Abstract
Objective
Cerebrospinal fluid (CSF) plays an important role in cortical development during the fetal stages. Embryonic CSF (E-CSF) consists of numerous neurotrophic and growth factors that regulate neurogenesis, differentiation, and proliferation. Mesenchymal stem cells (MSCs) are multi-potential stem cells that can differentiate into mesenchymal and non-mesenchymal cells, including neural cells. This study evaluates the prenatal and postnatal effects of CSF on proliferation and neural differentiation of bone marrow MSCs (BM-MSCs) at gestational ages E19, E20, and the first day after birth (P1).
Materials and Methods
In this experimental study, we confirmed the mesenchymal nature of BM-MSCs according to their adherence properties and surface markers (CD44, CD73 and CD45). The multi-potential characteristics of BM- MSCs were verified by assessments of the osteogenic and adipogenic potentials of these cells. Under appropriate in vitro conditions, the BM-MSCs cultures were incubated with and without additional pre- and postnatal CSF. The MTT assay was used to quantify cellular proliferation and viability. Immunocytochemistry was used to study the expression of MAP-2 and β-III tubulin in the BM-MSCs. We used ImageJ software to measure the length of the neurites in the cultured cells.
Results
BM-MSCs differentiated into neuronal cell types when exposed to basic fibroblast growth factor (b-FGF). Viability and proliferation of the BM-MSCs conditioned with E19, E20, and P1 CSF increased compared to the control group. We observed significantly elevated neural differentiation of the BM-MSCS cultured in the CSF-supplemented medium from E19 compared to cultures conditioned with E20 and P1 CSF group.
Conclusion
The results have confirmed that E19, E20, and P1 CSF could induce proliferation and differentiation of BM-MSCs though they are age dependent factors. The presented data support a significant, conductive role of CSF components in neuronal survival, proliferation, and differentiation.
Keywords: Cell Proliferation, Cerebrospinal Fluid, Mesenchymal Stromal Cells, Neural Differentiation
Introduction
The vertebrate central nervous system (CNS) develops from the neural tube filled with embryonic cerebrospinal fluid (E-CSF) and neuroepithelial cells adjacent to the E-CSF (1). CSF is mainly produced by the choroid plexus cells in the brain ventricles. During development and early postnatal stages, the CSF contains a high concentration of proteins in contrast to its low protein content in normal adults (2, 3). Evidence suggests that the E-CSF contains diffusible factors such as transforming growth factor-β (TGF-β), nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), and basic fibroblast growth factor (b-FGF). All of these factors are important for neurogenesis regulation (4, 5). b-FGF in the E-CSF of chick embryos at the early stages is involved in regulating neuro-ectodermal cell behavior, such as cell proliferation and neurogenesis (6). IGF2 signaling likely promotes proliferation of progenitor cells during cerebral cortical development. Researchers have reported that IGF2 expression in the rat CSF was temporally dynamic with a peak during periods of neurogenesis and decline in adulthood (7). HGF promotes the survival and migration of immature neurons. After birth, there is a rapid increase in HGF expression until day P2 that coincides with migration of neurons and glial in the cerebral cortex (4). The high concentration of proteins in the E-CSF compared to the adult CSF suggests that the protein in this fluid may partake in brain development. One of the functions attributed to embryonic and fetal CSF proteins is the generation of osmotic pressure inside the embryonic brain cavity, which is necessary for expansion of the cerebral primordium (8, 9). The CSF plays a number of key roles in neural development during embryonic and early days after birth. These roles include regulation of survival, proliferation, and differentiation of neuro-epithelial progenitor cells (10). It has been demonstrated that the CSF acts as a growth environment for cortical progenitor cells where the age of both the CSF and stem cells interact to ensure that different parts of the CNS develop at the correct times (11).
Stem cells are characterized by long-term selfrenewal capability and the ability to differentiate into multiple cell types in response to induction factors (12). The adult bone marrow (BM) contains two separate populations of stem cells. The hematopoietic stem cells (HSCs) give rise to all other blood cells via hematopoiesis (13, 14). Mesenchymal stem cells (MSCs) (13) are the non-hematopoietic part of the BM that consist of various heterogeneous populations (15). They are called BM derived from MSCs (BM-MSCs) or BM mesenchymal stromal cells (16).
