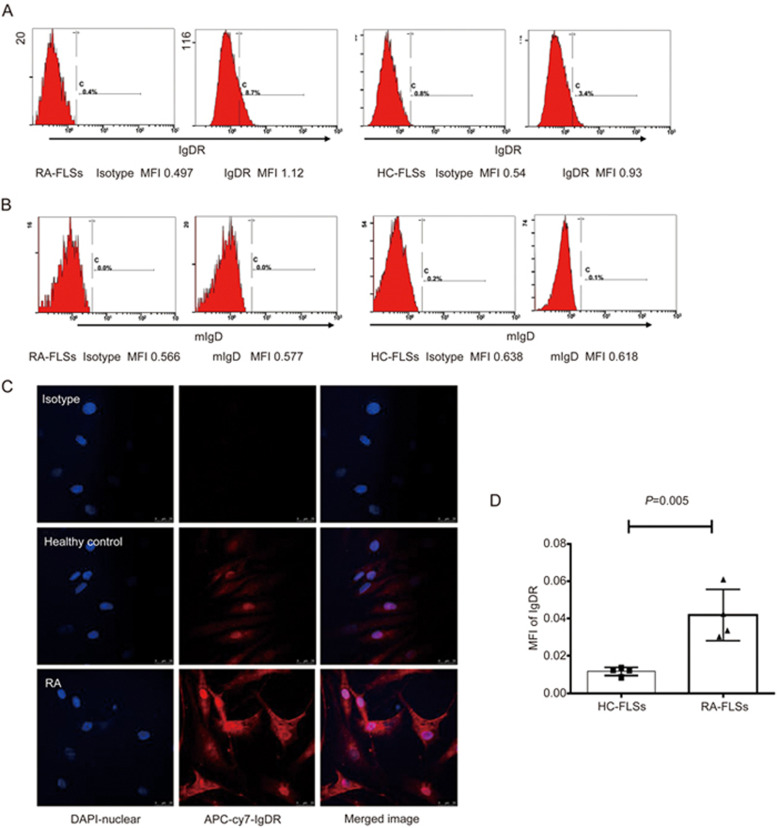
Flow cytometry was performed to identify the expression of IgD and IgDR on FLSs. As shown in Figure 1A, 8.7% of RA-FLSs and 3.4% of HC-FLSs were positively stained with biotinylated IgDR. The expression of IgDR was indicated by histograms. Additionally, mIgD was undetectable on the surface of FLSs (Figure 1B). We further characterized the subcellular localization of IgDR in RA-FLSs and healthy controls (HC-FLSs) by confocal immunofluorescence microscopy (Figure 1C). As expected, IgDR was primarily localized on the cell membrane and in the cytoplasm. The mean fluorescence intensity (MFI) of IgDR was higher on of RA-FLSs than on HC-FLSs (0.0419±0.007 vs 0.0117±0.001, P=0.005, Figure 1D).
Figure 1.
Expression of IgD and IgDR on FLSs. The expression of IgDR (A) and mIgD (B) was determined by flow cytometry and is indicated by the filled histograms. MFI: Mean fluorescence intensity. (C) Subcellular localization of IgDR in RA-FLSs and HC-FLSs, determined by confocal immunofluorescence microscopy. Colocalization was confirmed when the images were merged (right panel). Isotype matched control primary antibody staining is also shown in C (first line). (D) Quantification of IgDR expression by confocal microscopy analysis. Data represent the mean±SEM (n=4).
