Abstract
Background and Aims:
The aim is to evaluate the association between the use of intraoperative dexmedetomidine with an increase in recurrence-free survival (RFS) and overall survival (OS) after nonsmall cell lung cancer (NSCLC) surgery.
Material and Methods:
This was a propensity score-matched (PSM) retrospective study. Single academic center. The study comprised patients with Stage I through IIIa NSCLC. Patients were excluded if they were younger than 18 years. Primary outcomes of the study were RFS and OS. RFS and OS were evaluated using univariate and multivariate Cox proportional hazards models after PSM (n = 251/group) to assess the association between intraoperative dexmedetomidine use and the primary outcomes. The value of P < 0.05 was considered statistically significant.
Results:
After PSM and adjusting for significant covariates, the multivariate analysis demonstrated no association between the use of dexmedetomidine and RFS (hazard ratio [HR] [95% confidence interval (CI)]: HR = 1.18, 95% CI: 0.91–1.53; P = 0.199). The multivariate analysis also demonstrated an association between the administration of dexmedetomidine and reduced OS (HR = 1.28, 95% CI: 1.03–1.59; P = 0.024).
Conclusions:
This study demonstrated that the intraoperative use of dexmedetomidine to NSCLC patients was not associated with a significant impact on RFS and but worsening OS. A randomized controlled study should be conducted to confirm the results of this study.
Keywords: Dexmedetomidine, lung cancer, surgery
Introduction
Lung cancer is the leading cause of cancer-related mortality and the second most common oncological diagnosis in both women and men in the United States.[1] Although surgical resection has been proven effective, the rate of recurrence can be as high as 40% in patients with Stage I nonsmall cell lung cancer (NSCLC).[2] A major risk factor associated with poor survival in this patient population is high tumor stage at the time of diagnosis and surgery; however, other independent perioperative factors have been associated with increase or decrease in cancer recurrence of NSCLC, including opioid consumption, perioperative inflammatory status, blood transfusions, and the use of nonsteroidal anti-inflammatory agents or β-adrenergic blockers.[3,4,5,6] The findings that such short-term perioperative factors/interventions may impact long-term cancer outcomes, together with causal findings from animal studies, suggest that the perioperative period has a critical impact on the metastatic process, and through it determines long-term cancer outcomes.[6,7] Therefore, there has been a search for perioperative interventions that might modify risk or impact long-term outcome of patients with NSCLC.[4,8,9]
Dexmedetomidine is a potent and highly selective α2 adrenoreceptor agonist, which exert analgesic, sedative, and sympatholytic effects that are clinically manifested also in the form of reduced consumption of opioids and volatile anesthetics, and reduced intraoperative levels of circulating catecholamines.[10] Given that, pro-inflammatory and sympathetic responses to surgery, as well as the perioperative use of high opiate levels, were shown to promote cancer progression, dexmedetomidine could potentially reduce the pro-tumoral and pro-metastatic state associated with surgery by modulating inflammation and reducing the consumption of opioids and volatile anesthetics.[6,11,12,13,14,15] These potential beneficial effects of dexmedetomidine added to its recently demonstrated immune protective and anti-inflammatory properties, could be of beneficial impact in cancer surgery.[16,17] Unfortunately, recent evidence has also indicated that dexmedetomidine can promote tumor growth through the directly stimulating proliferation of cancer cells, and through modifying the tumor microenvironment.[18,19]
Based on the above rationalizations and findings and since dexmedetomidine is commonly used in the intraoperative period, we decided to investigate the effect of dexmedetomidine on recurrence-free survival (RFS) and overall survivals (OS) after NSCLC surgery. Specifically, we hypothesize that dexmedetomidine decreases survival in patients undergoing NCLC surgery.
Material and Methods
Study approval and waiver of written informed consent were obtained from the Institutional Review Board (IRB#PA11-1067) before the start of the study. Perioperative data were collected, stored, and managed in a Research Electronic Data Capture database from patients who underwent surgical resection for primary Stage I, II, or III NSCLC between January 2004 and December 2011 at The University of Texas MD Anderson Cancer Center. Patients 18 years of age or older who had surgery with the intention to cure were included in the analysis. Those who had palliative surgery or secondary malignancies were excluded from the analysis. The analyzed data included patient's age, gender, body mass index (BMI), the American Society of Anaesthesiology (ASA) physical status, tumor histology, tumor Stage (I, II, or III), type of surgery (thoracotomy versus thoracoscopy), duration of anesthesia, and administration of neoadjuvant and/or adjuvant chemotherapy and/or radiation.