MSCs are multi-potential cells that reportedly reside in virtually all postnatal organs and tissues. BM-MSCs are clonogenic, non-hematopoietic stem cells that exist in the BM and have the capability to differentiate into multiple mesoderm-type cell lineages such as osteoblasts, chondrocytes, and endothelial cells as well as non-mesoderm-type lineages (neuronal-like cells) (17-19). BM-MSCs are defined by their ability to adhere to plastic and express specific sets of surfacecell markers (20, 21). The identification and selection of stem cells within a given tissue or organ mainly relies on the presence of specific surface cell markers- CD29+, CD44+, CD73+, CD90+, CD105+, CD146+, PDGFR+, CD31-, CD34-, CD45-, and Stro-1- (14, 22). This investigation examines the differentiation of BMMSCs into neural cells by using E-CSF obtained at various gestational ages. This study aims to assess the ability of CSF to stimulate proliferation and neural differentiation of BM-MSCs for cell therapy applications.
Materials and Methods
In this experimental study, wistar rats were bred inhouse at the Research Facility of the Department of Biology, Kharazmi University after the Animal Use Committee at Kharazmi University provided Ethical approval. The animals were maintained in large rat cages at a constant temperature on a 12-hour light/dark cycle starting at 8 am and with free access to food and water. For timed mating, the individual male and female rats were paired in mating cages and checked regularly for the presence of a vaginal plug, which was taken as an indication of successful mating. This day was noted as embryonic day zero (E0). At specific gestational time points, the pregnant dams were euthanized by cervical dislocation, the uteri rapidly removed and placed on ice, and the fetuses were dissected out onto ice. A pregnant rat usually has 10-15 fetuses.
Collection of cerebrospinal fluid samples
We collected the CSF from the cisterna magna of the rat fetuses at E19, E20, and P1 using glass micropipettes and capillary action without aspiration. Samples containing undesirable blood contamination, visualized as a pink colour in the fluid, caused by damaging a blood vessel within the cisternal cavity, were discarded. All samples were collected into sterile microtubes and centrifuged at 4000 rpm. The clear supernatant was transferred into sterile tubes and stored at -40˚C. We used 200-300 pooled fetuses per age group for CSF analyses.
Total protein analysis
Samples were pooled according to specific prenatal and postnatal ages. Total protein concentration was measured using the Bradford protein assay with absorbance measured at 595 nm wavelength.
Preparation and culture of bone marrow mesenchymal stem cells
Male adult NMRI mice (6-8 weeks) were sacrificed, and we removed the femurs and tibias from both hind legs. After cleaning, the BM was flushed out with Dulbeccos’ modified Eagles’ medium (DMEM, Gibco, UK) using a syringe 0.5 ml with a needle. Cells were disaggregated by gentle pipetting through a decreasing needle bore size. The suspension of cells obtained was centrifuged at 1500 rpm at 25˚C for 5 minutes before resuspension in 1 ml of medium. Cells were seeded at a density of 104 cells/cm2 in a 25 cm2 plastic flask in DMEM, 15% fetal bovine serum (FBS, Gibco, UK), 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco, UK) then incubated at 37˚C and 5% CO2. After 48 hours, we removed any nonadherent cells by replacing the medium. We added fresh medium every 3 or 4 days. Passage-2 cells were used for the eperiments.
Differentiation of bone marrow mesenchymal stem cells
We induced adipogenic differentiation by culturing 90% confluent cultures in adipogenic induction medium that contained DMEM-LG with FBS, isobutylmethylanthine, insulin, deamethasone, and indomethacin. The medium was changed every third day. After 2 weeks, the cells were stained with oil red O to confirm adipogenic patterning (Sigma-Aldrich, USA). Osteogenic differentiation was confirmed after BM-MSCs were incubated in induction medium that included DMEM-LG with FBS, deamethasone, ascorbate, and β-glycerophosphate for approximately 14 days. In order to assess extracellular calcium deposits, the cultures were stained with 2% alizarin red S (Sigma-Aldrich, USA).
Surface antigen analysis of bone marrow
mesenchymal stem cells by flow cytometry Pasage-3 adherent cells were treated with 0.25% trypsin (Gibco, UK) and washed twice with PBS. Cells were incubated with the antibodies mouse anti-CD44, mouse anti-CD73, and mouse anti- CD45 (Abcam, UK) for 30 minutes at 4˚C and resuspended in 100 μl of PBS. (Gibco, UK) Unbound antibodies were removed by washing with PBS. After washing, the cells were incubated for 40 minutes at room temperature in the dark using FITC conjugated secondary antibody and re-suspended in PBS for FACS analysis. At least 1×106 cells per sample were analyzed with a flow cytometer.