Dose-dependent binding between IgD and IgDR
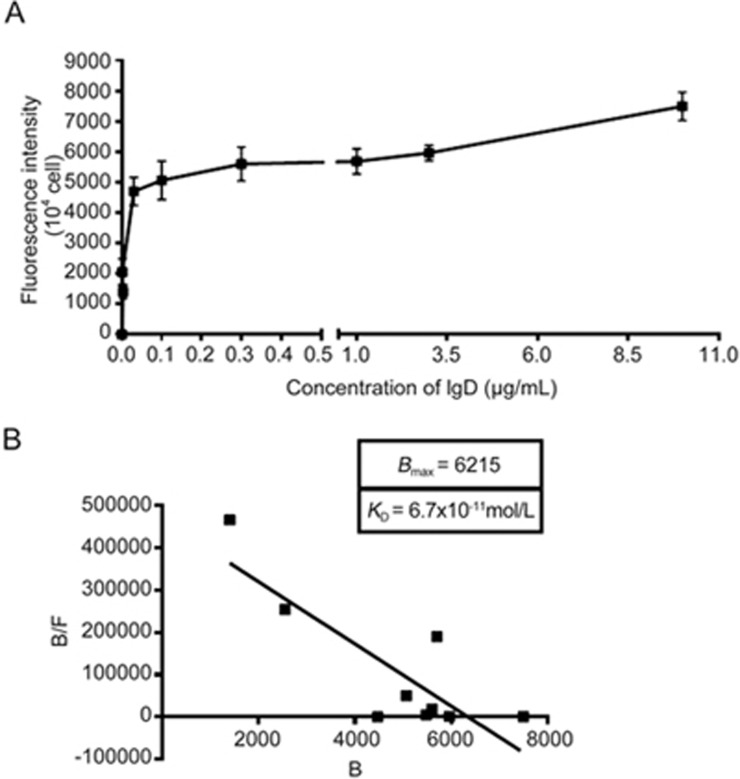
To determine whether the observed binding of IgD to IgDR on FLSs showed characteristics of receptor-ligand interaction, we determined receptor saturation and affinity of binding. The affinity of human IgD was detected with PE conjugated anti-IgD antibody. Binding of IgD to IgDR expressed on HC-FLSs was also determined with IgD as ligands. Our findings indicated that binding between IgD and IgDR was concentration-dependent (Figure 2A).
Figure 2.
Ligand binding assays. HC-FLSs expressing IgDR were incubated with human IgD for 2 h at 37 °C and washed three times. The cells with bound fluorescent ligands were determined by flow cytometry. Values for nonspecific binding, determined by isotype control, were subtracted. Data are plotted as Scatchard analyses and are representative of several independent experiments, n=4. (A) The specific binding vs IgD concentrations was plotted. (B) The Scatchard line was transformed from the specific binding values. Bmax and KD values were calculated by Scatchard plot analysis.
Binding affinity
We estimated the IgD binding affinity by using a fluorescence based receptor binding assay. The specific binding vs IgD concentration was determined (Figure 2A). The Scatchard line transformed from the specific binding data was plotted (Figure 2B). Bmax and KD values were calculated through Scatchard plot analysis as shown in Figure 2B. Moreover, the KD value was 6.7×10-11mol/L, which corresponded to a high-affinity binding, whereas the Bmax value was 6215 arbitrary units/104 cells (Figure 2B).
IgD stimulated IgDR expression in RA-FLSs
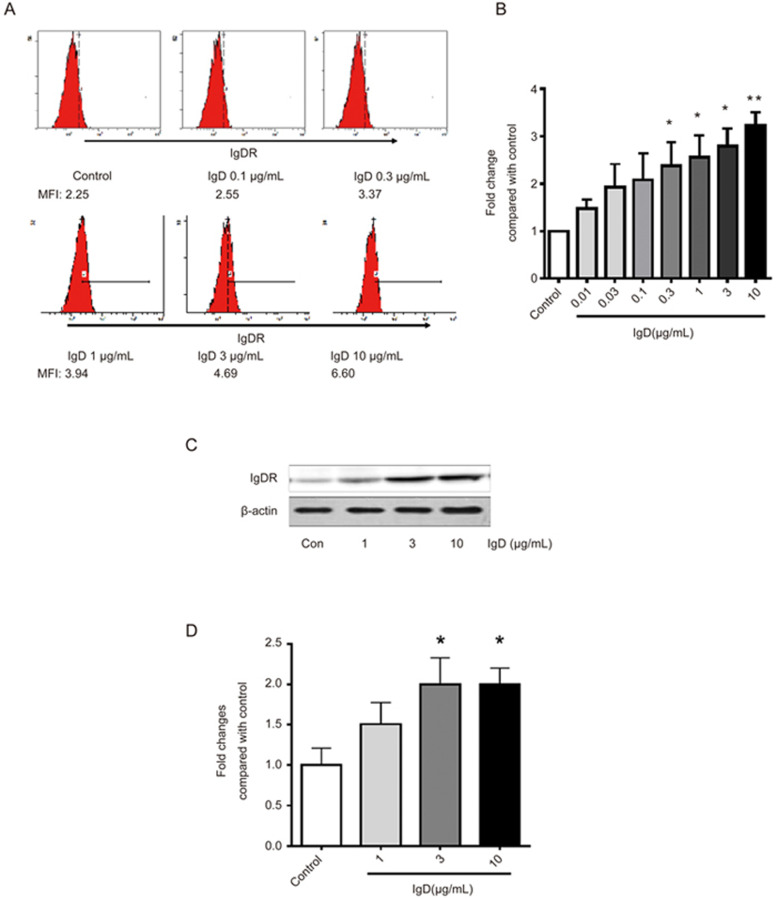
To examine whether IgD stimulated the expression of IgDR, flow cytometry and western blotting analysis were performed on RA-FLSs after they were co-cultured with IgD for 48 h. As shown in Figure 3A, the MFI of IgDR on the surface of FLSs was upregulated in the presence of increasing IgD concentration (Figure 3B). Western blot analysis showed increased levels of IgDR in the 70 kDa band after IgD stimulation (Figure 3C and 3D), a result consistent with the flow cytometry results.
