Chronically implanted electrodes were used to record neural activity across multiple nodes in the basal ganglia-thalamocortical circuit simultaneously in a nonhuman primate model of Parkinson’s disease, enabling within-subject comparisons of electrophysiological biomarkers between normal and parkinsonian conditions and different vigilance states. This study improves our understanding of the role of oscillatory activity and phase-amplitude coupling in the pathophysiology of Parkinson’s disease and supports the development of more effective DBS therapies based on pathophysiological biomarkers.
Keywords: Parkinson’s disease; local field potential; oscillations; phase-amplitude coupling; globus pallidus, subthalamic nucleus, primary motor cortex
Abstract
Oscillatory neural activity in different frequency bands and phase-amplitude coupling (PAC) are hypothesized to be biomarkers of Parkinson’s disease (PD) that could explain dysfunction in the motor circuit and be used for closed-loop deep brain stimulation (DBS). How these putative biomarkers change from the normal to the parkinsonian state across nodes in the motor circuit and within the same subject, however, remains unknown. In this study, we characterized how parkinsonism and vigilance altered oscillatory activity and PAC within the primary motor cortex (M1), subthalamic nucleus (STN), and globus pallidus (GP) in two nonhuman primates. Static and dynamic analyses of local field potential (LFP) recordings indicate that 1) after induction of parkinsonism using the neurotoxin MPTP, low-frequency power (8–30 Hz) increased in the STN and GP in both subjects, but increased in M1 in only one subject; 2) high-frequency power (~330 Hz) was present in the STN in both normal subjects but absent in the parkinsonian condition; 3) elevated PAC measurements emerged in the parkinsonian condition in both animals, but in different sites in each animal (M1 in one subject and GPe in the other); and 4) the state of vigilance significantly impacted how oscillatory activity and PAC were expressed in the motor circuit. These results support the hypothesis that changes in low- and high-frequency oscillatory activity and PAC are features of parkinsonian pathophysiology and provide evidence that closed-loop DBS systems based on these biomarkers may require subject-specific configurations as well as adaptation to changes in vigilance.
NEW & NOTEWORTHY Chronically implanted electrodes were used to record neural activity across multiple nodes in the basal ganglia-thalamocortical circuit simultaneously in a nonhuman primate model of Parkinson’s disease, enabling within-subject comparisons of electrophysiological biomarkers between normal and parkinsonian conditions and different vigilance states. This study improves our understanding of the role of oscillatory activity and phase-amplitude coupling in the pathophysiology of Parkinson’s disease and supports the development of more effective DBS therapies based on pathophysiological biomarkers.
identifying electrophysiological biomarkers that correlate with motor symptoms or disease severity will be instrumental in understanding the pathophysiology of Parkinson’s disease (PD) and developing more effective treatments. There is particular interest in incorporating such biomarkers into devices that could deliver closed-loop deep brain stimulation (DBS) tailored to the clinical state of individual patients (Johnson et al. 2016; Little et al. 2013; Rosa et al. 2015). Biomarkers derived from local field potentials (LFPs) are attractive for closed-loop DBS because they can be recorded continuously from brain structures via chronically implanted electrodes or DBS leads (Abosch et al. 2012; Brown and Williams 2005; Khanna et al. 2016; Little and Brown 2012; Swann et al. 2017).
Changes in LFP power across frequency bands in different structures of the basal ganglia-thalamocortical (BGTC) motor circuit as well as changes in coupling between the phase of low-frequency and the amplitude of high-frequency oscillations [phase-amplitude coupling (PAC)] have been proposed as biomarkers of PD. Studies have shown that the low-frequency (13–30 Hz) power of LFPs from macroelectrode recordings in the subthalamic nucleus (STN) and globus pallidus (GP) decreases after levodopa administration to PD patients and during voluntary movement (Brown et al. 2001; Brown 2003; Brown and Williams 2005; Kühn et al. 2009). Thus, elevated power (13–30 Hz) has been hypothesized to be an antikinetic mechanism of the motor circuit that in excess could be associated with motor impairment in PD (Kühn et al. 2006).
Other researchers have hypothesized that changes in the high-frequency side of the spectrum may also be associated with PD. For example, Foffani et al. (2003, 2005), showed that levodopa administration elicited an increase in power at ∼320 Hz in the STN of PD patients implanted with DBS leads, whereas Tsiokos et al. (2013) demonstrated that the power between 200 and 300 Hz in the internal segment of the globus pallidus (GPi) of PD patients was movement dependent. Both groups hypothesized that these high-frequency oscillations are required for normal information processing and motor control.
More recent studies hypothesize that PAC could be a robust biomarker of PD (Connolly et al. 2015; de Hemptinne et al. 2013; López-Azcárate et al. 2010;). PD patients exhibited a reduction of PAC measured in the STN after levodopa administration (López-Azcárate et al. 2010). The studies by de Hemptinne et al. (2013, 2015) showed that PD patients were more likely to exhibit significant measurements of PAC in the primary motor cortex (M1) than epilepsy and dystonia controls and that cortical PAC was reduced during therapeutic STN DBS. A study using LFPs from microelectrode recordings in the nonhuman primate MPTP model of PD showed that PAC in the pallidum progressively increased as a function of parkinsonism severity (Connolly et al. 2015).
The aforementioned studies illustrate that changes in oscillatory activity across frequencies as well as PAC may provide information on how parkinsonism alters the BGTC. It is not clear, however, how oscillatory activity and PAC change from the normal to the disease condition, whether changes are consistent across multiple nodal points within the BGTC of the same subject, or how these biomarkers are influenced by the vigilance state of the subject. Answers to these questions will lead to a better understanding of the pathophysiology of PD and inform the development of therapies that use electrophysiological biomarkers (e.g., closed-loop DBS) in different behavioral contexts. In this study, we characterized oscillatory activity and PAC in the STN, GP, and M1 of two nonhuman primates, before and after the induction of parkinsonism, during the awake and non-REM sleep states. A dynamic analysis is presented to assess how neural oscillations and PAC were altered across nodal points in the BGTC circuit in both the normal and the parkinsonian state during different states of vigilance (awake and sleep states). We found that basal ganglia oscillatory activity in the β-range was increased in both animals in the parkinsonian state. Additionally, high-frequency power in the STN was decreased in both animals in the parkinsonian state. PAC measurements, on the other hand, changed in both animals but in different sites [M1 in one animal, external segment of the globus pallidus (GPe) in the other]. Both oscillatory activity and PAC were significantly impacted by the state of vigilance. These findings improve our understanding of the role of low- and high-frequency oscillations and PAC measurements in the pathophysiology of PD and suggest that optimizing closed-loop DBS systems based on these putative biomarkers may require subject-specific configurations as well as the ability to adapt to changes in vigilance.
MATERIALS AND METHODS
Subjects
All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with US Public Health Service policy on the humane care and use of laboratory animals. Two adult female rhesus macaques [Macaca mulatta, subjects J (17 yr) and K (13 yr)] were used in this study. All surgery was performed using aseptic techniques under isoflurane anesthesia. Preoperative cranial CT and 7-T MRI images were incorporated into the Monkey Cicerone neurosurgical navigation program (Miocinovic et al. 2007) to facilitate surgical planning of a titanium cephalic chamber targeting the STN, GPe, and GPi. Extracellular microelectrode mapping confirmed the location of target nuclei. Each animal was then implanted in both the STN and GP with 8-contact scaled down versions of human DBS leads (0.5 mm contact height, 0.5 mm intercontact spacing, 0.625 mm diameter; NuMED). These methods are described in detail in a previous publication (Hashimoto et al. 2003). Each animal was subsequently implanted in the arm area of the primary motor cortex (M1) with a 96-channel Utah microelectrode array (Pt-Ir, 1.5-mm depth, 400-μm interelectrode spacing; Blackrock Microsystems), using surgical methods described previously (Maynard et al. 1997; Rousche and Normann 1992). Pt/Ir reference wires were placed between the dura and skull adjacent to the array. During array implantation surgery, M1 was identified based on sulcal landmarks (Fig. 1, A and E), and the arm area was localized based on intraoperative stimulation of the cortical surface using a stainless-steel ball electrode (Grass Technologies). Locations of DBS leads were verified in subject K histologically using frozen coronal sections (50-μm thick) that were imaged and visualized in Avizo 3D analysis software (FEI; see Fig. 1E). Histology is not yet available in subject J; however, DBS lead locations were approximated using fused preimplantation MRI and postimplantation CT images (Fig. 1A) together with microelectrode identification of target nuclei before implantation of the leads. In both animals, we verified that electrical stimulation in STN and GPi contacts produced improvements in parkinsonian motor signs.
