Abstract
Objective
Umbilical cord blood is used for transplantation purposes in regenerative medicine of hematological disorders. MicroRNAs are important regulators of gene expression that control both physiological and pathological processes such as cancer development and incidence. There is a new relation between p53 (tumor suppressor gene) and miR-145 (suppressor of cell growth) upregulation. In this study, we have assessed how adipose-derived stem cells (ADSCs) affect the expansion of hematopoietic stem cells (HSCs), as well as miR-145 and p53 expressions.
Materials and Methods
In this experimental study, we cultured passage-3 isolated human ADSCs as a feeder layer. Flow cytometry analysis confirmed the presence of ADSC surface markers CD73, CD90, CD105. Ex vivo cultures of cordblood CD34+cells were cultured under the following 4 culture conditions for 7 days: i. Medium only supplemented with cytokines, ii. Culture on an ADSCs feeder layer, iii. Indirect culture on an ADSCs feeder layer (Thin Cert™ plate with a 0.4 µm pore size), and iv. Control group analyzed immediately after extraction. Real-time polymerase chain reaction (PCR) was used to determine the expressions of the p53 and miR-145 genes. Flow cytometry analysis of cells stained by annexin V and propidium iodide (PI) was performed to detect the rate of apoptosis in the expanded cells.
Results
ADSCs tested positive for mesenchymal stem cell (MSC) markers CD105, CD90, and CD73, and negative for HSC markers CD34 and CD45. Our data demonstrated the differentiation potential of ASCs to osteoblasts by alizarin red and alkaline phosphatase staining. MTT assay results showed a higher proliferation rate of CD34+cells directly cultured on the ADSCs feeder layer group compared to the other groups. Direct contact between HSCs and the feeder layer was prevented by a microporous membrane p53 expression increased in the HSCs group with indirect contact of the feeder layer compared to direct contact of the feeder layer. p53 significantly downregulated in HSCs cultured on ADSCs, whereas miR-145 significantly upregulated in HSCs cultured on ADSCs.
Conclusion
ADSCs might increase HSCs proliferation and self-renewal through miR-145, p53, and their relationship.
Keywords: Adipose Cell, Hematopoietic Stem Cell, MicroRNA
Introduction
Umbilical cord blood is used for transplantation in regenerative medicine for hematological disorders. Improvement of hematopoietic reconstitution and engraftment potential of ex vivo-expanded hematopoietic stem cells has been unsuccessful due to the inability to generate an adequate amount of stem cells. Many studies report that control of in vitro hematopoietic stem cell (HSC) self-renewal is difficult. Hematopoietic cytokines fail to support reliable amplification of in vitro HSCs and additional factors appear to be needed (1). Recently, factors such as feeder layers are suggested to affect HSCs expansion (2). Expanded HSCs derived from cord blood cultured on a feeder layer of mesenchymal stem cells (MSCs) have reduced apoptosis rates (3). Adiposederived stem cells (ADSCs) show properties similar to that observed in bone marrow MSCs. Because of the ease of accessibility human, researchers consider ADSCs to be an attractive source for regenerative medicine (4). ADSCs are immunoprivileged, prevent severe graft-versus-host disease, and stable in culture (5). ADSCs show high intrinsic expression of self-renewal factors compared to bone marrow-derived MSCs (2). In the current study, we have used ADSCs as a feeder layer for HSC expansion because they produce various factors to support stem cell maintenance and cell growth.