Figure 3.
Effect of IgD on the expression of IgDR on RA-FLSs. RA-FLSs were stimulated with 0.1, 0.3, 1, 3, 10 μg/mL of IgD for 48 h. (A) Dose dependent induction of IgDR expression on FLSs was assayed by flow cytometry. MFI: Mean fluorescence intensity. (B) Bar graphs show quantitative evaluation of expression of IgDR by MFI. (C) Expression of IgDR in FLSs was assayed by Western blotting. (D) Bar graphs show quantitative evaluation of expression of IgDR by densitometry. Data represent the mean±standard error of the mean (n=3). *P<0.05, **P<0.01 vs control.
IgD enhanced FLSs viability
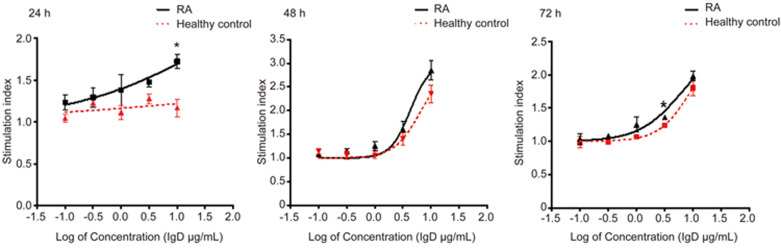
To determine the effect of excess IgD on the viability of the FLSs, RA-FLSs and HC-FLSs were cultured under different concentrations of IgD for various times before viability was measured. As expected, the viability of FLSs was increased in response to treatment with IgD. As shown in Figure 4 and Table 1, for RA-FLSs, IgD enhanced the viability of FLSs in a concentration-dependent manner after 48 h of treatment (P<0.05), with an EC50 value of 4.243 μg/mL (R2=0.897). After 72 h, enhanced viability was still significant (P<0.05), and the EC50 values were 3.316 μg/mL (R2=0.836). These results suggested that the optimal stimulation time was 48 h, and the optimal stimulation concentration ranged from 1 to 10 μg/mL. In addition, we measured the effect of IgD stimulation on RA-FLSs and HC-FLSs and found that IgD slightly enhanced RA-FLS viability after 24 h, whereas it had no significant effect on HC-FLSs. To a lesser extent, the viability of HC-FLSs was also increased after cultured with IgD for 48 h. Together, these results demonstrated that RA-FLSs are more sensitive to IgD than HC-FLSs are.
Figure 4.
The stimulating effect of IgD on RA-FLSs and HC-FLSs. RA-FLSs and HC-FLSs were cultured with 0.1, 0.3, 1, 3, 10 μg/mL of IgD for 24 h (A), 48 h (B), or 72 h (C). Data are expressed as the mean±SEM (n=4), SI=absorbance at 450 nm in the cell cultures with drug divided by the absorbance in the culture with medium alone. *P<0.05, **P<0.01 vs healthy control.