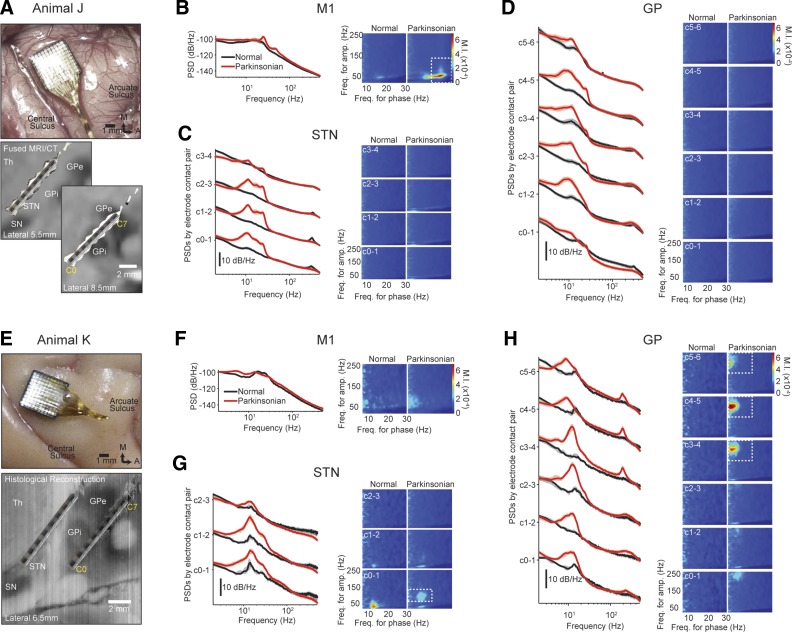
Fig. 1.
The effects of parkinsonism on oscillatory activity and phase-amplitude coupling (PAC) across all recorded structures in each subject. A and E: location of Utah array in the motor cortex (M1; top) and location of deep brain stimulation (DBS) leads in the subthalamic nucleus and internal and external segments of the globus pallidus [subthalamic nucleus (STN), internal segment of the globus pallidus (GPi), and external segment of the globus pallidus (GPe); bottom]. In animal J, contact pairs C2–3, C3–4, and C4–5 are estimated to be in the GPi, and the most dorsal usable contact pair, C5–6, straddled the border between GPe and GPi. Also shown are PSDs (left) and PAC comodulograms (right) in the M1 (B and F), STN (C and G), and globus pallidus (GP; D and H). Power spectral density (PSD) plots reflect median values, and shaded regions contain the 25th and 75th percentiles of the PSDs at each frequency. The white dashed boxes in the PAC comodulograms indicate regions associated with an observed increase in PAC in the parkinsonian condition.
MPTP Administration and Behavioral Assessments
Once data were collected in the normal state, animals were rendered parkinsonian by systemic (intramuscular) and intracarotid injections of the neurotoxin 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP). Subject K received six intramuscular injections (0.3–0.4 mg/kg each, total of 1.8 mg/kg). Subject J received three intramuscular injections and one intracarotid injection (0.3–0.4 mg/kg each, total of 1.4 mg/kg). For both subjects, data were gathered after a stable parkinsonian state was achieved, beginning ∼1 mo after the last MPTP injection and continuing for 1–2 mo. Motor symptoms were assessed using a modified Unified Parkinson’s Disease Rating Scale (mUPDRS), which rated axial motor symptoms (gait, posture, balance) as well as upper and lower limb rigidity, bradykinesia, akinesia, and tremor on the hemibody contralateral to neural recordings using a 0–3 scale (0 = normal, 3 = severe; maximum total score of 18). The results of these assessments are summarized in Table 1. Subject K received daily dopaminergic treatment (carbidopa/levodopa 25/100 mg tablets) at the end of the day’s experimental sessions to facilitate the animal’s care in its home enclosure. To minimize possible carryover effects of levodopa on physiological recordings, all recording sessions were conducted a minimum of 16 h after the last treatment dose.
Table 1.
Clinical ratings of parkinsonian animals
| mUPDRS Ratings, 0–3 |
||
|---|---|---|
| Motor Symptoms | Subject J (n = 15)* | Subject K (n = 25)* |
| Axiala | 2.0 (0.1) | 2.3 (0.1) |
| Rigidity | 1.0 (0.3) | 2.1 (0.2) |
| Tremor | 0.0 (0.0) | 0.3 (0.1) |
| Bradykinesia | 1.8 (0.1) | 2.4 (0.4) |
| Akinesia | 1.8 (0.2) | 2.5 (0.3) |
| Food retrieval | 1.5 (0.3) | 2.5 (0.3) |
| Total (≤18) | 8.2 | 12.1 |
Values are means (SD). mUPDRS, modified Unified Parkinson’s Disease Rating Scale. mUPDRS for nonhuman primates was used to assess motor symptoms in each animal, rating axial motor symptoms (gait, posture, balance) as well as upper and lower limb rigidity, bradykinesia, akinesia, and tremor (on the side contralateral to neural recordings) using a 0–3 scale (0 = normal, 3 = severe; maximum total score of 18).
axial symptoms are an average of posture, gait, and balance scores;
n = no. of observations, except for axial scores, which are n = 4 and 5 for subjects J and K, respectively.
Neuronal Recordings
Neurophysiological data were collected using a TDT workstation (Tucker Davis Technologies) operating at ∼25-kHz sampling rate. Signals were bandpass filtered (0.5–700 Hz) to extract local field potentials (LFPs) and downsampled to ∼3 kHz for analysis. LFPs from the STN and GP were created by subtracting recordings from adjacent contacts of the DBS leads (e.g., LFP C0–1 represents the signal created by subtracting contact 1 from contact 0). LFPs from contact C7 from the GP lead in both animals were noisy and highly variable across recording sessions and were not used for analysis. A mean M1 LFP was obtained by averaging recordings from all 96 channels in the array. All data were collected during a resting state while the animal was seated in a primate chair with its head fixed. Time periods with movement artifacts were identified by high-amplitude broadband power in the time-frequency spectrogram (spectral analysis described below) and excluded from further analysis.
Assessment of Subject Vigilance
We have observed that parkinsonian animals in particular are susceptible to periods of drowsiness and sleep during resting state recordings. This creates a potential confounding variable if data are combined irrespective of animal vigilance, but it also provides an opportunity to evaluate changes in neural activity during drowsiness and early stages of sleep. To differentiate periods of wakefulness and drowsiness/sleep, we assessed the animal’s vigilance using a combination of eye monitoring and analysis of instantaneous power of low-frequency oscillations in the M1. A video camera was focused on the animals’ eyes and synchronized to the neural recording system. Customized MATLAB (MathWorks, 2016) image segmentation algorithms were used to analyze video of one eye and calculate the eye-opening area in each video frame (i.e., total no. of pixels corresponding to the eyeball in a given frame). The eye-opening area was low-pass filtered (Butterworth, 0.2-Hz cutoff frequency, zero phase forward and reverse filtering) to eliminate blinks and normalized between 0 and 1. A value of 1 corresponds to the maximum eye opening observed in each video recording. The maximum eye opening in all videos was non-zero and corresponded to a period of time in which the animal had the eyes wide open. Normalized eye-opening estimates at each sample k are denoted by Ae(k). The state of the eyes at each sample, denoted by Se(k), was classified as open [Se(k) = 1], closed [Se(k) = 0], and undetermined [Se(k) = 0.5] as follows.
The eye state alone may not be a robust measure of the vigilance state because an animal could be fully awake while having the eyes closed. To reduce the inaccuracy when estimating the sleep state, we also examined the power of low-frequency oscillaons in M1. The rationale behind using M1 oscillatory activity as a measure of sleep is twofold. First, electroencephalography has revealed that rhesus macaques exhibit θ-waves (4–7 Hz), K-complexes, and slow and δ-oscillations (0.4–4 Hz) during the stages N1, N2, and N3 of non-rapid eye movement (NREM) sleep (Hsieh et al. 2008). Second, these low-frequency complexes and oscillations have large spatial correlation across cortical areas, including motor cortex, that can be captured via LFP recordings (Dehghani et al. 2012; Destexhe et al. 1999).
A measurement of low-frequency power at each sample k denoted by Pm1(k) was computed by bandpass filtering the M1 LFP in the 0.1- to 7-Hz range (Butterworth, zero-phase forward and reverse filtering), obtaining the amplitude envelope of the low-frequency oscillations via the Hilbert transform, and smoothing the envelope via a low-pass filter of cutoff 0.2 Hz (Butterworth, zero-phase forward and reverse filtering). A threshold Pt was used to classify, given the low-frequency power envelope, whether the animal was in the NREM sleep or awake state. The threshold Pt was computed as follows. First, a prolonged period of time in which the animal was in the awake state with its eyes open was selected by inspecting the video recordings. Then, the maximum value of the low-frequency power envelope during this period was selected as the threshold Pt. Thresholds were computed for each subject and disease condition.
The expression below summarizes how at each sample k the vigilance state Sv(k) was estimated based on combined measurements of eye state and low-frequency power in M1:
Measurements of both eye area and M1 power had to be consistent for the vigilance state to be defined as awake [Sv(k) = 1)] or sleep [Sv(k) = 0]. Values of Sv(k) = 0.5 were assigned to samples if there was uncertainty about the vigilance state of the subjects. Eye video was not available for subject K in the normal condition; hence only low-frequency power was used to estimate the vigilance state of this subject in the normal condition.