MicroRNAs, a large group of negative gene regulators, work through a post-transcriptional suppression mechanism. MicroRNAs play an important role in proliferation, differentiation, and apoptosis (6). They are short, noncoding RNAs, usually 18-25 nucleotides in length, which repress translation and cleave mRNA by base pairing to the 3′untranslated region of the target genes (7). Although various numbers of microRNAs have been studied in HSCs, there are few reports that pertain to the function of miR-145. Human miR-145 is broadly expressed in germline and mesoderm-derived tissues such as the breast (8), ovaries (9), testes, uterus, prostate, heart, and spleen (6). Sachdeva and Mo (6) have reported miR-145 mediated suppression of cell growth, invasion, and metastasis. Based on these findings, they proposed that as a tumor suppressor, miR-145 might be a valuable biomarker for cancer diagnosis. Starczynowski et al. (7) reported that deletion of chromosome 5q in patients with 5-q32-33 syndrome correlated with the loss of miR-145 and miR-146a, two microRNAs frequently observed in HSCs. It was reported that in various cancers, miR-145 prevents tumor angiogenesis and metastasis by targeting c-Myc (10, 11). In the present research, we have investigated the expression levels of p53 and miR-145 in HSCs after culture on feeder layers of ASDCs. It is well known that p53 upregulates miR-145 expression (12). Previous studies have shown the transcriptional induction of miR-145 by p53 in response to anticancer drugs or serum starvation. p53 induces expression of tumor suppressor miR- 145 (13, 14). In this study, we investigated the expression levels of p53 and miR-145 in HSCs after culture on a feeder layer of ADSCs.
Materials and Methods
Adipose-derived stem cell culture
We obtained human subcutaneous adipose tissue samples from donors who underwent abdominoplasty in Erfan Hospital Iran). The patient gave consent to use of donated samples in the present study. The tissue samples were processed according to a modified procedure by Zuk et al. (15), which included 0.075% collagenase II (Sigma-Aldrich, St. Louis, MO) for 30 minutes, followed by centrifugation at 150 g for 5 minutes. The pellet was washed three times in phosphate buffered saline (PBS, Gibco, Germany), then we seeded the cells at 105 cells/dish and cultured them in Dulbecco’s modified eagle’s medium (DMEM, Gibco, Germany), 10% fetal bovine serum (FBS, Gibco, Germany), and 100 U/ml penicillin/streptomycin. Human HSCs were obtained from Royan Institute. The Institutional Review Board and Ethical Committee of Royan approved the HSCs extraction method.
Proliferation and phenotype analyses
We used flow cytometry to detect ADSCs surface markers monoclonal antibodies were used for CD73, CD90, and CD105 markers. To enable differentiation into osteoblast cells, we used passage-4 ADSCs and a medium that consisted of high glucose DMEM, 10% FBS, 10 nM dexamethasone (Sigma-Aldrich, USA), 35 mg/mL of ascorbic acid, and 1 mM β-glycerophosphate (Chemicon, USA). Cells were incubated in 5% CO2 at 37˚C for 21 days. We used alizarin red to confirm differentiation into osteoblast cells. An alkaline phosphatase kit (Sigma- Aldrich) was used for alkaline phosphatase activity.
CD34+ cell isolation and culture (group design)
Mononuclear cells were separated with Ficoll (1.077 ± 0.001 kg/L, Sigma-Aldrich, USA). Next, we incubated these cells with anti-CD34 antibody labeled with Fe nanoparticles (America Milton Biotech), after which CD34+ cells were separated by manual cell separation using a MACS column (America Milton Biotech). Anti- CD34 were used to confirm the CD34 marker in isolated cells obtained from umbilical cord blood. After feeder layer preparation with mitomycin C the CD34+ cells were cultured under the following 4 culture conditions for 7 days: i. Stem span medium only supplemented with 100 ng/ml of the following cytokines: stem cell factor (SCF), thrombopoietin (TPO), and fetal liver tyrosine kinase 3 ligand (Flt-3L), ii. Direct culture on an ADSCs feeder layer, iii. Indirect culture on an ADSCs feeder layer (ThinCert™ plate with a 0.4 μm pore size), and iv. Control group of cells analyzed immediately after extraction.