Bone marrow mesenchymal stem cell culture and in vitro tests
All eperiments were carried out in triplicate. Passage-2 cells were seeded, (about 7×104 cells) in 24-well plates with media changes every 2-3 days. The attached cells were eposed to CSF (E19, E20, and P1) at 0, 3, 7, and 10% v/v concentrations in DMEM, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cell morphology was eamined for neurite outgrowths after one week. Cells in individual wells were photographed and further analyzed using ImageJ software (NIH) (3). A neurite was defined as when the cellular process was longer than the diameter of the cell body. The average length of the neurites was calculated from measurements of 10 cells in each of 6 wells for each age group of CSF tested. We used b-FGF as a positive promoter of BM-MSC proliferation and differentiation into neuronal phenotypes to compare to the effects of CSF.
MTT assay
Cell viability and/or proliferation were quantitatively determined by the 3-(4, 5-dimethyl 2 thiazolyl)-2,5- diphenyl 2 tetrazolium bromide (MTT) method. MTT is a yellow tetrazolium dye that responds to metabolic activity. Reductases in living cells reduce MTT from a pale yellow color to dark blue formazan crystals. In 24-well plates, the cells were seeded at 7×104 cells/ well in 500 μL DMEM without FBS. Following attachment, the cells were eposed to CSF (E19, E20, and P1) at concentrations of 0, 3, 7, and 10% (v/v). After 24 hours, we added 100 ml of MTT (Merck, Germany, 5 mg/mL in PBS) to each well and the cells were allowed to incubate for 4 hours at 37˚C. Finally, after removal of the supernatant, 2 ml dimethylsulfoide (DMSO, Merck, Germany) was added to each well to dissolve the blue substance. Absorbance was read at 570 nm in disposable cuvettes. All eperiments were carried out in triplicate.
Immunocytochemistry
The cells were fied in 4% paraformaldehyde in PBS for 15 minutes, permeabilized with 0.1% Triton -100 (Merck, Germany) for 30 minutes at room temperature, blocked with 5% BSA (Sigma-Aldrich, USA) in Tween 20 (Merck, Germany) in PBS (T-PBS, Gibco, UK) for 1 hour at room temperature, and then incubated at 4˚C overnight in the presence of either anti-ß-III tubulin (ABcam, UK, 1:100) or anti-MAP2 (ABcam, UK, 1:100). Negative controls, to verify the specificity of the antibodies, were obtained by omission of the primary antibodies, and incubating only with secondary antibodies. After three washes with T-PBS in PBS, we added FITC conjugated goat anti-mouse IgG (1:50, Abcam, UK) at room temperature for 1 hour. Cells were washed and photomicrographs were taken with a florescence microscope (Olympus, Japan).