Power Spectral Density and Phase-Amplitude Coupling Analysis
Typically, 5 min of resting state data were collected during each recording session. Data presented for animal J are from 20 sessions (19 days) in the normal state and 21 sessions (16 days) in the parkinsonian state. For animal K, data are from five sessions (5 days) in the normal condition and 15 sessions (15 days) in the parkinsonian condition. LFPs were grouped into individual data segments 15 s in duration, during which the animals were continuously in either the awake [Sv(k) = 1] or the sleep [Sv(k) = 0] state. A total of 516 segments were analyzed for subject J, which included 185 in the normal awake state, four in the normal sleep state, 312 in the parkinsonian awake state, and 15 in the parkinsonian sleep state. A total of 150 segments were analyzed for subject K, which included 25 in the normal awake state, five in the normal sleep state, 79 in the parkinsonian awake state, and 41 in the parkinsonian sleep state.
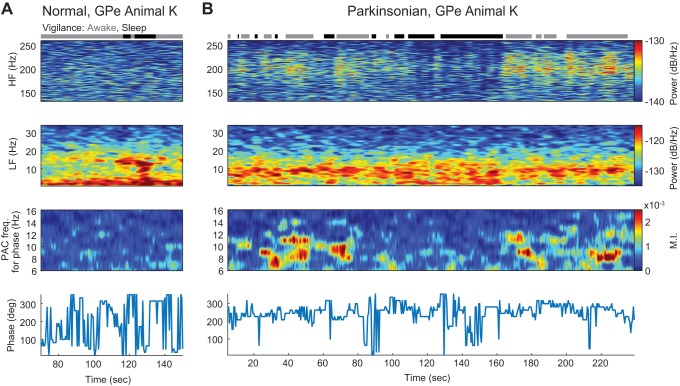
All analysis was performed using customized scripts in MATLAB (MathWorks, 2016). Power spectral densities (PSDs) of the 15-s LFP segments were computed via the Welch’s method. To compute the PSDs, we used 214 points in the fast Fourier transform, a Hamming window of 1.34 s (¼ 214 points), zero padding (¾ 214 points), and an overlap of 50%. The frequency resolution was ∼0.1863 Hz. The power in a frequency interval was computed as the sum of PSD values over the interval. We carried out a post hoc analysis of changes in power across conditions and vigilance states that were observed within particular frequency intervals, specifically 8–20, 20–30, 30–70, 70–145, 140–260, and 260–370 Hz. These intervals were used to quantify changes in power across frequency and statistical significance of differences between conditions (normal vs. parkinsonian) and vigilance state (awake vs. sleep). These frequency intervals were selected instead of traditionally defined frequency bands (e.g., α, 8–13 Hz; β, 13–30 Hz) to have the peaks of the PSDs inside the intervals entirely. We evaluated how oscillatory power evolved over time by using spectrograms computed via the multi-taper method and Chronux toolbox (Bokil et al. 2010). See Fig. 5 for an example.
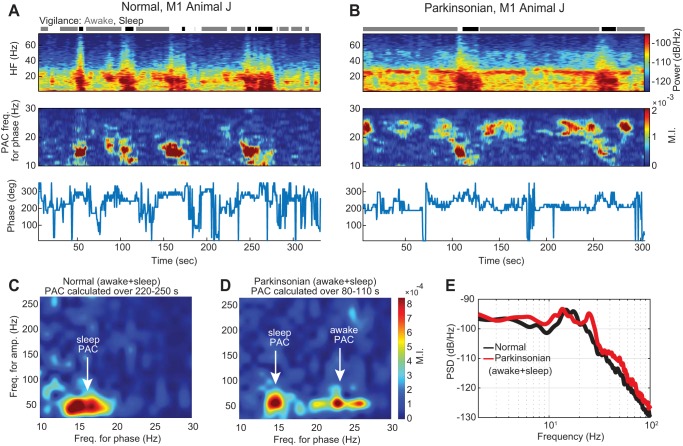
Fig. 5.
A and B: temporal dynamics of power and PAC for the M1 of subject J in both normal (A) and parkinsonian (B) conditions during epochs of sleep and wakefulness. Top: spectrograms in which power (color scale) across frequency (vertical axis) is shown over time (horizontal axis). Middle: PACograms that illustrate the MI values (color scale) across frequencies for phase fl (vertical axis) over time (horizontal axis) and with fixed frequencies for amplitude (fh in the interval between 50 − fl and 50 + fl Hz). Bottom: the preferred phase Φmax associated with MImax evolved over time. C and D: comodulograms in the normal and parkinsonian conditions, respectively, that show PAC calculated using 30-s recordings that contain time in both the sleep and awake states (220–250 s in the normal condition and 80–110 s in the parkinsonian condition). E: PSDs computed using the same 30-s recordings used for the comodulograms in C and D.
PAC between the phase of oscillations at low-frequency (fl) and the amplitude of oscillations at high-frequency (fh) was calculated for each 15-s LFP segment using a customized version of the modulation index (MI) presented by (Tort et al. 2010). Briefly, the original LFP signal was bandpass filtered (Butterworth 2nd order, zero phase forward and reverse filtering) with a center frequency fl and bandwidth of 1 Hz to obtain the low-frequency component. The narrow bandwidth of the filter was chosen to enable accurate calculation of the phase of the low-frequency component. The high-frequency component was obtained using a bandwidth of 2 fl. A value of 2 fl or greater is necessary to retain the envelope information of the high-frequency oscillations and thereby compute PAC accurately (Aru et al. 2015). The phase of the low-frequency component (ϕl) and amplitude of the high-frequency component (Ah) were computed through the Hilbert transform. The coupling between ϕl and Ah over a time segment was measured using the MI, which is the Kullback-Leibler distance between the uniform distribution and the distribution of Ah values (normalized between 0 and 1) at discretized values of ϕl (0, 10, 20, . . . , 350 degrees) (Tort et al. 2010). Note that the MI is not a function of the absolute amplitude of the low- or high-frequency oscillations. The phase associated with the MI was the value of ϕl in the distribution at which the maximum amplitude Ah was found.
Rectangular maps referred to as comodulograms were used to examine the MI values (color) across different frequencies for phase (fl; horizontal axis) and for amplitude (fh; vertical axis). Comodulograms were created at frequencies for phase in intervals of 8–100 Hz (1-Hz increments) and frequencies for amplitude in intervals of 20–400 Hz (5-Hz increments) for each data segment. The comodulograms presented here are the average of all data segment comodulograms in each condition and vigilance state. For a detailed visualization, comodulograms were displayed only within the subset of frequencies (fl and fh) where PAC was observed.
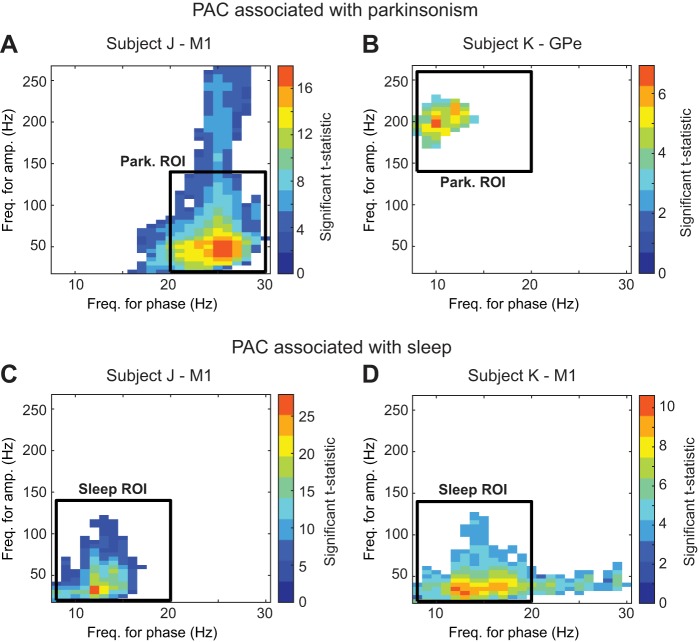
We carried out a post hoc analysis of changes in PAC across brain structures. We found that if PAC was observed in a particular brain structure, it was typically restricted to a bounded frequency region in the two-dimensional space of frequencies for phase and for amplitude. Rectangular frequency regions where changes in PAC were observed from one state to another were used for the statistical analysis. Regions will be further described in results. The average (MIavg) and maximum MI (MImax) in each selected frequency region were used to quantify with a scalar value the changes in PAC across conditions and vigilance states. Only MIavg values are presented in results section because MImax led to the same statistical results. To quantify the preferred phase of PAC in a specific frequency region of a comodulogram, we used the preferred phase associated with the frequency pair where MImax was observed. We denoted this phase by Φmax. The trend and variability of Φmax values over different data segments (and comodulograms) were quantified via their mean and standard deviation using circular statistics (Berens 2009). The distribution of preferred phase Φmax from different data segments in each condition and vigilance state was depicted using angle histograms, see Fig. 4A, for example.