Table 1. Comparison of stimulating effect of IgD on FLSs between RA patients and healthy controls.
| Group Time (h) | RA- FLSs | HC-FLSs | RA-FLSs | HC-FLSs | RA-FLSs | HC-FLSs |
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | ||||
| EC50 (μg/mL) of IgD | 66.3 | - | 4.243 | 6.674 | 3.316 | 5.242 |
| 95% Confidence intervals | 6.156∼715.8 | - | 3.287∼5.476 | 5.244∼8.495 | 2.211∼4.975 | 4.016∼6.843 |
| R2 | 0.519 | 0.096 | 0.897 | 0.880 | 0.836 | 0.899 |
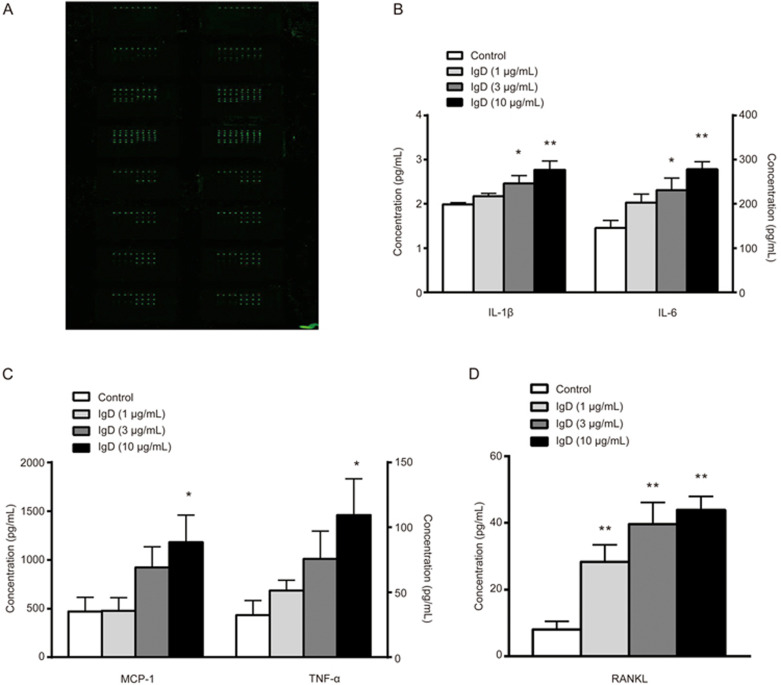
IgD stimulated cytokine and chemokine secretion in RA-FLSs
To further characterize the effects of IgD on RA-FLSs, we used a cytokine/chemokine antibody-based array to measure protein secretion by FLSs that were cultured in the presence or absence of IgD. Compared with that in unstimulated cells, secretion of IL-1β, IL-6, MCP-1 and TNF-α was significantly induced by IgD (10 μg/mL) (P<0.05), and secretion of RANKL was significantly induced by IgD (1 μg/mL) (P<0.01) (Figure 5).
Figure 5.
Effects of IgD on inflammatory cytokine production in RA-FLS culture supernatant. RA-FLS culture supernatants were collected and treated with IgD (1, 3, or 10 μg/mL) for 48 h. The glass slide was scanned with a microarray scanner (A), levels of IL-1β and IL-6 (B), MCP-1 and TNF-α (C), RANKL (D) were measured. Data represent the mean±SEM (n=4). *P<0.05, **P<0.01 vs control.
Discussion
In RA, FLSs play an important role in responding to a pro-inflammatory environment, such as that associated with infiltration of lymphocytes and dendritic cells. In addition, FLSs in RA can act as an effector cell population that participates in synovial inflammation and joint destruction18.
The immunoglobulin IgD accounts for approximately 1% of the normal serum immunoglobulins and may be involved in different immunological mechanisms1,4,19,20. Receptors for the Fc portion of immunoglobulin molecules have been described on a number of lymphoid cells in mammals, including T cells, B cells and monocytes/macrophages21,22. Coico et al have identified IgDR-positive T cells by developing a rosetting assay7, and their results have indicated that IgD is a ligand for IgD-specific receptors. In contrast to IgG and IgE receptors, the structural and functional characteristics of the IgD receptor remain unknown because the gene encoding the IgD receptor has not yet been identified. Although accumulating evidence indicates that activated FLSs are key players in RA joint destruction, the roles of IgD and IgDR expression on FLSs have not been described. In this study, biotinylated IgD and flow cytometric analysis was used to detect the expression of IgDR on human FLSs11. We demonstrated that IgDR, not mIgD, was expressed on the surfaces of FLSs. Furthermore, we confirmed the subcellular localization of IgDR in FLS and showed that expression of IgDR was higher on RA-FLSs than on HC-FLSs.