Intraoperative anesthetic care of the patients comprised volatile-opioid general anesthesia typically involving the use of a volatile anesthetic in oxygen, intravenous opioids, muscle relaxation with nondepolarizing agents, and normothermia. We also collected information on the total amount of dexmedetomidine administered intraoperatively. Postoperative management typically included patient-controlled epidural analgesia that was transitioned to a combination of an opioid plus acetaminophen or a nonsteroidal anti-inflammatory drug.
Statistical analysis
Descriptive statistics, including mean, standard deviation, median, and range, were calculated for continuous variables, such as age and BMI, and the frequency counts and percentages were presented for categorical variables, including gender, stage, and use (yes versus no) of dexmedetomidine. Fisher's exact test or Chi-square test was used to evaluate the association between two categorical variables. Wilcoxon rank sum test was used to evaluate the difference in a continuous variable between patient groups. Kaplan–Meier method was used for time-to-event analysis, including RFS and OS. Median time-to-event in months with 95% confidence interval (CI) was calculated. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. To adjust for potential selection bias, we conducted a propensity score matching (PSM) analysis. The propensity score is the conditional probability of receiving a specific treatment (dexmedetomidine) conditional on a set of observed covariates. In this study, we included the following prognostic covariates in the multicovariate logistic model to estimate the propensity scores: age, gender, ASA, stage of disease, type of surgery, and anesthesia duration. Two hundred and fifty-one patients who received dexmedetomidine (n = 251) and had nonmissing values for the covariates were matched with a 1:1 ratio to the patients who did not received dexmedetomidine (n = 251) and had nonmissing values for the covariates. Prognostic covariates by dexmedetomidine before PSM: Fisher's exact/Chi-square test for categorical variables or Wilcoxon rank sum test for continuous variables was used to assess the difference in covariate distribution between the dexmedetomidine group and nondexmedetomidine group. Univariate Cox proportional hazards models were fitted to evaluate the effects of continuous variables on time-to-event outcomes. Multivariable Cox proportional hazards models were used for multivariate analysis to include important and significant covariates. Estimating an overall probability of recurrence at 5 years of 0.4 and a reduction in the risk of 40% in patients treated with dexmedetomidine, we will need 237 patients in each group to demonstrate a statistically, significantly different (type 1 error: 0.05 and power, 1-β: 0.8) in survival.
Statistical software SAS 9.1.3 (SAS, Cary, NC, USA) and S-Plus 8.0 (TIBCO Software Inc., Palo Alto, CA, USA) were used for all the analyses.
Results
A total of 1404 patients were included in the analysis. The median age (interquartile range [IQR]) and BMI of the patients were 66.07 years (19–90) and 26.49 (23.6–54.91), respectively. There were 679 (48.4%) females and 1257 patients had an ASA physical status of 3–4 (89.5%). The most common type of histology was adenocarcinoma (n = 796, 56.7%) and approximately half (n = 767, 54.6%) of the patients had a Stage I cancer. Stage II NSCLC was found in 332 patients (23.7%) whereas 305 patients had Stage IIIa (21.7%). The median (IQR) duration of anesthesia was 263 min (223–315.5). The duration of anesthesia was significantly longer in patients with Stage IIIa (278 min [60–1100]) disease than those with Stage I (252 min [37–1190]) and Stage II (274 min [86–746], P < 0.001).