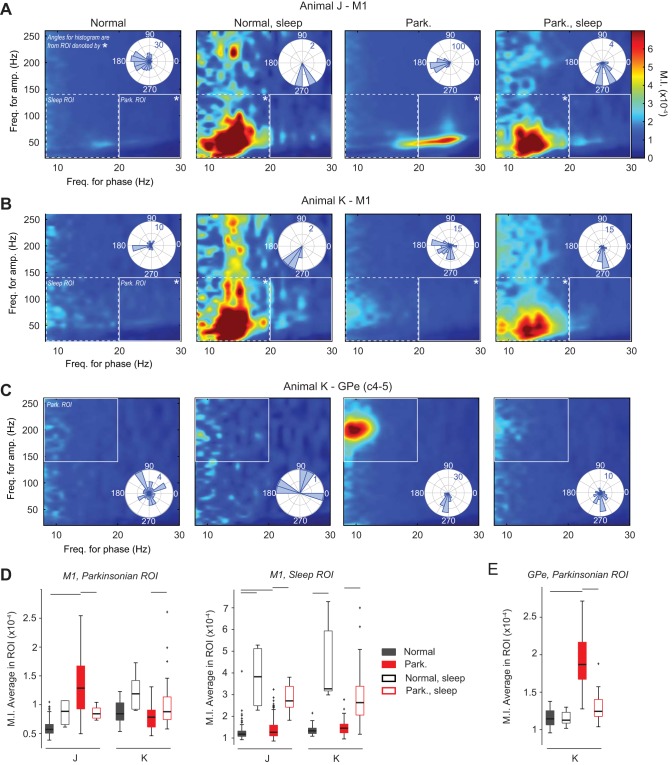
Fig. 4.
Effect of sleep and parkinsonism on PAC. A and B: comodulograms are displayed for the M1 of subjects J and K, respectively. The color in the comodulograms is the average MI value (MIavg) across frequencies for phase (horizontal axis) and for amplitude (vertical axis) from all data sets in a given condition. Region of interest (ROI) associated with the sleep or parkinsonian condition is indicated by dashed or solid white rectangles, respectively. *Polar histograms (top right corner of comodulograms) illustrate preferred phases (Φmax) calculated in the frequency regions. C: comodulogram and polar histograms (bottom right corner of comodulograms) for the GPe (C4–5) of subject K. D: box plots comparing the MIavg in selected M1 regions of interest (ROI) between conditions. The parkinsonian ROI is associated with the region where increased PAC was observed in subject J in the parkinsonian condition (frequency for phase: 20–30 Hz; frequency for amplitude: 20–140 Hz). The sleep ROI is associated with the region where increased PAC was observed in both subjects in the sleep state (frequency for phase: 8–20 Hz; frequency for amplitude: 20–140 Hz). Significance bars represent P < 0.01; Wilcoxon rank-sum test corrected for multiple comparisons, as described in Fig. 3. E: box plots comparing the MIavg values in the GPe between conditions. The parkinsonian ROI is associated with the region where increased PAC was observed in subject K in the parkinsonian condition (frequency for phase: 8–20 Hz; frequency for amplitude: 140–260 Hz).
To characterize the temporal dynamics of PAC during the awake resting state and as the animal transitioned to and from sleep, MI values were evaluated over time and visualized using time comodulograms, referred to as PACograms. In these PACograms, the vertical axis is the frequency for phase fl, the horizontal axis is the time, and the color is the MI value. The frequency for amplitude fh was selected as that where the maximum MI occurred as revealed by the static comodulograms. To create a PACogram, MI values in the frequencies of interest were calculated via 10-s moving windows and increments of 0.5 s. A time window of 10 s was above the minimum time needed to clearly capture PAC in the recorded signals. The preferred phase at each time was that associated with the maximum MI over the frequencies for phase fl.
Measurements of PAC may reflect not only true oscillatory coupling but also nonsinusoidal signal shapes, including sawtooth, triangular, and sharp signal deflections (Gerber et al. 2016; Jensen et al. 2017; Kramer et al. 2008; Lozano-Soldevilla et al. 2016). A periodic nonsinusoidal signal in particular can be decomposed into its fundamental frequency and harmonics. The phase locking between the fundamental frequency and its harmonics can lead to spurious measurements of PAC, which are stronger at the first harmonic frequencies. We assessed whether the measurements of PAC from our LFP recordings were due to nonsinusoidal shapes by examining the shape of the LFP trace coupled to high-frequency oscillations. The shape of the signal was estimated by averaging segments of the raw signal locked to the local maxima of the high-frequency amplitude envelope. We computed an amplitude-triggered average of the LFP trace for each day of recording and each state (normal, normal sleep, parkinsonian, parkinsonian sleep). The median and interquartile ranges of the average shapes over all recording days were computed to illustrate the trend and variability of the data.
Statistics
We conducted a post hoc statistical analysis of changes in power and PAC. Nonparametric tests were performed to determine whether the difference between scalar measurements of power and PAC across conditions were significant. These tests were better suited than parametric tests because of the small number of samples in the sleep state and because data were not normally distributed. Pairwise differences between measurements of power within a particular frequency interval and MI averages within a frequency region in two different conditions were assessed via the Wilcoxon rank-sum test. P values from this test were corrected for the post hoc comparisons (no. of hypotheses) using the Bonferroni method. In the analysis of power and PAC, we corrected for n = k × r total comparisons resulting from the pairwise comparisons made (k = 4) and frequency regions evaluated (r; Table 2). We assumed that the difference between measurements in the two conditions was significant when corrected (P < 0.01). Significant differences between preferred phase measurements in the two conditions were evaluated via a nonparametric test for equal medians using the circular statistics MATLAB toolbox (Berens 2009).
Table 2.
Changes in oscillatory activity and PAC due to parkinsonism and sleep in each subject (J and K)
| Normal to Parkinsonian (Awake) |
Normal to Parkinsonian (Sleep) |
Awake to Sleep (Normal) |
Awake to Sleep (Parkinsonian) |
|||||
|---|---|---|---|---|---|---|---|---|
| Location (Feature) | Subject J | Subject K | Subject J | Subject K | Subject J | Subject K | Subject J | Subject K |
| Oscillatory activity (power) | ||||||||
| M1 | ||||||||
| 8–20 Hz | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| 20–30 Hz | ↑ | ↓ | ↑ | ↑ | ↑a | ↑ | ||
| 30–70 Hz | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| STN | ||||||||
| 8–20 Hz | ↑ | ↑ | ↑ | ↑a | ↓ | ↓ | ||
| 20–30 Hz | ↑ | ↑ | ↑ | ↓ | ↓ | |||
| 260–370 Hz | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |
| GPi (8–20 Hz) | ↑ | ↑ | ↑ | ↑ a | ↑ | ↓ | ↓ | |
| GPe* | ||||||||
| 8–20 Hz | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↓ | |
| 140–260 Hz | ↑ | ↑ | ↓ | ↓ | ||||
| PAC | ||||||||
| M1 | ||||||||
| Parkinsonian ROI | ↑ | ↑ | ↑ | ↓ | ↑c | |||
| Sleep ROI | ↑b | ↑ | ↑ | |||||
| STN (Parkinsonian ROI) | ↑ | ↓ | ||||||
| GPe (parkinsonian ROI)* | ↑ | ↓ | ||||||
PAC, phase-amplitude coupling; M1, motor cortex; STN, subthalamic nucleus; GPi, internal segment of the globus pallidus; GPe, external segment of the globus pallidus; ROI, region of interest. Summary of the effects of parkinsonism and vigilance state on oscillatory activity and PAC in each subject. The recording locations are the M1 of each subject and contact pairs in the STN (subject J: C1–2; subject K: C0–1), GPi (subject J: C2–3; subject K: C0–1), and GPe (subject J: C5–6 border GPe/i; subject K: C4–5). Oscillatory activity in frequency bands with notable changes due to parkinsonism or sleep; for clarity, not all possible frequency band comparisons are shown. Arrows denote significant increase (↑) or decrease (↓) in a given measure based on a Wilcoxon rank-sum test, P < 0.01 (see materials and methods) based on the following 4 comparisons: from normal to parkinsonian condition in the awake state, from normal to parkinsonian condition in the sleep state, from awake to sleep state in the normal condition, and from awake to sleep state in the parkinsonian condition. PAC measurements (average modulation index) in a given ROI are also shown. In M1, PAC associated with the parkinsonian condition (subject J) was compared in an ROI 20–30 Hz for phase and 20–140 Hz for amplitude, and PAC associated with sleep in both subjects was compared in an ROI 8–20 Hz for phase and 20–140 Hz for amplitude. In STN, PAC associated with the parkinsonian condition (subject K) was compared in an ROI 8–20 Hz for phase and 65–140 Hz for amplitude. In GPe, PAC associated with the parkinsonian condition (subject K) was compared in an ROI 8–20 Hz for phase and 140–260 Hz for amplitude.
Contact pair within GPe in animal K and in animal J the most dorsal usable contact pair that straddled the border between GPe and GPi;
difference is statistically significant, with a level P < 0.02 instead of the prescribed level P < 0.01;
increase is due to parkinsonian-related PAC that extends into the sleep ROI (see Fig. 4A, left and middle right plots);
increase is due to sleep-related PAC that extends into the parkinsonian ROI (see Fig. 4B, middle right and right plots).
We also carried out an a priori statistical assessment of PAC comodulograms to verify the inference reached via the post hoc selection of PAC regions of interest and subsequent statistical analysis. We used a cluster-based, nonparametric permutation test using the FieldTrip Toolbox in MATLAB (Maris and Oostenveld 2007; Oostenveld et al. 2011). A description of this method and related results are presented in the appendix.