To investigate whether IgD binding to FLSs had the characteristics of a receptor-ligand interaction, we established a fluorescence based receptor binding assay based on a radioactive ligand receptor binding assay and using a fluorescence labeled antibody15,23,24. We found that after shorter periods in culture, human FLSs bound IgD in a manner characteristic of receptor-ligand interactions. The binding affinity of IgD to IgDR on RA-FLSs was found to be high (KD=0.067 nmol/L) and similar to the KD for binding to T cells (data not shown). In fact, the binding affinity of IgD was similar to the KD for binding of canakinumab to IL-1β in RA patients (KD=0.227 nmol/L)25 and was 800 times higher than that reported for human IgG1 (KD=528 nmol/L)26.
In recent studies, we have found that the serum level of sIgD from RA patients was significantly higher (91.93 μg/mL) than that from healthy controls (19.8 μg/mL). Moreover, these findings indicate that increased sIgD may play an important role in the pathogenesis of RA. Among the various pathological mechanisms that affect the synovium of RA, FLSs have been reported to play an important role in both the propagation of inflammation and joint damage27,28. FLSs induce the activation and accumulation of inflammatory cells and secreted growth factors, thus promoting angiogenesis29. In addition, RA-FLSs proliferate and migrate from the affected synovium to healthy synovium30. Our flow cytometry and Western blot results showed that IgD significantly increased the expression of IgDR in RA-FLSs. Further studies are necessary to investigate whether increased IgD may influence the function of FLSs and whether IgD might promote the pathological process in RA via upregulation of IgDR expression.
We have previously shown that IgD stimulation improves viability and promotes IgDR expression in Daudi cells and human PBMCs10,11. Indeed, we found that IgD enhanced the viability of human FLSs in a concentration-dependent manner. Moreover, the stimulation of viability of RA-FLSs by IgD was stronger than that of HC-FLSs at the same time point. In addition, the response of RA-FLSs to IgD was accelerated compared with that in HC-FLSs. These results indicated a close relationship between the response to IgD and expression of IgDR, thus suggesting that IgD-IgDR participates in joint destruction in RA.
Upon synoviocyte activation, several pro-inflammatory cytokines are essential for the development of RA31. FLSs are key cells of the invasive synovium that may induce inflammation by activating cytokine and chemokine production14. Cytokines, such as IL-1β, IL-6 and TNF-α, stimulate RA-FLS invasion and increase the production of matrix metalloproteinases (MMPs), thus aggravating synovial inflammation and resulting in joint destruction32. By secreting CCL2 (MCP-1), activated resident synoviocytes recruit monocytes into the joints, thereby initiating inflammatory responses33. The immunoglobulin IgD has been shown to enhance the release of various pro-inflammatory cytokines from human PBMCs, including IL-6, IL-1 and TNF-α, in vitro4,11,34. Additionally, it enhances IL-6 release by KU812 cells2. To our knowledge, the action of IgD on cytokine and chemokine release by RA-FLSs has not been investigated. In this study, IL-1β, IL-6, TNF-α and MCP-1 were found to be released from activated FLSs in RA patients and appear to be the important participants in the pathophysiology of RA after treatment with IgD. How does IgD influence the function of FLSs? There are several possible explanations: first, IgD modulates the function of FLSs via interaction with IgDR. However, the signaling pathways downstream of IgDR on FLSs are still unknown. Second, IgD modulates the function of FLSs by upregulating the production of TNF-α, IL-1β, and IL-6. In turn, IL-1β promotes proliferation by MAPK and NF-κB activation35,36,37 while increasing the production of IL-1β, IL-6, and RANKL. IL-6 stimulates RANKL expression through IL-6/IL-6R/JAK pathway38. In RA patients, we have previously reported that IgD concentration is correlated with serum RANKL concentration11. The inflamed synovium is an optimal microenvironment for RANKL activation, which is essential for the differentiation of monocyte/macrophages into mature osteoclasts39. In patients with RA, FLSs have been shown to produce RANKL, which may promote osteoclast development40. Our present results showed that RANKL may be a key molecule downstream of the IgD-IgDR signaling pathway. IgD might enhance the level of RANKL in RA thereby leading to osteoclastogenesis at the site of pannus formation in the RA synovium. Further elucidation of IgDR and its signaling pathway is necessary to provide a clearer understanding of the molecular mechanisms by which FLS activation is regulated.