Statistical analysis
Statistical analysis was performed by one-way ANOVA and the Tukey test. P<0.05 were considered significant.
Results
BM-MSCs are typically isolated from BM based on their preferential adherence to plastic. After two or three passages, BM-MSCs became homogeneous in appearance with two distinct populations of large flattened cells and spindle-shaped cells (Fig .1A)
Fig.1.
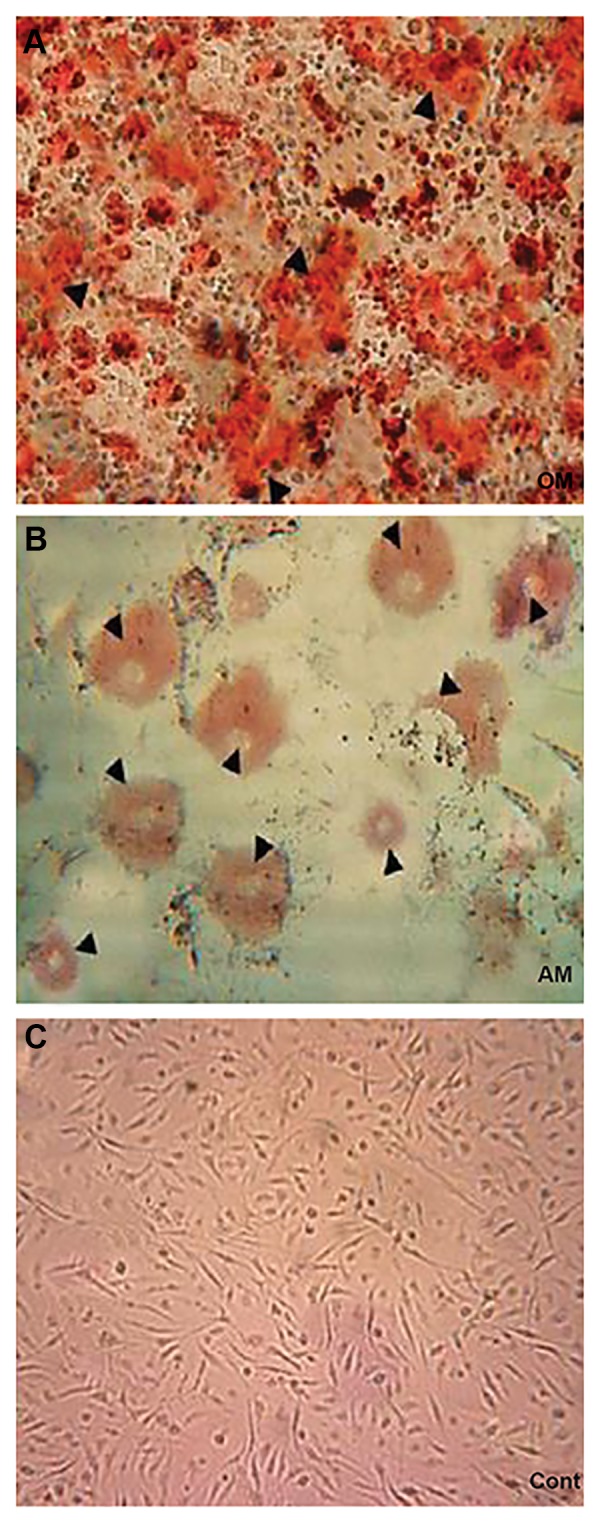
Characterization of bone marrow mesenchymal stem cells (BM-MSCs) and tests for multipotential characteristics. A. Cells grown in osteogenic medium (OM) medium with no defined cytokines to promote differentiation (×100), B. Cells grown in adipogenic medium (AM) stained with oil red O (×200). The open arrow heads show fat cells, and C. Cells grown in control medium (Cont) and stained with alizarin red (×100). The open arrow heads demonstrate mineralization of the extracellular calcium deposits.
Adipogenic and osteogenic differentiation
We induced adipogenic and osteogenic differentiation to confirm the differentiation potential of the BMMSCs. BM-MSCs showed adipogenic differentiation (lipid vacuoles stained with oil red O) after treatment for 2 weeks in adipogenic medium (AM, Fig .1B). Mineralization of the etracellular calcium deposits were assessed by alizarin red S staining (Fig .1C).
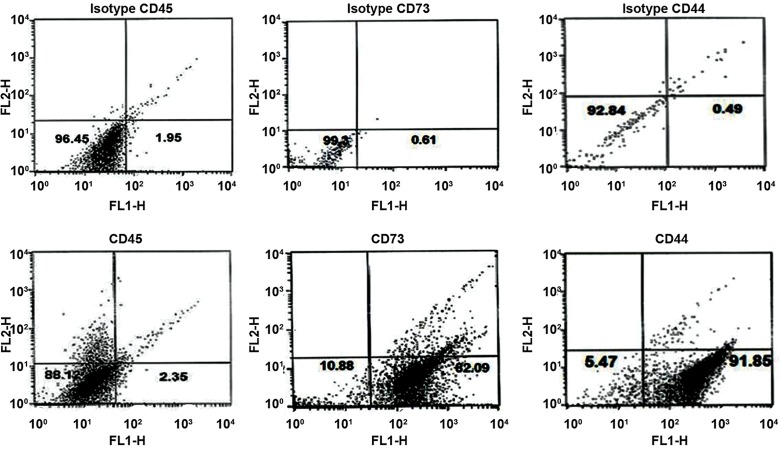
Bone marrow mesenchymal stem cell surface marker expressions
Flow cytometry analysis of the P3 surface antigens revealed that the BM-MSCs were positive for CD44 and 73, and negative for CD45 (Fig .2).
Fig.2.
Flow cytometry analysis of bone marrow mesenchymal stem cell (BMMSC) surface markers. The BM-MSCs suspension was immunostained for CD44, CD73, and CD45. Cell surface analysis of BM-MSCs by FACS revealed that BM-MSCs were positive for CD44 and CD73 but negative for CD45.
Total protein concentration
The CSF from E19 rat fetuses had a slightly higher mean total protein concentration (1.6 ± 0.1 mg/ml) compared to the E20 (1.5 mg/ml) and postnatal P1 (1.07 mg/ml) CSF.
Neural differentiation of bone marrow mesenchymal stem cells induced by pre- and postnatal cerebrospinal fluid
Three days following CSF treatment, the spindle shaped BM-MSCs elongated and the neurites of the neuron-like cells became longer after 7 days. Inverse microscopic eamination of BM-MSCs showed the presence of neuron-like cells in the cell cultures treated with E19, E20, and P1 CSF and b-FGF (positive control) compared to the control group (without CSF, Fig.3.), BM-MSCS cultured in E19 CSF-supplemented medium had significantly greater neural differentiation compared to the E20 and P1 groups.