MTT assay
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was used to assess cell viability for all groups. This assay measures the amount or ratio of cell proliferation. It is a colorimetric assaydependent on the reduction of the tetrazolium salt, MTT, to form blue formazan crystals. After incubation, we removed the overlying culture medium and added MTT. Next, the cells were incubated for 4 hours in an incubator in CO2 at 37˚C. Isopropanol acid was added and we read the optical density (OD) of the obtained solution at 630nm as the reference wavelength and 570 nm as the measurement wavelength using the ELISA reader. Oneway ANOVA was used for data analysis.
Annexin V evaluation of apoptotic cells
We used an Apoptosis kit (Bioscience, USA) for apoptosis analysis. At culture day 14, we treated 1×104 cells resuspended in 1x binding buffer with fluorochromeconjugated annexin V for 10 minutes. Next, cells were washed and resuspended in 1x binding buffer. A propidium iodide (PI) solution was added and fluorescence of the stained cells was analyzed by flow cytometry.
Reverse transcription and real-time polymerase chain reaction
RNA was extracted from sample cells using TRIzol (Fermentas, Germany). The cDNA was synthesized using a cDNA synthesis kit (Fermentas, Germany) based on the manufacturer’s instructions.Primers were designed according to the NCBI website and synthesized by Bioneer Company. SYBER green master mix was used for the polymerase chain reaction (PCR) reactions (Applied Biosystems, USA). The real time quantitative (qRT) PCR program was performed with a melting cycle for 5 minutes at 95˚C followed by 10 seconds at 95˚C, 40 cycles of melting, 15 seconds at 60˚C (annealing), and 30 seconds at 72˚C (extension). The sequences for GAPDH, p53, and MiR-145 are as follows:
-
p53:
F: 5′-TCCTCAGCATCTTATCCGAGTG-3́
R: 5́-AGGACAGGCACAAACACGCACC-3 ´
-
GAPDH:
F: 5′-ATGGGGAAGGTGAAGGTCG-3 ´
R: 5 ´-GGGGTCATTGATGGCAACAATA-3 ´
-
miR-145:
F: 5′-GTCCAGTTTCCCABGGAA-3′
R: 5́-TGACCCCAGGTAACTCTGAGTGT-3 ´
Statistical analysis
Data are presented as mean standard deviation (SD). We used the two-way ANOVA and Duncan test for data analysis. Differences were considered significant at P<0.05. In the present study, all experiments were repeated 3 times.
Results
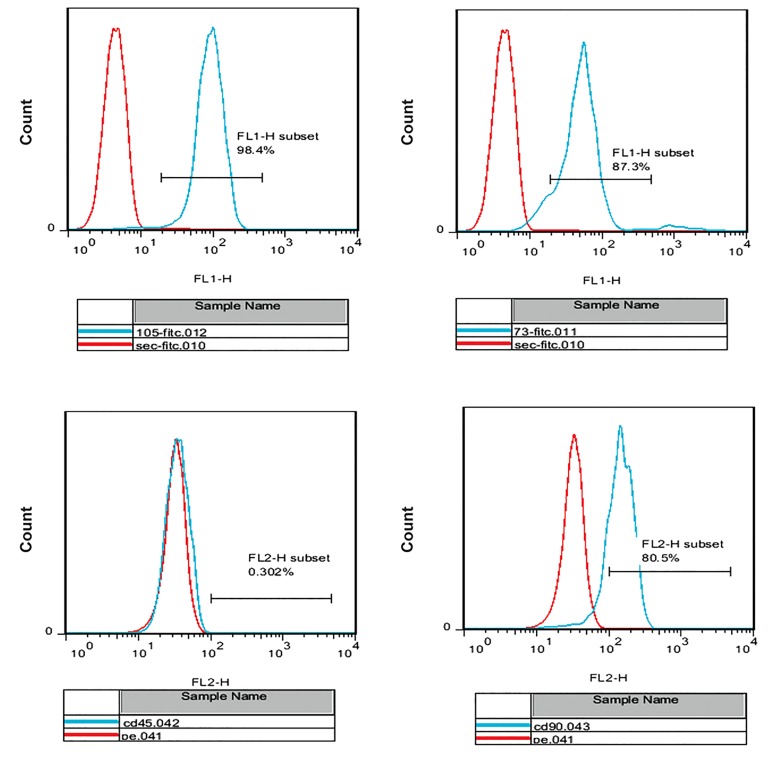
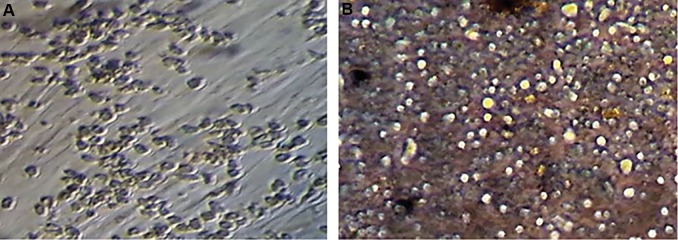
We performed flow cytometry analyses of the ADSC surface antigen markers, which resulted inpositive reactions for CD105 (98.4%), CD90 (80.5%), and CD73 (87.3%) antibodies. ADSCs were negative for CD45 (0.302%) (Fig .1). Cultured hematopoietic stem cells on an adipose-derived stem cell feeder layer after 2 and 7 days have shown in Figure 2. We performed alizarin red staining to assess the ability of ADSCs to differentiate osteoblast cells. The results confirmed the osteogenic potential of the ADSCs (Fig .3).
Fig.1.
Flow cytometry analysis of adipose-derived stem cell (ADSCs), markers showed positive expressions of 98.4% of ADSCs, CD105+, 87.3% cells are CD73+, 80.5% are CD90 and 0.303% are CD45 positive.
Fig.2.
Cultured hematopoietic stem cells on an adipose-derived stem cell feeder layer. A. After 2 days and B. After 7 days.
Fig.3.
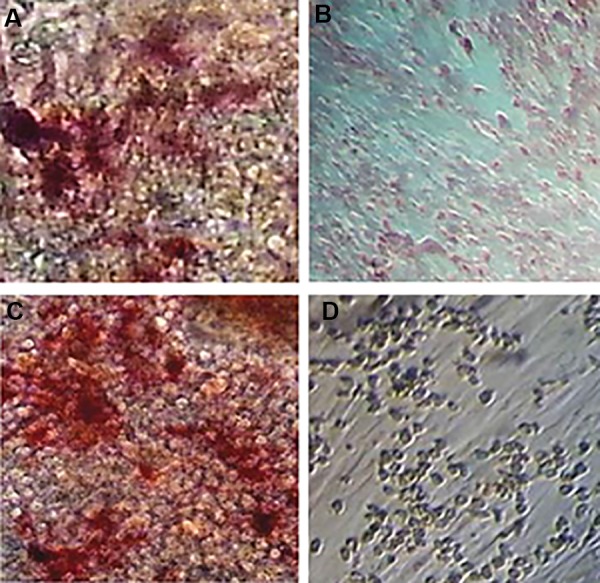
Osteogenic differentiation of adipose-derived stem cell, 200. A. Positive reaction in osteoblastic differentiated cells with alizarin red staining, B. Undifferentiated cells, C. Osteoblast differentiated cells with increased alkaline phosphatase activity, and D. Undifferentiated cells.
