Abstract
Ludwig's angina is potentially lethal, rapidly spreading cellulitis of the floor of mouth and neck. The anticipated difficult airway becomes even more challenging when it occurs in children. In children, the larynx is positioned relatively higher in the neck, and one does not have the option for blind nasal intubation or awake fiberoptic, which otherwise is the technique of choice in adult patients. We present the clinical course of 16 children and highlight various problems encountered during the anesthetic management of six children who required emergency surgical drainage under general anesthesia.
Keywords: Difficult airway, Ludwig's, Ludwig's angina
Introduction
Ludwig's angina is potentially lethal, rapidly spreading cellulitis of the floor of mouth and neck. Although modern dental care and use of antibiotics for oral infections have markedly reduced its occurrence, it still remains anesthesiologist's nightmare. Early recognition and prompt management are important to avoid life-threatening acute airway obstruction.[1] It is predominantly seen in middle-aged individuals and has been rarely reported in children.[2] However, children with Ludwig's angina (LA) are at high risk for airway obstruction because larynx is positioned relatively higher in the neck than in adults. We present the clinical course of 16 children and highlight various problems encountered during the anesthetic management of six children who required emergency surgical drainage under general anesthesia (GA).
Case Report
After approval from the institutional review board, we retrospectively analyzed clinical course and management of LA in 16 pediatric patients (2010-2014), who presented to the emergency room. Of 16, six underwent incision and drainage (I&D) under GA at our hospital. Demographic variables of the presenting patients have been summarized in Table 1. Primarily, infants and toddlers were surgically drained which included one infant, four toddlers, and one child above 3 years. All children were presented with fever and bilateral brawny and tender neck swelling [Table 1 and Figure 1]. Fourteen patients (87%) had lingual elevation, 12 (75%) patients had restricted neck movements, 11 (68%) had odynophagia, 10 (62%) had trismus, and five (31%) patients presented with respiratory symptoms (dyspnea and tachypnea). All children were started on intravenous antibiotics, and 10 children improved with medical management. These children, who did not respond to antibiotics, were irritable, unable to manage secretions and showed some features of respiratory distress underwent emergent I&D under GA.
Table 1.
Demographic profile and management of children with Ludwig’s angina

Figure 1.
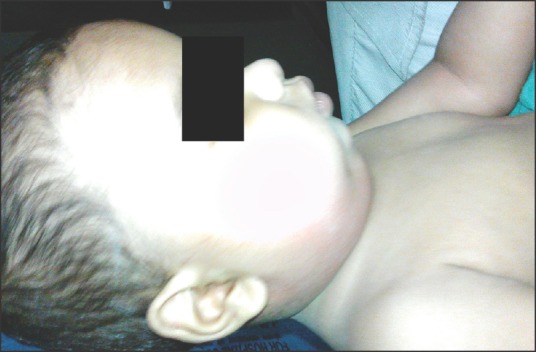
Severe swelling of the bilateral submandibular and anterior cervical area secondary to Ludwig's angina in an 8-month-old child
After preliminary clinical evaluation and preoperative investigations (hemogram, X-ray soft tissue neck), patients were taken up for surgical drainage. All patients had an intravenous assess secured and standard monitoring (heart rate, noninvasive blood pressure, pulse oximetry, and end-tidal carbon dioxide) applied in the operating room. Injection glycopyrrolate (5 μg/kg) and dexamethasone (0.1 mg/kg) were administered before the induction of GA. Inhalational induction with sevoflurane (2-4%) was done in five patients while incremental intravenous supplementation with thiopentone (2-3 mg/kg) was used in one patient. In one patient, superficial incision of the most prominent portion of the swelling was given to ease the expected difficult laryngoscopy. After successful bag and mask ventilation, direct laryngoscopy was performed to visualize the glottis, and trachea was intubated after administering suxamethonium 1.5 mg/kg. Anesthesia was maintained with rocuronium, fentanyl, and inhalational agent. In two patients, intraoral drainage of the abscess had to be done. The intraoperative period was uneventful in all the cases. The patients were transferred to intensive care unit for elective ventilation, in view of gross airway edema. The patients were extubated after the subsidence of airway edema as evidenced by an audible leak around the endotracheal tube. One child was extubated within 24 h. The remaining five were extubated over next 48 h. All the children were shifted to the ward on the 3rd postoperative day and discharged within a week of admission.
Discussion
Ludwig's angina is a rapidly progressive severe soft tissue infection of the neck and the floor of the mouth.[3] This review provides an overview of various anatomical considerations, risk factors, management, and anesthetic implications of LA in the pediatric population.