Fig.3.
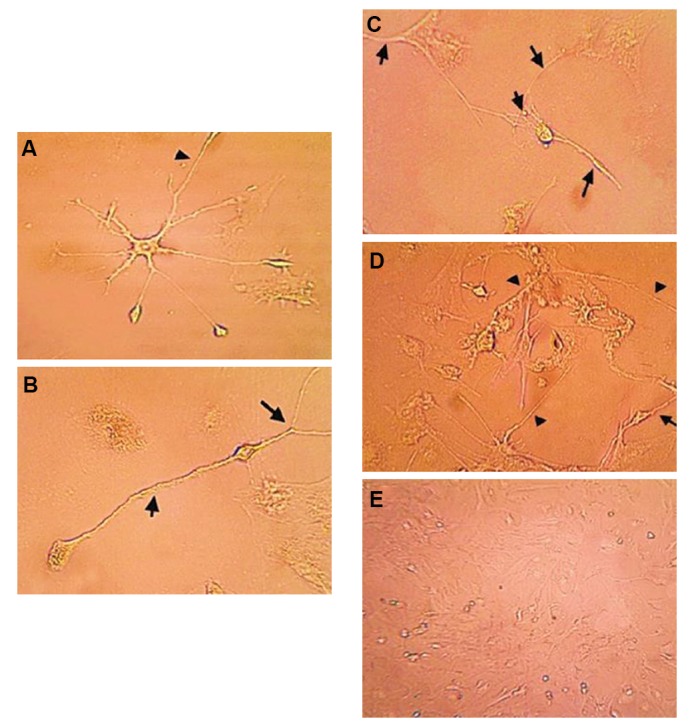
Bone marrow mesenchymal stem cells (BM-MSCs) after 7 days in culture with the addition of 10% pre- and postnatal cerebrospinal fluid (CSF) photographed with phase-contrast optics. A. Culture with E19 CSF (×400), B. Culture with E20 CSF (×400), C. Culture with P1 CSF (×400), D. Basic fibroblast growth factor (b-FGF, positive control) (×400), and E. Control group (×200) without pre- and postnatal CSF. There was significantly greater neural differentiation of BM-MSCS cultured in CSFsupplemented medium from E19 compared to E20 and P1.
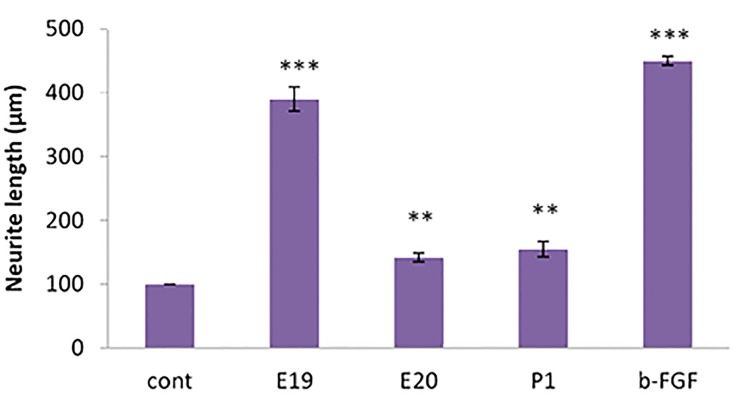
Measurement of neurite outgrowth
The cells had a significantly greater average neurite outgrowth compared to the controls when cultured in the presence of b-FGF (P<0.001), E19 (P<0.001), E20 (P<0.01), and P1 (P<0.01) for 7 days (Fig .4). Figure 4 shows that the length of the neurites in treated cells was much greater in the presence of b-FGF compared to CSF (E19, E20, and P1). b-FGF (positive control) promoted neurite outgrowth.
Fig.4.
In vitro neurite growth from bone marrow mesenchymal stem cells (BM-MSCs). Cells were cultured for 7 days in media alone (cont) or media supplemented with 10% CSF from E19, E20, and P1 CSF or 10 ng/ml basic fibroblast growth factor (b-FGF) as the positive control. Neurite length was measured for at least 10 representative cells from each of 6 wells for each age of CSF tested. The E19 treated cells had greater density and neurite length compared to the E20 and P1 treated cells. **; P<0.01 and ***; P<0.001 compared to the control culture.