RESULTS
Assessment of Severity of Parkinsonism
Average mUPDRS clinical ratings of the two parkinsonian animals are shown in Table 1. Both animals exhibited cardinal parkinsonian motor signs of rigidity, bradykinesia, and akinesia. As is typical for MPTP-treated rhesus monkeys, minimal resting tremor was observed. Subject K was more severely affected than animal J, as reflected by its modestly higher mUPDRS scores.
Oscillatory Activity and PAC Are Altered in the Parkinsonian Condition
The induction of parkinsonism caused significant changes in power and PAC measurements from LFP recordings in the M1, STN, and GP of both animals (Fig. 1 and Table 2). Results for each animal are presented individually, focusing in this section on recordings collected in the awake state.
Subject J.
In the parkinsonian condition, oscillatory activity was associated with an increase in power in the 8- to 20-, 20- to 30-, and 30- to 70-Hz bands in the M1 (P < 0.001; Fig. 1B, left). The changes in basal ganglia nuclei were even more prominent. In all recordings within the STN, the power increased in the 8- to 20- and 20- to 30-Hz bands (C0–1, C1–2, C2–3, P < 0.001; Fig. 1C, left). A peak in the STN PSDs centered at ∼335 Hz was observed in the normal condition in all STN contacts but vanished in the parkinsonian condition (C0–1, C1–2, C2–3, 260–370 Hz band, P < 0.001). All recording sites on the GP lead except for C0–1 exhibited an increase in power in the 8- to 20- and 20- to 30-Hz bands in the parkinsonian condition (P < 0.01; Fig. 1D, left).
In animal J, PAC measurements increased in the parkinsonian condition solely in M1, with a maximum MI observed at a frequency for phase fl ≈ 24 Hz and a frequency for amplitude of fh ≈ 50 Hz (Fig. 1B, right, and Fig. A1A). This increase in PAC was verified via the a priori statistical test presented in the appendix. To determine the significance of the PAC difference between normal and parkinsonian conditions using scalar measurements, we defined a region of interest (20–30 Hz for frequencies for phase and 20–140 Hz for frequencies for amplitude) where PAC was observed and the average MI (MIavg) values were computed. MIavg values were significantly higher in the parkinsonian condition than in the normal condition in the M1 (median MIavg: normal = 0.06 × 10−3, parkinsonian = 0.13 × 10−3, P < 0.001). The mean (SD) of the preferred phase of PAC in M1 across all data segments in the parkinsonian condition was 193 (30)°. PAC was not observed within the STN or GP in either the normal or parkinsonian condition of animal J (Fig. 1, C and D, right).
Subject K.
Similar to animal J, the induction of parkinsonism was associated with a significant increase in power in the 8- to 20- and 20- to 30-Hz bands in the STN (C0–1, C1–2, C2–3, P < 0.001; Fig. 1G, left) as well as an increase in power in the 8- to 20-Hz band in all GP contact pairs (P < 0.001; Fig. 1H, left). A reduction in power near ∼335 Hz was observed in the STN PSDs in the parkinsonian compared with the normal condition (C0–1, C1–2, C2–3, 260- to 370-Hz band, P < 0.001), although this difference was not as salient as that observed in the STN of animal J. There was a peak at ∼200 Hz in the PSDs from all GP contact pairs that emerged in the parkinsonian condition, particularly in contacts in or near GPe (140- to 260-Hz band, P < 0.001). Unlike animal J, in which 20–30 Hz power in the M1 increased following the induction of parkinsonism, in animal K the parkinsonian condition was associated with a modest but significant reduction in M1 power in the same band (P < 0.001; Fig. 1F, left).
In animal K, PAC emerged in the parkinsonian condition most prominently in the GPe recordings (Fig. 1H, right and Fig. A1B). The maximum MI occurred at fl ≈ 10 Hz and fh ≈ 200 Hz (C4–5; median MIavg: normal = 0.12 × 10−3, parkinsonian = 0.19 × 10−3, P < 0.001). The mean (SD) preferred phase associated with the maximum MI in the GPe was 254 (29)°. Interestingly, PAC in the STN was present in the normal awake condition (contact pair C0–1), with the maximum MI at fl ≈ 14 Hz and fh ≈ 35 Hz. The mean (SD) of the preferred phase was 262 (68)°. In the parkinsonian condition, however, PAC decreased in this frequency region (C0–1; median MIavg: normal = 0.2 × 10−3, parkinsonian = 0.16 × 10−3, P < 0.01) and appeared within another region with MImax at fl ≈ 16 Hz and fh ≈ 95 Hz (C0–1; median MIavg: normal = 0.15 × 10−3, parkinsonian = 0.2 × 10−3, P < 0.001). The mean (SD) of the PAC preferred phase in the parkinsonian condition was 317 (47) °.
Correlation Between Power and PAC
The frequencies for phase and/or amplitude where maximum MI occurred were the same frequencies where peaks were located in the PSDs (Fig. 1, B and H). To further examine the relationship between the MI values and the power in specific frequency bands, the Spearman correlation between these variables was computed (Fig. 2). In the M1 of subject J, there were significant correlations between low-frequency power and MI values and between high-frequency power and MI values (Fig. 2, A and B, respectively). In the GPe of subject K, no significant correlation between low-frequency power and MI values was found (Fig. 2C), but there was a significant correlation between high-frequency power and MI values (Fig. 2D). It is important to recall that the method used to compute MI values does not explicitly depend on the amplitude of the oscillations for phase or amplitude.
Fig. 2.
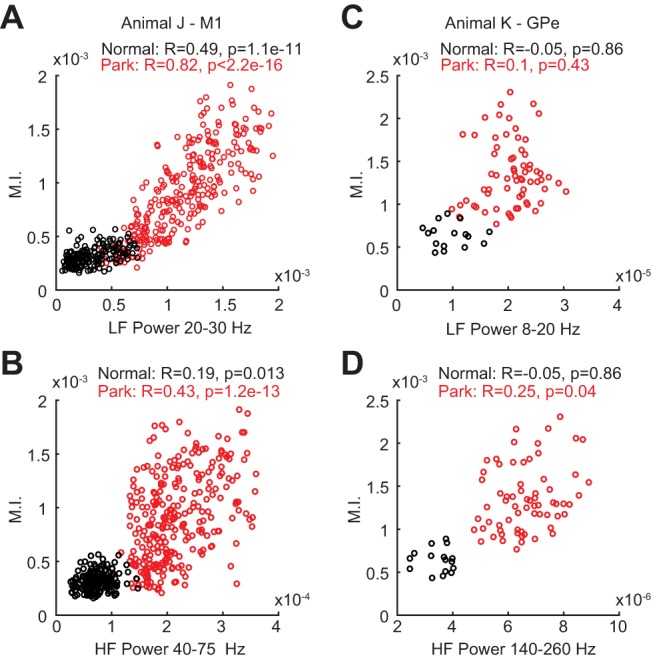
Correlation between PAC modulation index (MI) values and the power in frequency bands [low-frequency (LF) for phase and high-frequency (HF) for amplitude] where maximum PAC occurred in the parkinsonian condition. A: scatterplot of LF power (20–30 Hz) vs. MI values in the M1 of subject J. B: scatterplot of HF power (40–75 Hz) vs. MI in the M1 of subject J. C: scatterplot of LF power (8–20 Hz) vs. MI values in the GPe of subject K (contacts C4–5). D: scatterplot of HF power (140–260 Hz) vs. MI in the GPe of subject K.
Effect of Vigilance on Oscillatory Activity and PAC
In both animals the vigilance state of the subjects significantly influenced how oscillatory activity and PAC were expressed in the studied brain structures (Figs. 3 and 4). Note that the “sleep” state classification is defined based on an analysis of the low-frequency power recorded in M1 and degree of eye opening (see materials and methods), and no attempt was made at classifying particular sleep stages.
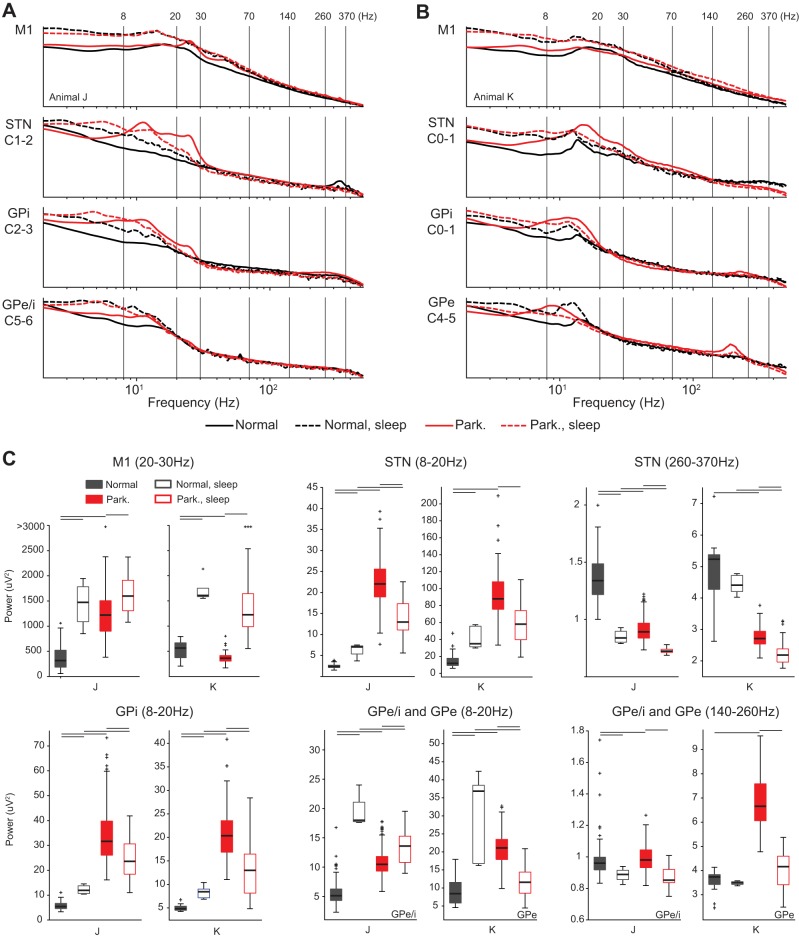
Fig. 3.