Anatomical considerations
The complex anatomical relationships in the head and neck must be understood to appreciate the natural history of infections spreading within the head and neck. The attachment of the fascial layers to neck structures forms potential spaces within the neck. The primary site of infection in Ludwig's angina is submandibular space, which is divided into two spaces by mylohyoid muscle, a superior sublingual and an inferior submaxillary space.[4] The spread of infection is halted anteriorly by the mandible and inferiorly by the mylohyoid muscle [Figure 2]. The infectious process expands superiorly and posteriorly, elevating the floor of the mouth and the tongue. The hyoid bone limits the process inferiorly, and swelling spreads to the anterior aspect of the neck, causing distortion, and “bull neck” appearance.[5] The tongue becomes massively swollen, immobile and displaced superiorly against the palate and posteriorly into hypopharynx, which results in insidious airway compromise and puts the patient in danger of respiratory obstruction. If untreated, the infection may spread posteriorly along the styloglossus muscle into pharyngomaxillary space, from there to retropharyngeal space and then track into the superior mediastinum.[5]
Figure 2.
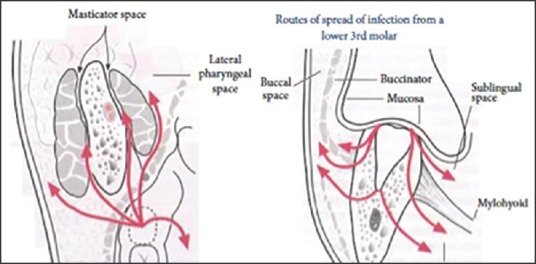
Route of spread of Ludwig's angina from lower teeth (taken from http://www.exodontia.info )
Pathogenesis
LA is predominantly seen in middle-aged individuals with poor dentition. Review of literature indicates 24-30% incidence of LA in children.[5] The predisposing factors are dental caries, recent dental extraction, immunosuppression, diabetes mellitus, sickle cell anemia, oral mucosal lacerations, submandibular sialadenitis, and mandibular fractures.[4] While it can be idiopathic when predisposing factor remains unidentified.[5] Odontogenic infection, especially of the 2nd and 3rd lower molar tooth is the most common cause in adults,[6] whereas in children the deep neck space infections subsequent to upper respiratory tract infection (URI) in the majority of the cases.[5] In our series, history of URI was present in eight children, whereas there was an odontogenic cause in four children and no cause in the remaining children.
The common organisms cultured in pediatric patients responsible for LA in most cases are Group A beta-hemolytic streptococci and Staphylococcus aureus.[3] Many abscesses are polymicrobial and contain Gram-positive, Gram-negative, anaerobic organisms, and hence they respond to broad spectrum antibiotics.[6] Meaningful bacteriologic data were not possible, as all of our patients had been treated with antibiotics prior to hospitalization. In the preantibiotic era, aggressive treatment with surgical decompression to preserve the oropharyngeal airway was the first choice. However these days, aggressive administration of intravenous antibiotics early in the course of cellulitis is often curative, obviating I&D. However, surgical exploration remains the choice for cases where an abscess is suspected, or there is no response to antibiotic therapy. In our series, the resolution of cellulitis was achieved in 10 out of 16 patients with only six requiring surgical drainage.
Clinical features
LA can present with fever, tachycardia, pain on swallowing, and a characteristic brawny and tender induration of the floor of the mouth. The primary site of infection in LA is submandibular space. The tongue becomes massively swollen, immobile and displaced superiorly against the palate and posteriorly into hypopharynx, which results in airway compromise and puts the patient in danger of respiratory obstruction.[1] Table 1 lists symptoms elicited from the 16 patients with LA. Fever and brawny tender neck swelling were present in all the patients. Restricted neck movements and trismus were observed in 12 (75%) and 10 (62%) patients, respectively. Five patients were admitted with respiratory complaints. Periodic physical examinations as a means of monitoring the airway are mandatory for the early diagnosis of impending respiratory failure. Warning signs that warrant early airway intervention include increased work of breathing such as marked tachypnea with shallow respiration, use of accessory muscles, dyspnea, orthopnea, stridor, and patients’ adoption of sniffing position. Such patients may not always require intubation, however, should be observed in an intensive care unit for frequent airway evaluations.