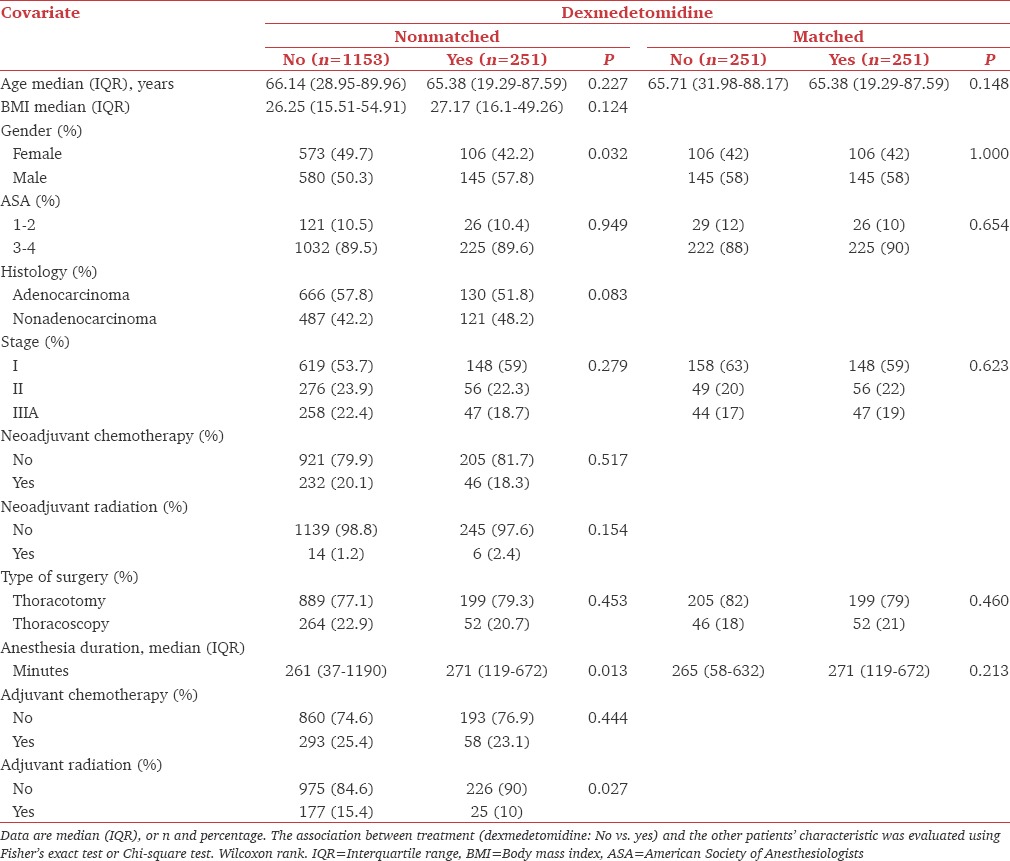
Two hundred and fifty-one (17.9%) patients received dexmedetomidine intraoperatively with a median (IQR) intraoperative consumption of 100 μg (57.47–140). Patients who received dexmedetomidine had also a significantly longer median (IQR) duration of anesthesia (271 min [119–672]) than those who did not (261 [37–1190, P = 0.013]); however, the use of dexmedetomidine was comparable between patients with different stages of disease (P = 0.279) and those who had thoracotomies or thoracoscopies (P = 0.453). As shown in Table 1, patients who received dexmedetomidine were more likely to be men (P = 0.032), had a longer duration of anesthesia (P = 0.013) and did not have adjuvant radiation (P = 0.01).
Table 1.
Clinicopathological characteristics of patients who had nonsmall cell lung cancer with general anesthesia with or without dexmedetomidine
Recurrence-free and overall survivals
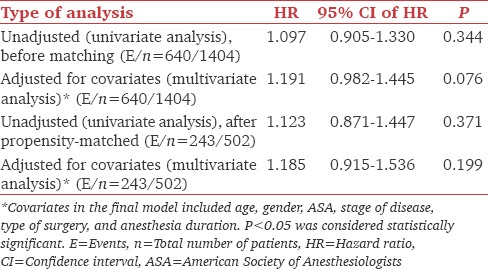
The median (95% CI) RFS time of the overall population of patients was 72.7 months (67.97–82.92) and the 5 years RFS rate (95% CI) was 0.55 (0.53–0.58). The median (95% CI) RFS time of the patients who did and did not receive dexmedetomidine was 71.35 (53.78–98.39) and 72.83 (66.69–86.86), respectively. The unadjusted univariate analysis (log-rank test) demonstrated no differences in 5 years RFS rate (95% CI) between both groups of patients (dexmedetomidine group: 0.53 [0.47–0.49] vs. nondexmedetomidine group: 0.56 [0.53–0.59], P = 0.343). After adjusting for age, gender, ASA physical status, stage of disease, and anesthesia duration, the multivariate analysis of nonmatched (hazard ratio [HR] = 1.19, 95% CI: 0.98–1.44; P = 0.076) and matched patients (HR = 1.18, 95% CI: 0.91–1.53; P = 0.199) showed that the use of intraoperative dexmedetomidine was not an independent predictor of RFS [Figure 1 and Table 2]. Using the median dose of dexmedetomidine as a cutoff, we divided patients who received the drug into two groups: Lower or equal than the median or higher than the median. After adjusting for age, gender, ASA physical status, and stage of the disease, the multivariate Cox proportional hazard model analysis demonstrated that the effect of dexmedetomidine dose was not statistically significant as a predictor of RFS (HR = 1.213, 95% CI: 0.850–1.732; P = 0.287).