A and B: effect of parkinsonism and vigilance state on the PSDs in the M1 and selected contact pairs within the STN, GPe, and GPi of subjects J (A) and K (B). Recording locations are the M1 for both subjects (top), STN for both subjects (upper middle), and GPi for both subjects (lower middle) and the border between GPi and GPe for subject J (denoted GPe/i) and GPe for subject K (bottom). Median values are shown. C: box plots comparing the power in selected frequency bands of the 4 conditions (normal awake and sleep, parkinsonian awake and sleep). Significance bars represent P < 0.01, Wilcoxon rank-sum test corrected for multiple comparisons. Comparisons were normal awake vs. normal sleep, normal awake vs. parkinsonian awake, normal sleep vs. parkinsonian sleep, and parkinsonian awake vs. parkinsonian sleep. Comparisons of normal awake vs. parkinsonian sleep and normal sleep vs. parkinsonian awake were not of interest, and significance tests are not shown.
The predominant effect of the sleep state on the PSDs across all three recording sites in both subjects (normal and parkinsonian) was an increase in the low-frequency power (0.5- to 8-Hz band; Fig. 3, A and B). Differences between normal and parkinsonian conditions were less pronounced in the sleep state compared with the awake state. For example, in M1 the sleep state was associated with an increase in power across most frequency bands in both the normal and parkinsonian conditions, and the sleep state PSDs in both conditions were similar (Fig. 3, A and B, top). In the STN and GPi, however, the effect of the sleep state on the 8- to 20-Hz frequency band differed between the normal and parkinsonian conditions. In both subjects, the sleep state in the normal condition was associated with an increase in power in the 8- to 20-Hz range in the STN and GPi, whereas in the parkinsonian condition the sleep state was associated with an overall decrease in power in the 8- to 20-Hz band (Fig. 3, upper and lower middle). In the GPe of animal K, elevated power in the frequency band centered at ∼200 Hz and associated with the parkinsonian condition was decreased during the sleep state (Fig. 3B). Statistical significance of the aforementioned differences in power is presented in the boxplots of Fig. 3C and summarized in Table 2.
Elevated PAC measurements emerged in the M1 of the two subjects during the sleep state in both the normal and parkinsonian conditions (Fig. 4, A and B, and Fig. A1, C and D). This sleep-related PAC in M1 was centered at ∼13.5 Hz for phase and ∼50 Hz for amplitude in both subjects. The mean preferred phase Φmax (standard deviation) associated with the sleep state in the normal condition was 280 (15) ° in subject J and 237 (12)° in subject K and in the parkinsonian condition was 280 (34)° in subject J and 271 (36)° in subject K. The frequency region of the sleep-related PAC was different from the PAC that emerged in the parkinsonian awake state of subject J (centered at a frequency for phase of ∼24 Hz and a frequency for amplitude of ∼50 Hz). Additionally, the preferred phase of the PAC associated with the sleep state was significantly different from that associated with the awake parkinsonian state of subject J (Φmax: sleep-parkinsonian = 280°, awake-park = 193°, P < 0.001; nonparametric test for equal medians, circular statistics).
PAC measurements associated with the awake parkinsonian condition decreased significantly in the sleep state for the M1 of subject J (Fig. 4, A and D) and the GPe of subject K (Fig. 4, C and E). Statistical significance of the aforementioned differences in PAC measurements across conditions and vigilance states is presented via boxplots in Figs. 4, D and E, and summarized in Table 2.
Dynamics of Oscillations and PAC
Power and MI measurements in the M1 of subject J and the GPe of subject K changed dynamically during transitions between the awake and sleep states as well as during periods in which these states remained constant. In subject J, during epochs of sleep in both the normal and parkinsonian condition, power in the 8- to 50-Hz band increased as compared with the power during epochs of wakefulness (see spectrograms and PACograms of Fig. 5, A and B). PAC (fl in the 8- to 20-Hz band and fh in the band between 50 − fl and 50 + fl Hz) increased during the epochs of sleep in both the normal and parkinsonian conditions. This result confirmed that elevated PAC measurements in the M1 emerged during the sleep state. PAC measurements associated with the parkinsonian condition (fl in the 20- to 30-Hz band and fh in the band between 50 − fl and 50 + fl Hz) were elevated during epochs in the resting awake state but exhibited fluctuations not captured in the static analysis. During the sleep state epochs, however, PAC associated with the parkinsonian condition was consistently suppressed. The preferred phases of PAC associated with the sleep state or the parkinsonian awake state were consistent when MI values were elevated but highly variable otherwise (Fig. 5, A and B, bottom). The temporal dynamics of PAC are further illustrated in Supplemental Movie S1 (Supplemental Material for this article can be found online at the Journal of Neurophysiology website), which shows M1 PAC comodulograms calculated with a sliding time window (0.2-s steps) during periods of wakefulness and sleep in animal J.
We emphasize here that if the data were not properly classified to be in the awake or sleep states, the interpretation of the results could have been different. To illustrate this, an example is presented in Fig. 5, C and D, in which average comodulograms were computed during a time period that included awake and sleep state epochs. In the parkinsonian condition, the comodulogram has elevated MI values in frequency regions associated with both parkinsonism and sleep.
In the GPe of subject K, the power in the 8- to 20-Hz and 160- to 240-Hz bands was higher in the parkinsonian awake than in the normal awake state (spectrograms in Fig. 6, A and B, top). The high-frequency spectrogram shows that power in the 160- to 240-Hz band, which was elevated in the parkinsonian condition, was suppressed during sleep state epochs. The low-frequency spectrogram shows that power in the 8- to 20-Hz frequency band increased in the normal condition during sleep. Power in this frequency band was persistently elevated in the parkinsonian condition and in fact decreased, rather than increased, during sleep state epochs (see also Fig. 3C, bottom middle). The observed power in the 8- to 20-Hz frequency band may reflect a coincident increase in sleep-related oscillations and a decrease in parkinsonian-related oscillations (similar to the decrease observed in parkinsonian-related high-frequency oscillations), resulting in a net decrease in oscillatory activity in this frequency range.
Fig. 6.
Temporal dynamics of power and PAC for the GPe (C4–5) of subject K in both normal (A) and parkinsonian (B) conditions during epochs of sleep and wakefulness. Top: time-frequency spectrograms showing the high-frequency (HF) and low-frequency (LF) bands that correspond to the frequencies for amplitude and phase, respectively, where maximum phase amplitude occurred. Lower middle: PACograms that illustrate the MI values (color scale) across frequencies for phase fl (vertical axis) over time (horizontal axis) and with fixed frequencies for amplitude (fh in 140–260 Hz). Lower middle and bottom: illustrations of how the preferred phase Φmax associated with MImax evolved over time.
The PACograms (Fig. 6, A and B) indicate that PAC in the GPe of subject K was negligible in the normal condition during both awake and sleep epochs. In the parkinsonian condition, PAC emerged (fl in 8–20 Hz and fh in 160–240 Hz) in the awake state epochs. During these awake epochs, the MI values and frequencies of maximum MI fluctuated. During the sleep state epochs, this PAC was consistently suppressed. Independent of the vigilance or pathological state, the phase associated with the maximum MI was consistent only during the epochs in which the MI values were elevated but variable and inconsistent otherwise (Fig. 6, A and B, bottom).