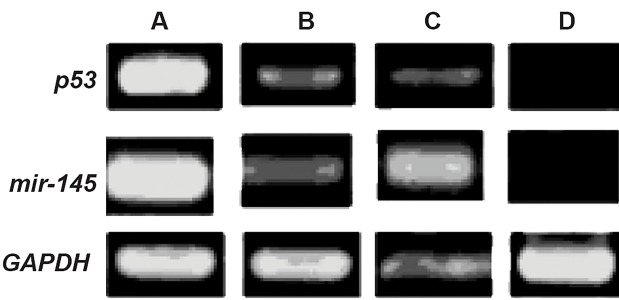
We found that p53 expressed less than the other groups. Our results showed lower expression of the p53 gene on the ThinCert™ plate with 0.4 μm pore size compared to HSCs cultured directly on the ADSCs feeder layer. The microporous membrane prevented direct contact between HSCs and the feeder layer. Consequently, there was increased p53 expression compared to cells that had direct contact with the ADSC feeder layer (Fig .4).
Fig.4.
Analysis of p53 and miR-145 expressions by reverse transcription PCR in A, B, C, and D groups. A; Fresh CD34+ cells, B; CD34+ cells cultured in the presence of cytokines, C; CD34+ cells indirectly cultured on feeder layer, and D; CD34+ cells directly cultured on feeder layer. GAPDH group was considered the control group.
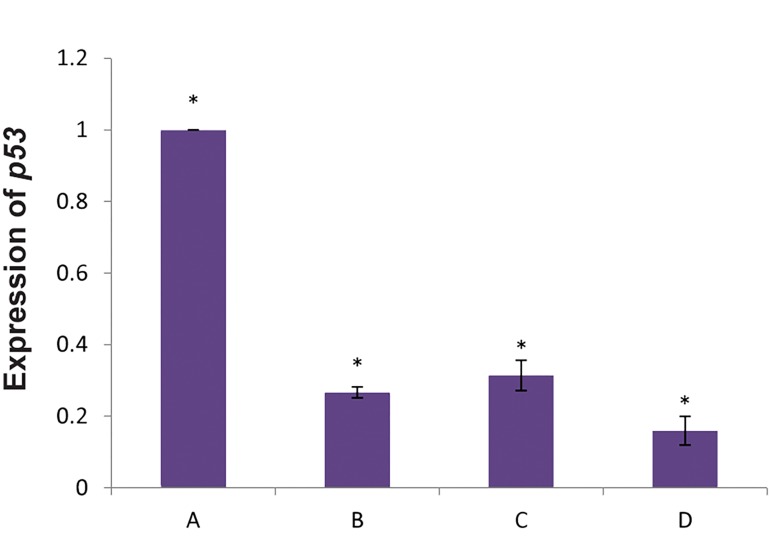
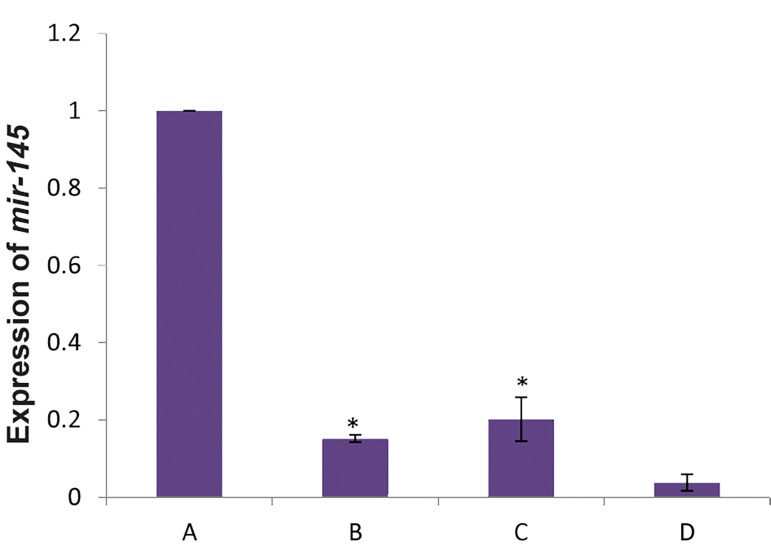
Results of (qRT) PCR analysis were the same as RTPCR analysis. We observed the highest expression of the p53gene in CD34+HSCs (P<0.05). There was lower p53expression in the presence of the ADSC feeder layer compared to the other experiments (P<0.05, Fig .5). Analysis of miR-145 expression in fresh CD34+ cells by real-time polymerase chain reaction compared to the other groups has shown in Figure 6.