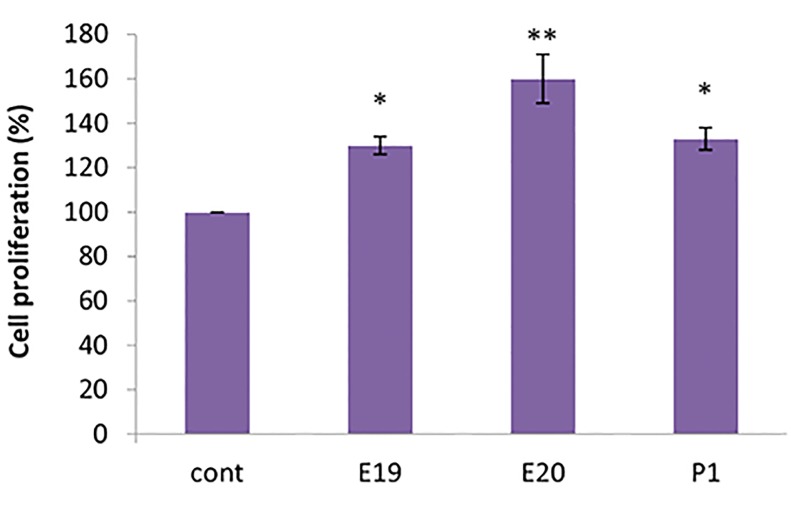
Cell viability/proliferation
Viability and cell proliferation of BM-MSCs cultured in CSF-supplemented medium from E20 rat fetuses had greater viability and cell proliferation compared to the other treated groups. We observed increased cell proliferation of BM-MSCs cultured in E19, E20, and P1 CSF at the 10% concentration compared to the 3 and 7% concentrations. We concluded that the effective CSF concentration for proliferation of BMMSCs was 10% (Fig .5).
Fig.5.
Cell proliferation of bone marrow mesenchymal stem cells (BM-MSCs) cultured in pre- and postnatal cerebrospinal fluid (CSF)- supplemented medium from the 10% concentrations of E19, E20, and P1. Results are expressed as a percentage of control levels. All cultures with added CSF had higher viability, which was significant with E20 CSF. *; P<0.05 and **; P<0.01.
Immunocytochemical characteristics of differentiated bone marrow mesenchymal stem cells
β-III tubulin and MAP-2 expressed in BM-MSCs in media supplemented with E19, E20, and P1 CSF, and in cells cultured with b-FGF (Fig .6).
Fig.6.
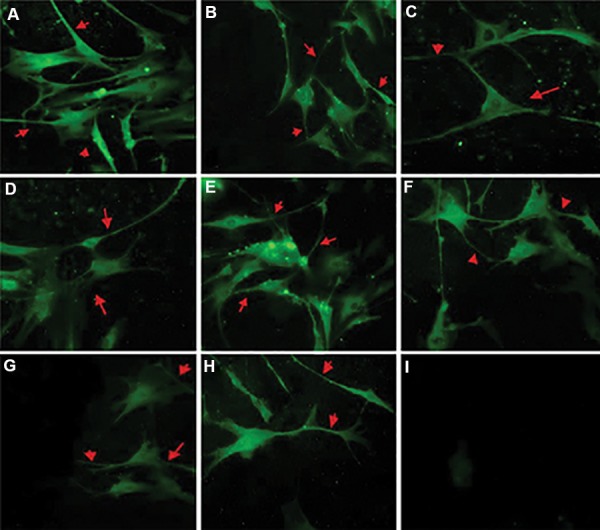
Cerebrospinal fluid (CSF) induced neuronal differentiation in the bone marrow mesenchymal stem cells (BM-MSCs). A. β-III tubulin expression in BMMSCs cultured with 10 ng/ml basic fibroblast growth factor (b-FGF) (×400), B. 10% E19 CSF (×400), C. 10% E20 CSF (×400), D. 10% P1 CSF (×400), E. MAP-2 expression in BM-MSCs cultured with 10 ng/ml b-FGF (×400), F. 10% E19 CSF (×400), G. 10% E20 CSF (×400), H. 10% P1 CSF (×400), and I. Control (×200) cultures without added CSF cells that show no stain when primary antibody was omitted. Red arrows indicate positive expression of β-III tubulin and MAP-2 in neuronlike cells and neurites. The highest expression level for these markers was detected in E19.
Discussion
It is widely agreed that stem cells are a substantial component of cell-based therapies as relatively high numbers of healthy cells are needed (15, 23). Currently, osteogenic and adipogenic differentiation are the most common methods used to identify and analyze cell populations and multi-lineage differentiation (24, 25). Our study results have demonstrated that BM-MSCs are capable of multi-lineage differentiation. The general strategy for identifying in vitro cultivated BM-MSCs is to analyze the epression of surface-cell markers such as CD44, CD45, and CD73. The FACS eperiments have indicated that BM-MSCs were positive for CD44 and CD73, and negative for CD45, a cell-surface marker associated with lymphohematopoietic cells (22). Therefore, we have observed no evidence of hematopoietic precursors in the cultures. Neurogenesis in the normal rat brain is a process that includes proliferation, migration, and differentiation. Days E19 and E20 coincide with migration of immature neurons and differentiation of migrated neurons (26). Studies show that undifferentiated cells migrate and neural differentiation form during the early postnatal stage (27). We have selected E19, E20, and P1 for CSF sampling.