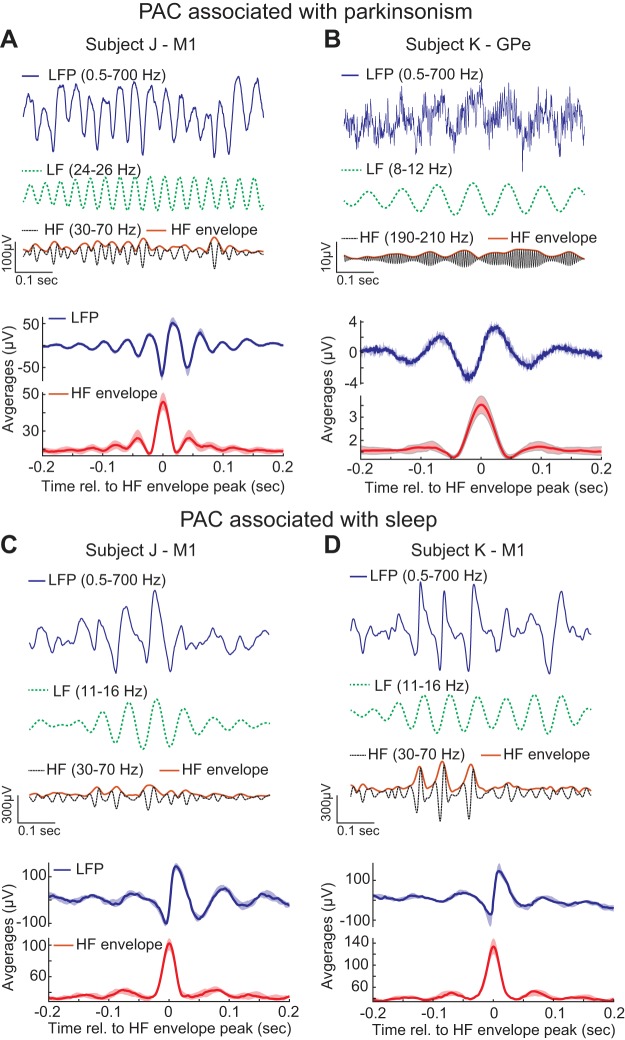
PAC and Signal Morphology
A known issue of PAC measurements is that they may not necessarily reflect true oscillatory coupling but rather result from nonsinusoidal oscillatory waveforms or sharp deflections in the raw LFP trace (Cole et al. 2017; Gerber et al. 2016; Jensen et al. 2017; Kramer et al. 2008; Kühn et al. 2009; Lozano-Soldevilla et al. 2016). To address this issue, LFP traces were examined along with event-related averages of the LFP data triggered by the peaks in the high-frequency amplitude envelope (Kramer et al. 2008).
In animal J, the M1 LFP traces and amplitude-triggered averages (Fig. 7A) in the awake state exhibited a sharp peak at the trough of the oscillation. Therefore, one possibility is that elevated PAC measurements in the M1 associated with parkinsonism could be the result of the LFP morphology but not true oscillatory coupling. It is also possible that the LFP traces resembled the superposition of oscillations at 24 Hz and oscillations at 50 Hz whose amplitudes were actually coupled to the phase of the 24-Hz oscillations.
Fig. 7.
Local field potential (LFP) signal morphologies associated with PAC measurements related to parkinsonism in the M1 of subject J (A) and GPe of subject K (B) as well as sleep in the M1 of subject J (C) and M1 of subject K (D). Raw LFP traces and their low- (LF) and high-frequency (HF) filtered components are shown from a selected recording session during periods with elevated PAC measurements along with amplitude-triggered averages of LFPs calculated from all data sets. The amplitude-triggered averages illustrate the shape of the LFP signal relative to the local maxima in the HF envelope (median and interquartile range shown).
In animal K, the LFP traces and amplitude-triggered averages (Fig. 7B) suggest that elevated PAC measurements in the GPe associated with parkinsonism were sinusoidal (10 Hz). Such a sinusoidal morphology indicates that elevated PAC measurements could be associated with true oscillatory coupling. The time series of the low- and high-frequency components also provide evidence of true oscillatory coupling, which is more likely to occur at a preferred phase (∼270°) but is not persistent over time.
The LFP traces associated with sleep-related PAC do exhibit nonsinusoidal morphologies (Fig. 7, C and D), which may be the cause of elevated PAC measurements during the sleep state. These waveforms are not artefactual or the apparent result of electrical or mechanical noise but instead likely reflect physiological processes arising in the sleep state. For example, sleep spindles associated with NREM sleep have characteristically sharp waveforms that occur in the same frequency range (11–16 Hz) (Iber 2007; Rechtschaffen and Kales 1968).
DISCUSSION
Effect of Parkinsonism on Oscillatory Activity in the STN, GP, and M1
The most consistent finding between animals was that low-frequency power (8–30 Hz) increased within the STN and GP after MPTP administration. This finding supports the hypothesis that β-oscillations within the basal ganglia are pathophysiological markers of parkinsonism (Brown et al. 2001; Brown 2003; Brown and Williams 2005; Giannicola et al. 2010) and are related to motor symptoms (Kühn et al. 2009). Based on these studies, increased β-power in the parkinsonian state might be expected. A novel aspect of the present study, however, is the use of neural recordings from chronically implanted electrodes across both normal and parkinsonian conditions, which are performed simultaneously across STN, GPi, and M1, providing a within-subject comparison that is unattainable in human patient studies. Our results provide support for the pathognomonic role of basal ganglia β-oscillations in parkinsonism, although no study has yet demonstrated a causal relationship between β-power and motor signs, and this hypothesis remains controversial. Although features of β-band activity correlate with clinical state in some studies (Kühn et al. 2006; Little and Brown 2012; Neumann et al. 2016), variability in β-power across subjects and little correlation with symptom severity are features of some human (Rosa et al. 2011; Weinberger et al. 2006) and animal studies (Connolly et al. 2015; Devergnas et al. 2014). In addition, β-oscillations have been shown to be present in dystonia patients, suggesting that these oscillations are not solely associated with Parkinson’s disease (Wang et al. 2016).
We found that M1 β-power was similar in the normal conditions of both animals but increased only in animal J after MPTP administration. These results suggest that elevated β-power is not necessarily observed across all nodal points in the motor circuit in the parkinsonian condition. An intriguing explanation for the difference in M1 neural activity between subjects arising after induction of parkinsonism is that the effects in M1 are not solely the direct result of basal ganglia dopaminergic cell loss but likely also reflect subject-specific compensatory mechanisms and cortical plasticity in response to the loss of dopamine in the BGTC network. Future studies with greater numbers of animals and chronic recordings of cell populations throughout the period of MPTP lesion and in the days and weeks thereafter will be necessary to better understand these changes in M1 activity.
Power in the 260- to 370-Hz range was elevated in the STN in the normal condition in both animals but disappeared in the parkinsonian condition. This finding supports the potential role of STN high-frequency oscillations (HFO) in normal brain functioning, as suggested by prior studies showing that PD patients with STN DBS leads had increased activity in that range after levodopa administration (Foffani et al. 2003, 2005). The cellular mechanisms underlying HFOs in the STN are not well understood. Although mean discharge rates have been reported to be increased in the parkinsonian state (Bergman et al. 1994; Miller and DeLong 1987), there are no studies that have identified a direct positive correlation between the rate of spiking activity, HFOs, or worsening of PD motor signs. Although Foffani et al. (2003) suggested that the origin of HFOs may involve complex mechanisms such as reverberating STN cell activity due to intrinsic membrane properties, synchronized phase-shifted recruitment of STN cells, or subthreshold fluctuations due to coordinated presynaptic input, what underlies these oscillations remains unclear.
Effect of Parkinsonism on PAC in the STN, GP, and M1
Elevated PAC in cortical and subcortical structures has been hypothesized to reflect abnormal information processing in the parkinsonian brain (Connolly et al. 2015; de Hemptinne et al. 2013, 2015; López-Azcárate et al. 2010; Özkurt et al. 2011). Here, we compare our results with previous studies reporting PAC in the STN, GP, and M1.
STN.
Studies in PD patients have found β-HFO PAC in the STN (Yang et al. 2014) and shown that it can be suppressed after levodopa administration (López-Azcárate et al. 2010; Özkurt et al. 2011), suggesting a relationship between STN PAC and motor symptoms in PD. In a more recent study, PAC was found in a subset of PD patients and was also observed in dystonia patients, highlighting intersubject variability and casting doubt on STN PAC as a biomarker specific to PD (Wang et al. 2016). Although we observed a modest increase in β-γ (70–140 Hz) PAC in one animal (animal K), we did not see an increase in the other. Thus, β-γ PAC was not a consistent feature in the STN in the parkinsonian state.
GP.
We found that PAC emerged in the GP of subject K after MPTP administration and was localized in the GPe and GPe/i border. These findings support a previous report by Connolly et al. (2015), that β-HFO coupling emerges in the parkinsonian state in the GP. Together, these results support the potential role of PAC in the GP in the pathophysiology of PD, although there were several differences between these studies. Our previous study found the phase of coupling to be highly variable between recordings and observed γ-HFO coupling in the normal and parkinsonian conditions, in the current study we found that PAC occurred at consistent phases and did not observe γ-HFO coupling. Methodological differences might account for these discrepancies given that in the previous study we used paired microelectrode recordings within the pallidum, with the location and distance between electrodes varying from day to day. Changes in PAC were not observed in the GP of the other animal (animal J) in the current study, which was possibly due to differences in electrode location.
M1.