Figure 1.
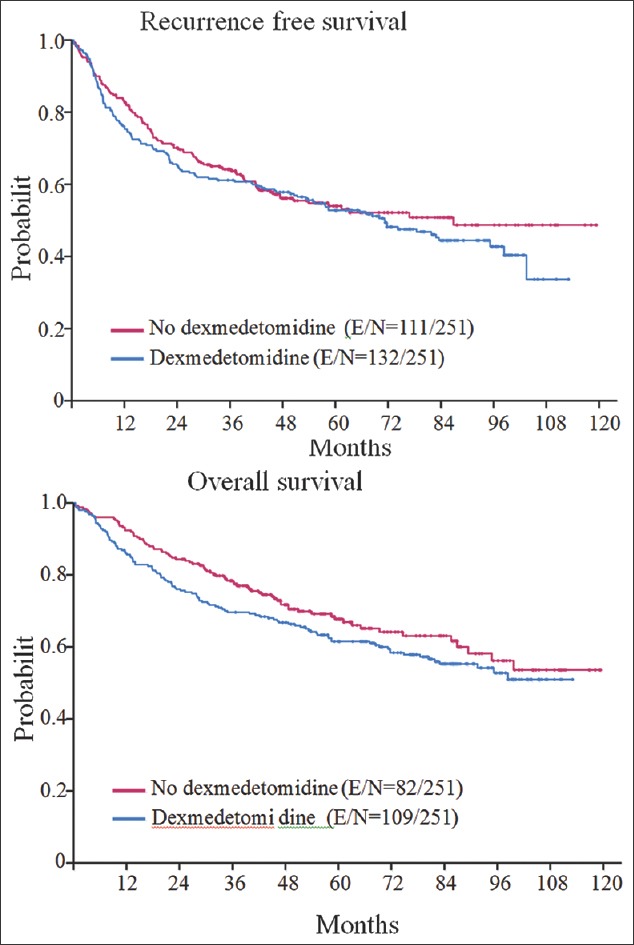
Kaplan–Meyer curves for recurrence-free survival (top) and overall survival (bottom). The figure depicts the Kaplan–Meier curves for recurrence-free survival (top) and overall survival (bottom) of the matched population of patients. As it can be appreciated patients who received dexmedetomidine had shorter overall survival than those who did not received the drug. Recurrence-free survival was similar in both groups of patients
Table 2.
Univariate and multivariate analysis for recurrence-free survival
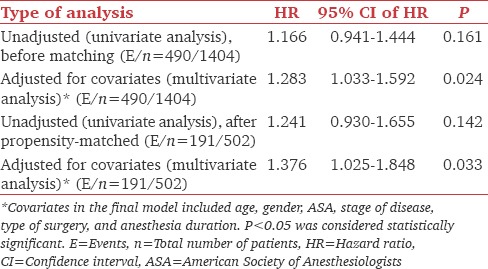
The median OS time of the overall population was 109.03 months (95% CI: 98.4–NA) and the median follow-up time for the censored observations was 58.2 months (range: 0.69–125.0 months). The median OS rate at 5 years was 0.68 (95% CI: 0.65–0.71) in the nondexmedetomidine patients and 0.62 (95%CI: 0.56–0.68, P = 0.16). The multivariate analysis of the unmatched population of patients demonstrated that the use of dexmedetomidine was associated with a 28% increase in risk of death after adjusting for age, gender, stage of disease, and anesthesia duration (HR = 1.28, 95% CI: 1.03–1.59; P = 0.024) [Figure 1]. The association still remained statistically significant when we included in the analysis only matched patients (HR = 1.37, 95% CI: 1.02–1.84; P = 0.024). The analysis also demonstrated that the dose of dexmedetomidine administered intraoperatively was not a significant predictor of OS (HR = 1.24, 95% CI: 0.84–1.84; P = 0.264) [Table 3].
Table 3.