In the present study, the E19, E20, and P1 CSF treatments induced BM-MSCs to differentiate into cells that had a neuronal phenotype and enhanced proliferation of BMMSCs relative to the control group. The most critical substances of the CSF are its protein components; their quality and quantity can change during CNS development (28).The present study has shown that CSF from E19 rat fetuses has a protein concentration of approximately 1.6 mg/ml which decreased to 1 mg/ml in P1 CSF. E19 has a high protein concentration compared to other age groups, whereas P1 has the lowest protein concentration. Total protein in CSF increased from birth to a peak concentration between 5 and 10 days, after which it declined rapidly (29). Growth factors are important for development of the cerebral corte, including FGF, TGF-β, NGF, BDNF, NT- 3, IGFs which are found in fetal CSF. Proteomic studies have shown the presence of mitogenic factors in CSF (30). Based on evidences, the CSF plays an important role as a neural stem cell niche and provides a microenvironment for regulation of neuroepithelial cells (31). The proteomic composition of fetal CSF suggests that it contains all of the secretory factors, growth factors, cytokines, etracellular matriproteins, and adhesion molecules, as well as numerous other materials and nutrients. These components are sufficient to maintain neural stem cell survival, and promote proliferation and differentiation of the progenitor cells into mature cells (32). Studies have reported great similarities in the composition of proteins in mammalian CSF such as humans, rats, and mice (6).
We hypnotized that the addition of different concentrations of CSF (E19, E20, P1) into the culture media would enable a better microenvironment to induce neural differentiation of BM-MSCs. The experimental groups had greater absorbance values compared to the control group, which indicated the improvements in cell proliferation and viability of BM-MSCs. These results demonstrated that prenatal and postnatal CSF had the potential to induce differentiation under in vitro culture conditions. In this study, we observed that β-III tubulin and MAp2 expression significantly increased in BM-MSCs cultured with CSF-supplemented medium compared with the control group. Based on these evidences, CSF promoted neuronal differentiation and proliferation of BM-MSCs in an age-dependent manner.
The in vitro survival, proliferation, and neuronal differentiation of BM-MSCs depend on certain growth factors which must be present in the CSF in order to obtain the effects observed in this study (11). Our knowledge about the role of the CSF in brain development and details of CSF functions helped us to understand how normal brain develop and to develop strategies and treatments to prevent neurodevelopmental abnormalities and neuropathological conditions. We concluded that regulation of CSF-born growth factors might be involved in some neurodegenerative diseases, such as MS and Alzheimers’ (33). We have provided evidence which suggests that the CSF induces proliferation and neural differentiation of BM-MSCs in an age-dependent manner. However, further eperiments are needed to identify effective CSF components in neural differentiation of BM-MSCs.
Conclusion
Our results demonstrated that stimulatory effects of CSF on BM-MSCs can altered in an age-dependent manner. This finding confirms that the CSF is a powerful growth medium which promotes brain development. The CSF provides a rich environment for differentiation of BMMSCs in vitro. The functions of the CSF components and their role in BM-MSC differentiation should be further researched to improve cell therapy.
Acknowledgments
We express our gratitude for financial support by the Department of Biology, Faculty of Science, Kharazmi University, Tehran, Iran. The authors declare no conflict of interest related to this work.
Author’s Contributions
M.N., R.S.H., J.A.M.; Contributed to conception and designed the study. R.S.H.; Contributed to all experimental work, data and statistical analysis, and interpretation of data. S.I.; Performed editing and revising and the manuscript. All authors were involved in writing and editing the manuscript and preparation of figures.