Our results suggested that excessive IgD in RA may enhance viability and cytokine/chemokine release by FLSs and improve FLS activation via cross-linking with IgDR. Therefore, the IgD-IgDR interaction may be a promising target for development of therapeutic agents for RA treatment.
Abbreviations
FLSs, fibroblast-like synoviocytes; IgA, immunoglobulin A; IgDR, IgD Fc receptor; mIgD, membrane IgD; MCP-1, monocyte chemotactic protein; MFI, mean fluorescence intensity; MMPs, matrix metalloproteinases; PBMCs, peripheral blood mononuclear cells; PVDF, polyvinylidene fluoride; RA, rheumatoid arthritis; RANKL, nuclear factor-κB ligand; sIgD, secreted IgD; SLE, systemic lupus erythematosus.
Author contribution
Yu-jing WU designed and performed experiments and wrote the manuscript; Wen-sheng CHEN and Heng-shi CHEN participated in study design, performed the statistical analysis and revised the manuscript; Jin DONG and Xing DAI carried out the flow cytometry assays; Ying WANG collected all donors' clinical samples and prepared the FLSs; Yan CHANG and Xiao-yi JIA carried out the confocal microscopy analysis; Ling-ling ZHANG and Qiong HUANG performed the statistical analysis and helped to revise the manuscript; Wei WEI conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No 81330081, 81473223, 81573443, and 81603121), Grants for Scientific Research of BSKY (No XJ201629) from Anhui Medical University and the Anhui Province Natural Science Foundation in the University (No KJ2015A317).
References
- Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev 2010; 237: 160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechet B, Meseri-Delwail A, Arock M, Wijdenes J, Lecron JC, Sarrouilhe D. Immunoglobulin D enhances interleukin-6 release from the KU812 human prebasophil cell line. Gen Physiol Biophys 2003; 22: 255–63. [PubMed] [Google Scholar]
- Rostenberg I, Penaloza R. Serum IgG and IgD and levels in some infectious and noninfectious diseases. Clin Chim Acta 1978; 85: 319–21. [DOI] [PubMed] [Google Scholar]
- Chen K, Xu W, Wilson M, He B, Miller NW, Bengtén E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol 2009; 10: 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wei Z, Yan B, Huang T, Gou K, Dai Y, et al. Establishment of a transgenic mouse model with liver-specific expression of secretory immunoglobulin D. Sci China Life Sci 2012; 55: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth PM. Fc Receptors: Introduction. Immunol Rev 2015; 268: 1–5. [DOI] [PubMed] [Google Scholar]
- Coico RF, Xue B, Wallace D, Pernis B, Siskind GW, Thorbecke GJ. T cells with receptors for IgD. Nature 1985; 316: 744–6. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kubagawa H, Ohno T, Cooper MD. Characterization of an IgM Fc-binding receptor on human T cells. J Immunol 1993; 151: 6933–41. [PubMed] [Google Scholar]
- Lakshmi TSM, Wu Y, Toporovsky I, Lima V, Coico RF. IgD receptor-mediated signal transduction in T cells. Cell Immunol 2001; 207: 110–7. [DOI] [PubMed] [Google Scholar]
- Dai X, Wu Y, Jia X, Chang Y, Wu H, Wang C, et al. hIgD promotes human Burkitt lymphoma Daudi cell proliferation by accelerated G1/S transition via IgD receptor activity. Immunol Res 2016; 64: 978–87. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen W, Chen H, Zhang L, Chang Y, Yan S, et al. The elevated secreted immunoglobulin D enhanced the activation of peripheral blood mononuclear cells in rheumatoid arthritis. PLoS One 2016; 11: e0147788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lakshmi TSM, Lima V, Coico R. Facilitated antigen presentation by B cells expressing IgD when responding T cells express IgD-receptors. Cell Immunol 1999; 192: 194–202. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–24. [DOI] [PubMed] [Google Scholar]
- Miyabe Y, Miyabe C, Iwai Y, Yokoyama W, Sekine C, Sugimoto K, et al. Activation of fibroblast-like synoviocytes derived from rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic acid receptor 1 cascade. Arthritis Res Ther 2014; 16: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Li KJ, Siao SC, Chen YH, Wu TH, Tsai CY, et al. The binding affinity and molecular basis of the structure-binding relationship between urinary Tamm-Horsfall glycoprotein and tumor necrosis factor-alpha. Molecules 2012; 17: 11978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JR, Jacocks HM, Niven SC, Poli MA, Baden DG, Bourdelais AJ. Development and utilization of a fluorescence-based receptor-binding assay for the site 5 voltage-sensitive sodium channel ligands brevetoxin and ciguatoxin. J AOAC Int 2014; 97: 307–15. [DOI] [PubMed] [Google Scholar]