Fig.5.
Analysis of p53 gene expression in fresh CD34+ cells by real-time polymerase chain reaction compared to the other groups.
A; p53 gene expression in fresh CD34+ cells, B; Expression of p53 in CD34+ cells in the presence of cytokines, C; Expression of p53 in CD34+ cells indirectly cultured on the feeder layer, D; Expression of p53 in CD34+ cells directly cultured on the feeder layer. Fresh CD34+ cells showed significant increase in p53 gene expression compared to the other groups, and *; P<0.05.
Fig.6.
Analysis of miR-145 expression in fresh CD34+ cells by real-time polymerase chain reaction compared to the other groups.
A; miR-145 expression in fresh CD34+ cells, B; Expression of miR-145 in CD34+ cells in the presence of cytokines, C; Expression of miR-145in CD34+ cells indirectly cultured on the feeder layer, D; Expression of miR-145 in CD34+ cells directly cultured on the feeder layer. Fresh CD34+ cells showed significant increase in miR-145 expression compared to the other groups, and *; P<0.05.
Discussion
Our results have shown that HSCs had higher selfrenewal in the presence of the ADSCs feeder layer compared to the other groups. Because of insufficient numbers of HSCs, expansion of these cells is important for clinical applications. Recently, it was reported that a bone marrow MSC feeder layer along with cytokines such as SCF and TPO increased proliferation of HSCs (2). Glettig and Kaplan (16) reported that different feeder layers for HSCs limited the differentiation of these cells. Our data revealed that the expression of p53 as a selfrenewal inhibitor gene in HSCs cultured on a feeder layer was lower than the other groups. Tumor suppressor p53 has been shown to direct regulation of a number of microRNAs such as the miR-34 family and miR-145 (17). Sachdeva et al. (13) reported suppression of c-Myc by p53-induced miR-145. miR-145 was reported to inhibit various cancers by targeting several protein coding genes such as c-Myc. p53 represses c-Myc through induction of the tumor suppressor miR-145 (14). Suzuki et al. (18) reported that a central tumor suppressor, p53, enhanced the post-transcriptional maturation of several microRNAs with growth-suppressive function, including miR-143 and miR-145, and miR-16-1.
Suh et al. (19) have found that miR-145 is regulated by DNA methylation and p53 gene mutation in some cancers, and p53 increased the expression level of miR-145. Dong et al. (17) established a new link between p53 and miR-145 in tumor growth regulation and metastasis in ovarian carcinoma. There have been no comprehensive studies on the role of microRNAs in HSCs. Our findings showed lower expression levels of p53 and miR-145 in HSCs cultured on ADSCs compared to the groups without feeder layers. In terms of the tumor suppressive role of miR-145 and p53, reduced expression of these two genes in the present study indicated that ASDCs could cause growth induction by inhibition of apoptosis. Downregulation of p53 and consequently miR-145 in HSCs could cause increased proliferation of HSC. On the other hand it has been shown that miR-145 is induced during differentiation, and it directly silences stem cell self-renewal and pluripotency (20). The results of the present study suggested that suppression of miR-145 of HSCs cultured on ASCs altered the p53-mediated cell cycle arrest.
Our results showed that the expression of miR-145 and p53 gene on a Thin Cert™ plate with 0.4 μm pore sized groups were lower than HSCs cultured directly on the ASCs feeder layer group. It has been shown that direct contact between HSCs and a feeder layer was critical for expansion of cells (2). da Silva et al. (21) reported that direct contact of HSCs and a feeder layer could increase HSC self-renewal. Alakel et al. (22) showed that direct contact between HSCs and a bone marrow MSCs feeder layer could improve self-renewal of HSCs and can affect migratory behavior of HSCs.