References
- 1.Vera A, Stanic K, Montecinos H, Torrejón M, Marcellini S, Caprile T. SCO-spondin from embryonic cerebrospinal fluid is required for neurogenesis during early brain development. Front Cell Neurosci. 2013;7:80–80. doi: 10.3389/fncel.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orešković D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010;64(2):241–462. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Nabiuni M, Rasouli J, Parivar K, Kochesfehani HM, Irian S, Miyan JA. In vitro effects of fetal rat cerebrospinal fluid on viability and neuronal differentiation of PC12 cells. Fluids Barriers CNS. 2012;9(1):8–8. doi: 10.1186/2045-8118-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabiuni M, Shokohi R, Moghaddam P. CSF protein contents and their roles in brain development. Zahedan J Res Med Sci (ZJRMS) 2015;17(9):e1042–e1042. [Google Scholar]
- 5.Salehi Z, Mashayekhi F. The role of cerebrospinal fluid on neural cell survival in the developing chick cerebral cortex: an in vivo study. Eur J Neurol. 2006;13(7):760–764. doi: 10.1111/j.1468-1331.2006.01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Alonso MI, Santiago C, Moro J, De la Mano A, Carretero R, et al. Early embryonic brain development in rats requires the trophic influence of cerebrospinal fluid. Int J Dev Neurosci. 2009;27(7):733–740. doi: 10.1016/j.ijdevneu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69(5):893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gato A, Martin P, Alonso MI, Martin C, Pulgar MA, Moro JA. Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J Exp Zool A Comp Exp Biol. 2004;301(4):280–289. doi: 10.1002/jez.a.20035. [DOI] [PubMed] [Google Scholar]
- 9.Dziegielewska KM, Knott GW, Saunders NR. The nature and composition of the internal environment of the developing brain. Cell Mol Neurobiol. 2000;20(1):41–56. doi: 10.1023/A:1006943926765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327(2):263–272. doi: 10.1016/j.ydbio.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Miyan JA, Zendah M, Mashayekhi F, Owen-Lynch PJ. Cerebrospinal fluid supports viability and proliferation of cortical cells in vitro, mirroring in vivo development. Cerebrospinal Fluid Res. 2006;3:2–2. doi: 10.1186/1743-8454-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lairson LL, Lyssiotis CA, Zhu S, Schultz PG. Small molecule- based approaches to adult stem cell therapies. Annu Rev Pharmacol Toxicol. 2013;53:107–125. doi: 10.1146/annurev-pharmtox-011112-140300. [DOI] [PubMed] [Google Scholar]
- 13.Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, Xinwen Z. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31(9):916–923. doi: 10.1016/j.cellbi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamous M, Al-Zoubi A, Khabaz MN, Khaledi R, Al Khateeb M, Al- Zoubi Z. Purification of mouse bone marrow-derived stem cells promotes ex vivo neuronal differentiation. Cell Transplant. 2010;19(2):193–202. doi: 10.3727/096368910X492599. [DOI] [PubMed] [Google Scholar]
- 16.Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401–412. doi: 10.3349/ymj.2011.52.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21(5):527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 18.Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas J, et al. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. 2011;33(10):1083–1093. doi: 10.1179/1743132811Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Expression of neural markers by undifferentiated rat mesenchymal stem cells. J Biomed Biotechnol. 2012;2012:820821–820821. doi: 10.1155/2012/820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson A, Shehadeh LA, Yu H, Webster KA. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics. 2010;11:229–229. doi: 10.1186/1471-2164-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni WF, Yin LH, Lu J, Xu HZ, Chi YL, Wu JB, et al. In vitro neural differentiation of bone marrow stromal cells induced by cocultured olfactory ensheathing cells. Neurosci Lett. 2010;475(2):99–103. doi: 10.1016/j.neulet.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Karaoz E, Aksoy A, Ayhan S, Sarıboyacı AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132(5):533–546. doi: 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 23.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats-similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95(7):3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25(7):1610–1617. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- 25.Zheng YH, Xiong W, Su K, Kuang SJ, Zhang ZG. Multilineage differentiation of human bone marrow mesenchymal stem cells in vitro and in vivo. Exp Ther Med. 2013;5(6):1576–1580. doi: 10.3892/etm.2013.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16(Suppl 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- 27.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 28.Yuan X, Desiderio DM. Proteomics analysis of human cerebrospinal fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815(1-2):179–189. doi: 10.1016/j.jchromb.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Dziegielewska K, Habgood M, Jones SE, Reader M, Saunders NR. Proteins in cerebrospinal fluid and plasma of postnatal Monodelphisdomestica (grey short-tailed oposum) Comp Biochem Physiol B. 1989;92(3):569–576. doi: 10.1016/0305-0491(89)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res. 2007;6(9):3537–5348. doi: 10.1021/pr070247w. [DOI] [PubMed] [Google Scholar]
- 31.Johansson PA, Cappello S, Götz M. Stem cells niches during development- lessons from the cerebral cortex. Curr Opin Neurobiol. 2010;20(4):400–407. doi: 10.1016/j.conb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM, Monuki ES. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci. 2013;33(45):17553–17559. doi: 10.1523/JNEUROSCI.3258-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]