Options for airway management
Airway management in these patients could be very challenging. Patient factors such as supraglottic edema, nuchal rigidity, and trismus may interfere with securing an airway in an emergent situation. The published recommendations on airway management are based on anecdotal experiences and, therefore, are quite variable. The various techniques described for anesthetic management are elective tracheostomy under local anesthesia,[7] awake fiberoptic[7] or blind nasal intubation,[7] direct laryngoscopy and intubation under intravenous, or inhalational anesthesia with or without muscle relaxant.[8] Tracheostomy, although considered an established method of securing the airway under local anesthesia, is not without complications. This includes inability to operate in uncooperative children without anesthesia, difficulty in operating on edematous tissue, fear of life-threatening mediastinal invasion and is associated with significant morbidity.
Awake fiberoptic and blind nasal intubation using topical anesthesia have been successfully used in adults,[7] but these methods may not feasible in this age group. Successful intubations under controlled conditions using intravenous or inhalational technique with or without muscle relaxant, dexamethasone, and adrenaline nebulization have been reported in adults.[9] Mehrotra and Mehrotra used cervical plexus block for surgical decompression of airway without endotracheal intubation in an adult patient.[10]
Anticipated problems
The main problem in pediatric patients with LA is to induce anesthesia and achieve intubation without the loss of airway. No clinical criteria have been validated until date for preoperative assessment of difficult airway in pediatric age group. Selection of endotracheal tube is also important since no formulae for endotracheal tube size apply to LA due to unique configuration of laryngeal inlet. It is clear that intravenous induction would be extremely dangerous as neither successful intubation nor ventilation of lungs could be guaranteed. Hence, the preferred choice is an inhalational induction with sevoflurane/halothane that maintains spontaneous respiration until laryngoscopy is performed. Intubation without muscle relaxant may precipitate laryngospasm and rupture of abscess due to direct trauma ultimately leading to airway catastrophe. Judicious use of muscle relaxant is warranted in such situations, and suxamethonium provides the best intubating conditions. Alternatively, laryngoscopy may be performed under deep inhalational anesthesia, and muscle relaxant should only be administered once vocal cords are visualized. Therefore, such decisions should be taken very carefully in each patient by an experienced anesthesiologist. The use of ketamine without securing airway should be highly discouraged because if the abscess ruptures, one can land into catastrophic airway sequelae. In our institution, patients with large abscess are first given a superficial horizontal incision in the submandibular area after ensuring adequate mask ventilation. However, a deep incision may lead to rupture of contents into the oral cavity and cause catastrophic airway sequel.
To conclude, such patients should be taken up urgently in the presence of a consultant anesthesiologist for drainage well before severe airway compromise. A superficial horizontal incision in the submandibular area can facilitate mask ventilation and early establishment of definitive airway.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kremer MJ, Blair T. Ludwig angina: Forewarned is forearmed. AANA J. 2006;74:445–51. [PubMed] [Google Scholar]
- 2.Kurien M, Mathew J, Job A, Zachariah N. Ludwig's angina. Clin Otolaryngol Allied Sci. 1997;22:263–5. doi: 10.1046/j.1365-2273.1997.00014.x. [DOI] [PubMed] [Google Scholar]
- 3.Srirompotong S, Art-Smart T. Ludwig's angina: A clinical review. Eur Arch Otorhinolaryngol. 2003;260:401–3. doi: 10.1007/s00405-003-0588-9. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AH, Pai SD, Bhattarai B, Rao ST, Ambareesha M. Ludwig's angina and airway considerations: A case report. Cases J. 2008;1:19. doi: 10.1186/1757-1626-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HW, O’Neill A, Cunningham MJ. Ludwig's angina in the pediatric population. Clin Pediatr (Phila) 2009;48:583–7. doi: 10.1177/0009922809333095. [DOI] [PubMed] [Google Scholar]
- 6.Kavarodi AM. Necrotizing fasciitis in association with Ludwig's angina – A case report. Saudi Dent J. 2011;23:157–60. doi: 10.1016/j.sdentj.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovassapian A, Tuncbilek M, Weitzel EK, Joshi CW. Airway management in adult patients with deep neck infections: A case series and review of the literature. Anesth Analg. 2005;100:585–9. doi: 10.1213/01.ANE.0000141526.32741.CF. [DOI] [PubMed] [Google Scholar]
- 8.Neff SP, Merry AF, Anderson B. Airway management in Ludwig's angina. Anaesth Intensive Care. 1999;27:659–61. doi: 10.1177/0310057X9902700323. [DOI] [PubMed] [Google Scholar]
- 9.Saifeldeen K, Evans R. Ludwig's angina. Emerg Med J. 2004;21:242–3. doi: 10.1136/emj.2003.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrotra M, Mehrotra S. Decompression of Ludwig angina under cervical block. Anesthesiology. 2002;97:1625–6. doi: 10.1097/00000542-200212000-00040. [DOI] [PubMed] [Google Scholar]