Conclusion
miR-145 appears to increase proliferation of HSC cultured on ADSCs by impairing p53 function. Defining the role of ADSCs in controlling the HSC self-renewal through reduced miR-145 and p53 may lead to the treatment and prevention of hematopoietic disorders. Improvement of HSCs self-renewal direct cultured on ADSCs is associated with reduced expression of miR-145 and p53.
Acknowledgments
This project was supported financially by Kharazmi University. The authors express their appreciation to all participants. There is no conflict of interest.
Author’s Contributions
F.T.; Contributed to the design and implementation of the research, to the direction of project, and to the writing of the manuscript. F.A.; Participated to all experimental work, data and statistical analysis, and interpretation of data. A.S.; Supervised to carry out their work. M.B.S.; Contributed molecular experiments and RTqPCR analysis. All authors read and approved the final manuscript.
References
- 1.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 2.Foroutan T. Increased miR33 expression in expanded hematopoietic stem cells cultured on adipose stem cells feeder layer. Iran J Ped Hematol Oncol. 2016;6(2):106–114. [Google Scholar]
- 3.Mehrasa R, Vaziri H, Oodi A, Khorshidfar M, Nikogoftar M, Golpour M, et al. Mesenchymal stem cells as a feeder layer can prevent apoptosis of expanded hematopoietic stem cells derived from cord blood. Int J Mol Cell Med. 2014;3(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Saidi R, Rajeshkumar R, Shariftabrizi A, Zimmerman A, Walter O. Human adipose-derived mesenchymal stem cells promote liver regeneration. J Invest Surg. 2015;28(6):303–308. doi: 10.3109/08941939.2015.1006379. [DOI] [PubMed] [Google Scholar]
- 5.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7(2):269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 6.Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010;2(2):170–180. [PMC free article] [PubMed] [Google Scholar]
- 7.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat Med. 2010;16(1):49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 8.Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, et al. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One. 2012;7(3):e33778–e33778. doi: 10.1371/journal.pone.0033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummel R, Hussey DJ, Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46(2):298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Song L, Yang J, Duan P, Xu J, Luo X, Luo F, et al. MicroRNA-24 inhibits osteosarcoma cell proliferation both in vitro and in vivo by targeting LPAATβ. Arch Biochem Biophys. 2013;535(2):128–135. doi: 10.1016/j.abb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Liang W, Gao B, Fu P, Xu S, Qian Y, Fu Q. The miRNAs in the pathgenesis of osteosarcoma. Front Biosci (Landmark Ed) 2013;18:788–794. doi: 10.2741/4142. [DOI] [PubMed] [Google Scholar]
- 12.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106(9):3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera-Merchan A, Cerrato C, Luengo G, Dominguez O, Piris MA, Serrano M, et al. MiR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell Cycle. 2010;9(16):3277–3285. doi: 10.4161/cc.9.16.12598. [DOI] [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Ashjian P, Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glettig DL, Kaplan DL. Extending human hematopoietic Stem Cell Survival in vitro with adipocyte. Biores Open Access. 2013;2(3):179–185. doi: 10.1089/biores.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in highgrade serous ovarian carcinoma. Oncotarget. 2014;5(21):10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460(7254):529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 19.Suh So, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32(5):772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137(4):647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 21.da Silva CL, Gonçalves R, Crapnell KB, Cabral JM, Zanjani ED, Almeida-Porada G. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005;33(7):828–835. doi: 10.1016/j.exphem.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Alakel N, Jing D, Bornhauser M, Ehninger G, Ordemann R. Direct contact with mesenchymal stromal cells affects migratory behavior and gene expression profile of CD133+ hematopoietic stem cells during ex vivo expansion. Exp Hematol. 2009;37(4):504–513. doi: 10.1016/j.exphem.2008.12.005. [DOI] [PubMed] [Google Scholar]