Univariate and multivariate analysis for overall survival
Discussion
The primary finding of the present study is that the intraoperative use of dexmedetomidine is associated with a decrease in OS after surgery for NSCLC. Dexmedetomidine has anti-inflammatory effects and has been shown to be protective against the deleterious effects of ischemia-reperfusion.[20,21] In terms of clinical cardiac outcomes, the use of dexmedetomidine appears to be associated with lower mortality in the setting of cardiac surgery and a trend toward reduced cardiac complications after noncardiac surgery.[22,23,24] Moreover, a recent randomized controlled trial in patients who had gastrectomies for gastric cancer indicates that dexmedetomidine, given intraoperatively, has strong immunomodulatory properties that are observed as an improvement in the Th1/Th2 ratio and a reduction in pro-inflammatory cytokines (interleukin-6 and tumor necrosis factor).[16] Similar results were reported in another randomized controlled trial that measured the inflammatory response in patients who had cardiac surgery with cardiopulmonary bypass.[25] Overall, given the reported anti-inflammatory, immune protective, and morphine-sparing effects of dexmedetomidine, our results are unexpected.
Several nonexclusive alternatives may underlie our observation that dexmedetomidine use was associated with decreased OS in our patient population. First, dexmedetomidine may have been used in patients with significantly more severe comorbidities that were not captured in our database; therefore, there were a larger number of noncancer-related deaths in the dexmedetomidine group of patients. Second, other studies in the perioperative care setting have shown improvements in outcomes after the administration of dexmedetomidine for longer durations than in the present study.[16,22] Alternatively, dexmedetomidine may have indeed potentiated the progression of minimal residual disease during and immediate following surgery. Specifically, dexmedetomidine could have led to the progression of micrometastasis (dormant or proliferating) into macrometastasis, which is ultimately the leading cause of death in patients with NSCLC. This can be hypothesized based on the fact that the multivariate analysis of nonmatched patients showed an important trend toward an association between the use of dexmedetomidine and reduced RFS. Although the contribution of α2-adrenoreceptor activation or inhibition in NSCLC cells proliferation and growth is unknown, recent studies investigating the effects of dexmedetomidine on breast cancer cells demonstrated that it can induce tumor growth and metastasis through two independent mechanisms: (a) direct activation of α2-adrenoreceptors expressed on the cancer cells or (b) activating α2-adrenoreceptors on host stromal cells.[18,19] Dexmedetomidine is also an imidazoline receptor agonist.[26] Activation of this receptor increases the formation of hypoxia-inducible factor and vascular endothelial growth factor, which are proteins involved in proliferation and growth of NSCLC cells.[27] Thus, it is possible to speculate that dexmedetomidine can trigger the growth of NSCLC cells through these cellular mechanisms. In addition, dexmedetomidine can induce tumor growth by inducing immune suppression. Although, in our patient population, we did not assess the effect of very low doses of dexmedetomidine on survival, it worth noticing that Inada et al. demonstrated that the chronic administration of subhypnotic doses decreased the function of cytolytic lymphocytes and enhanced tumor growth in animals inoculated with lymphoma tumor cells.[28]
As for any retrospective study, unknown confounding factors are the major limitations. Therefore, our results should be taken with caution. Although our PSM analysis was based on clinical demographic, surgical, and tumor staging variables, a number of comorbidities and intraoperative variables might have still differed between the patients who did and did not receive dexmedetomidine. For instance, we did not account in the analysis for the consumption of opioids or volatile anesthetics intraoperatively, hemodynamics and depth of anesthesia. Although, dexmedetomidine has sparing effects on volatile anesthetic consumption, if the depth of anesthesia was not measured it is possible to speculate that those patients in the dexmedetomidine group had significant deeper states of anesthesia along with intraoperative arterial hypotension (a known adverse effect of dexmedetomidine), which have been associated with higher mortality rate after noncardiac surgery.[29] Our analysis also indicated that patients in the dexmedetomidine group underwent longer surgical procedures (albeit based on anesthesia duration times – 271 vs. 263 min); however, we adjusted for the anesthesia time in the multivariable analysis of unmatched and matched patients. Furthermore, we believe that a difference of 8 min in the duration of anesthesia after matching is unlikely to explain our findings. Moreover, the use of dexmedetomidine was not different among patients who had thoracotomy versus thoracoscopies or had different stages of disease; hence, we believe that the difference in OS was not due to these confounding factors. Finally, we did not analyze the effect of dexmedetomidine on each stage of cancer or histology. Although we have previously shown that perioperative interventions might have a different impact on survival according to the stage of disease,[3] in the present study, we did not perform such analysis because of the limited number of patients in the dexmedetomidine group.