- Wang D, Hu S, Zhu J, Yuan J, Wu J, Zhou A, et al. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J Cell Mol Med 2013; 17: 1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev 2010; 233: 256–66. [DOI] [PubMed] [Google Scholar]
- Rigante D. The truth on IgD in the ploy of immune surveillance and inflammation. Immunol Res 2016; 64: 632–5. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol 2011; 29: 273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RS. Fc receptor-like molecules. Annu Rev Immunol 2007; 25: 525–60. [DOI] [PubMed] [Google Scholar]
- Leech MT, Morand EF. Fibroblasts and synovial immunity. Curr Opin Pharmacol 2013; 13: 565–9. [DOI] [PubMed] [Google Scholar]
- Chang SK, Mihalcik SA, Jelinek DF. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol 2008; 180: 7394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Quagliata JM, Patel S, Han JK, Arakelov S, Rao TD, Shulman MJ, et al. The IgA/IgM receptor expressed on a murine B cell lymphoma is poly-Ig receptor. J Immunol 2000; 165: 2544–55. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin Pharmacokinet 2012; 51: e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbaxani B, Dostalek M, Gardner I. Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity. Mol Immunol 2013; 56: 660–74. [DOI] [PubMed] [Google Scholar]
- Tough DF, Prinjha RK, Tak PP. Epigenetic mechanisms and drug discovery in rheumatology. Clin Med (Lond) 2015; 15: s64–71. [DOI] [PubMed] [Google Scholar]
- Miossec P. Rheumatoid arthritis: still a chronic disease. Lancet 2013; 381: 884–6. [DOI] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 2010; 233: 233–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 2010; 237: 264–83. [DOI] [PubMed] [Google Scholar]
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 2008; 118: 3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum 2012; 64: 2499–503. [DOI] [PubMed] [Google Scholar]
- Scanu A, Oliviero F, Gruaz L, Sfriso P, Pozzuoli A, Frezzato F, et al. High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res Ther 2010; 12: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Göertz J, Daha MR, van der Meer JW. Immunoglobulin D enhances the release of tumor necrosis factor-alpha, and interleukin-1 beta as well as interleukin-1 receptor antagonist from human mononuclear cells. Immunology 1996; 88: 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012; 119: 651–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liu S, Yua D, Zhang N. Therapeutic effect of total saponins from Dioscorea nipponica Makino on gouty arthritis based on the NF-κB signal pathway: An in vitro study. Pharmacogn Mag 2016; 12: 235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HX, Chen JY, Wang QT, Sun WY, Liu LH, Zhang LL, et al. Expression and function of β-arrestin 2 stimulated by IL-1β in human fibroblast-like synoviocytes and the effect of paeoniflorin. Int Immunopharmacol 2012; 12: 701–6. [DOI] [PubMed] [Google Scholar]
- Choe JY, Park KY, Park SH, Lee SI, Kim SK. Regulatory effect of calcineurin inhibitor, tacrolimus, on IL-6/sIL-6R-mediated RANKL expression through JAK2-STAT3-SOCS3 signaling pathway in fibroblast-like synoviocytes. Arthritis Res Ther 2013; 15: R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani K, Itoh K, Nakajima K, Kuroki H, Katsuragawa Y, Mochizuki M, et al. Rheumatoid arthritis fibroblast-like synoviocytes express BCMA and are stimulated by APRIL. Arthritis Rheum 2007; 56: 3554–63. [DOI] [PubMed] [Google Scholar]
- Stanford SM, Svensson MN, Sacchetti C, Pilo CA, Wu DJ, Kiosses WB, et al. Receptor protein tyrosine phosphatase α-mediated enhancement of rheumatoid synovial fibroblast signaling and promotion of arthritis in mice. Arthritis Rheumatol 2016; 68: 359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]