Conclusions
The use of dexmedetomidine is not associated with an improvement in survival of patients undergoing NSCLC surgery. Prospective randomized controlled trials are needed to properly assess the effects of dexmedetomidine on NSCLC patients and other cancers, and animal studies should further elucidate potential mediating mechanisms to be tested in cancer patients.
Financial support and sponsorship
This work was partially supported by a Cancer Center Support Grant from the University of Texas – MD Anderson Cancer Center, Houston, TX, USA.
Dr. Cata has received in the past funds from Hospira, Inc., (consultant and research grant).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Goodgame B, Viswanathan A, Zoole J, Gao F, Miller CR, Subramanian J, et al. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol. 2009;4:1370–4. doi: 10.1097/JTO.0b013e3181b6bc1b. [DOI] [PubMed] [Google Scholar]
- 3.Cata JP, Keerty V, Keerty D, Feng L, Norman PH, Gottumukkala V, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–8. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F, et al. Perioperative blood transfusions and survival in patients with non-small cell lung cancer: A retrospective study. BMC Anesthesiol. 2013;13:42. doi: 10.1186/1471-2253-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, et al. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S650–60. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 6.Elias KM, Kang S, Liu X, Horowitz NS, Berkowitz RS, Frendl G. Anesthetic selection and disease-free survival following optimal primary cytoreductive surgery for stage III epithelial ovarian cancer. Ann Surg Oncol. 2015;22:1341–8. doi: 10.1245/s10434-014-4112-9. [DOI] [PubMed] [Google Scholar]
- 7.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 8.Cata JP, Gottumukkala V, Thakar D, Keerty D, Gebhardt R, Liu DD. Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. J Clin Anesth. 2014;26:3–17. doi: 10.1016/j.jclinane.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Cata JP, Jones J, Sepesi B, Mehran RJ, Rodriguez-Restrepo A, Lasala J, et al. Lack of association between dexamethasone and long-term survival after non-small cell lung cancer surgery. J Cardiothorac Vasc Anesth. 2016;30:930–5. doi: 10.1053/j.jvca.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 11.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69:3727–30. doi: 10.1158/0008-5472.CAN-08-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 13.Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J, et al. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PLoS One. 2014;9:e91577. doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: A novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, et al. In vivo suppression of NK cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–19. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Xu X, Liu H, Ji F. Effects of dexmedetomidine on patients undergoing radical gastrectomy. J Surg Res. 2015;194:147–53. doi: 10.1016/j.jss.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–6. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzone A, Piñero CP, Castillo LF, Sarappa MG, Rojas P, Lanari C, et al. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155:494–504. doi: 10.1038/bjp.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and a2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013;6:1262–72. doi: 10.1158/1940-6207.CAPR-13-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin T, Begeç Z, Toprak HI, Polat A, Vardi N, Yücel A, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res. 2013;183:385–90. doi: 10.1016/j.jss.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55:1272–8. doi: 10.1111/j.1399-6576.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- 22.Ji F, Li Z, Young N, Moore P, Liu H. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2014;28:267–73. doi: 10.1053/j.jvca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–84. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: A meta-analysis of randomised controlled trials. Anaesthesia. 2008;63:4–14. doi: 10.1111/j.1365-2044.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- 25.Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69:693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Ding T, Yu L, Zhong Y, Dai H, Yan M. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J Pharm Pharmacol. 2012;64:120–7. doi: 10.1111/j.2042-7158.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Xu S, Jiao F, Ren T, Li Q. Vascular endothelial growth factor B coordinates metastasis of non-small cell lung cancer. Tumour Biol. 2015;36:2185–91. doi: 10.1007/s13277-014-2829-5. [DOI] [PubMed] [Google Scholar]
- 28.Inada T, Shirane A, Hamano N, Yamada M, Kambara T, Shingu K. Effect of subhypnotic doses of dexmedetomidine on antitumor immunity in mice. Immunopharmacol Immunotoxicol. 2005;27:357–69. doi: 10.1080/08923970500240883. [DOI] [PubMed] [Google Scholar]
- 29.Sessler DI, Sigl JC, Kelley SD, Chamoun NG, Manberg PJ, Saager L, et al. Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]