We found a significant increase in β-γ PAC measurements in the M1 of one subject, consistent with recent findings suggesting elevated PAC in the M1 in PD (de Hemptinne et al. 2013, 2015) based on subdural electrocorticography recordings. We did not observe significant M1 PAC in the other subject. Intersubject variability is not always emphasized, but it is notable between patients in the aforementioned studies as well. Additionally, we observed a strong correlation between low-frequency power (8–20 Hz) and PAC MI, indicating that power may be a proxy measurement of PAC strength. This correlation, together with the sharp peaks in the LFP traces (Fig. 7), provides evidence that elevated PAC measurements in M1 associated with parkinsonism (and sleep) may be caused by the LFP morphology rather than true oscillatory coupling. Cole et al. (2017) reanalyzed the recordings presented in de Hemptinne et al. 2015 and suggested that the sharpness of sawtooth-like β-waveforms in LFP traces was the driver for increases in PAC measurements. Such sharp, nonsinusoidal waveforms are physiological and posited to reflect the synchrony of synaptic inputs (Cole et al. 2017, Sherman et al. 2016).
Overall, PAC measurements were not consistent features in any one nodal point (M1, STN, or GP) in the BGTC circuit, and additional studies are necessary with a larger cohort of animals to better characterize the consistency of PAC across structures and subjects. However, PAC measurements were elevated in at least one nodal point in each animal. Although one could argue that disruptions in signal processing in the motor circuit require alterations in only one nodal point, whether the presence of abnormal PAC measurements anywhere in the circuit is sufficient for development of the parkinsonian motor signs remains unclear.
Effects of Vigilance on Putative Biomarkers
Sleep was associated with significant increases in low-frequency power (<8 Hz) across STN, GP, and M1 in both the normal and parkinsonian conditions. In the normal condition, sleep was also associated with an increase in power in the 8- to 30-Hz range in the STN and GPi, whereas in the parkinsonian condition, sleep was associated with an overall decrease in power, albeit one still greater than in the normal condition (Fig. 3). It is notable that PAC observed in the awake parkinsonian condition in the M1 and GPe of subjects J and K, respectively, was also suppressed during sleep. A possible explanation of the decrease in 8–30 Hz power in the STN and GP and PAC in the M1 when asleep is the reduction of parkinsonian motor signs that has been observed during sleep (Askenasy 1993; Askenasy and Yahr 1990). An alternative explanation for PAC reduction is that PAC measurements associated with parkinsonism remain elevated during sleep, but phase measurements to compute PAC are degraded due to the superposition of low-frequency sleep waves with the pathological oscillations.
It is important to highlight that if M1 PAC were calculated during periods of sleep, it could be misinterpreted as being related to the subject’s parkinsonian condition. This is particularly relevant when considering parkinsonian subjects that often suffer from daytime sleepiness (Ondo et al. 2001; Tandberg et al. 1999) and resting state recordings that may be prone to include periods of somnolence. It is also notable that in both the parkinsonian-awake and normal-sleep conditions there was a marked increase in power in low-frequency bands across structures. These findings lead to the intriguing possibility that the awake parkinsonian brain is functioning in some ways similarly to the sleeping brain. Previous observations by our group of sleep-like bursting activity in the thalamus in awake parkinsonian animals (Elder and Vitek 2001) leads one to consider the potential role of these altered sleep-like patterns of activity in disrupting subcortical-cortical as well as cortical-cortical signal processing leading to the development of motor signs and alteration in sleep patterns reported in patients with PD (Ondo et al. 2001; Tandberg et al. 1999).
Although the effects of vigilance on oscillatory activity and PAC were statistically significant and validated via spectrograms and PACograms, we must acknowledge the large asymmetry between the number of recordings obtained in the awake and sleep state (see materials and methods). We were able to collect relatively few sleep compared with awake state data segments, particularly in the normal condition. Future studies using overnight wireless recording techniques in a larger number of animals will be needed to fully characterize the effects of sleep and particular non-REM and REM sleep stages on neural activity in the basal ganglia and motor cortex in the normal and parkinsonian conditions.
Temporal Dynamics
The average comodulogram and PSD reflect the preponderance of PAC and power that exists in the parkinsonian awake state, but the temporal dynamics, which may be relevant to understand the relationship between PAC and power and fluctuating motor symptoms, are lost upon averaging. Oscillatory activity and PAC are nonstationary phenomena whose measurements can fluctuate even within a given vigilance state, as illustrated in the PACograms (Figs. 5 and 6 and Supplemental Movie S1). How motor symptoms correlate with oscillatory activity and PAC over time is particularly relevant to gain a better understanding of PD pathophysiology but will require the development of techniques that are able to quantify individual motor PD symptoms in real time.
Movement preparation and execution can suppress oscillatory power and PAC in the motor circuit (Cassidy et al. 2002; de Hemptinne et al. 2015). According to our records, the PACograms and spectrograms illustrating the dynamics of oscillatory power and PAC in M1 and GPe (Figs. 5 and 6) were created using data sets in which the arm contralateral to the recording sites was not moving. Therefore, one could argue that the execution of arm movements was not influencing the fluctuations in oscillatory power and PAC. However, undetected muscle activation and movement preparation could influence these fluctuations. Future studies intended to assess the relationship between PAC fluctuations and symptoms should also characterize how muscle activation and movement planning and execution alter PAC measurements.
Implications for Biomarker-Based Closed-Loop DBS
Closed-loop control strategies have been proposed to increase the efficacy and energy efficiency of DBS therapy by adjusting stimulation based on pathophysiological biomarkers. In particular, the power of β-oscillations in the STN (Little and Brown 2012; Priori et al. 2013) and PAC in M1 (de Hemptinne et al. 2015) have been proposed for closed-loop DBS.
Low-frequency power measured from STN and DBS electrodes, which consistently increased after MPTP administration in the studied animals, is an attractive biomarker for use in closed-loop DBS because additional sensing electrodes may be unnecessary. Nevertheless, algorithms may still need to account for behavioral context. For example, β-triggered closed-loop DBS may be less effective than traditional DBS because of changes in movement-related β-activity (Johnson et al. 2016). Our data also suggest that the contribution of sleep to β-band oscillatory activity could affect how stimulation is delivered in a closed-loop scheme.
PAC measurements associated with the parkinsonian condition occurred in the M1 and GPe, but these locations were not consistent across subjects. This inconsistency highlights the notion that closed-loop DBS based on PAC may need to be subject specific, with relevant biomarkers varying according to each patient’s unique pathophysiology, symptom profile, or electrode location.
Closed-loop systems based on M1 PAC may also need to differentiate whether PAC measurements are associated with sleep or with parkinsonism. The frequency region and preferred phase of PAC could potentially be used to make this differentiation. The strong correlation between low-frequency power and PAC MI values in the M1 suggest that power could be used to quantify PAC strength and thereby reduce algorithmic complexity associated with the computation of PAC. Nevertheless, the preferred phase of PAC, which may be useful for closed-loop DBS therapies (Holt et al. 2016), cannot be quantified using the low-frequency oscillations only. Finally, we cannot discount the possibility that M1 PAC measurements are caused by nonsinusoidal LFP shapes (Fig. 7). If M1 PAC does not reflect true oscillatory coupling, it may be more appropriate to use features of low-frequency waveforms, such as waveform sharpness (Cole et al. 2017) or low frequency power, as biomarkers for closed-loop DBS.
Study Limitations
Differences in DBS lead placement, with GP DBS being more anteromedial and STN DBS being more dorsolateral in subject K, could have influenced the observed intersubject variability in the basal ganglia recordings. Given its level of severity, one subject (subject K) received daily doses of levodopa/carbidopa at the end of the experimental sessions (materials and methods). Although all recordings were taken after overnight withdrawal and the animal at a baseline parkinsonian state, we cannot exclude the possibility of the medication regimen influencing our findings in this animal. Another possible source of variability could be the difference in MPTP administration between animals, with subject J receiving intramuscular and intracarotid injections and subject K receiving only intramuscular injections. Additionally, the difference in severity of motor signs between subjects, with subject K more severe than subject J, could also be a source of variability in the data.
GRANTS
The research reported in this publication was funded by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (R01-NS077657, R01-NS037019, P50-NS098573), and University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) Initiative Postdoctoral Fellowships to D. Escobar and L. A. Johnson.
DISCLOSURES
J. L. Vitek serves as a consultant for Medtronic, Boston Scientific, and Abbott and serves on the scientific advisory board for Surgical Information Systems.
AUTHOR CONTRIBUTIONS
D.E., L.A.J., J.Z., M.D.J., K.B.B., and J.L.V. conceived and designed research; D.E., L.A.J., and S.D.N. analyzed data; D.E., L.A.J., K.B.B., G.F.M., and J.L.V. interpreted results of experiments; D.E. and L.A.J. prepared figures; D.E., L.A.J., and J.L.V. drafted manuscript; D.E., L.A.J., M.D.J., K.B.B., G.F.M., and J.L.V. edited and revised manuscript; D.E., L.A.J., S.D.N., J.Z., M.D.J., K.B.B., G.F.M., and J.L.V. approved final version of manuscript; L.A.J., S.D.N., and J.Z. performed experiments.
Supplementary Material
ACKNOWLEDGMENTS
Present address of K. B. Baker: Dept. of Neurosciences, Cleveland Clinic Foundation, Cleveland, OH 44195.
APPENDIX
An a priori statistical assessment of PAC comodulograms was performed to verify the inference reached via the post hoc statistical analysis presented in results. This assessment was performed using a cluster-based, nonparametric permutation test and the FieldTrip Toolbox (Maris and Oostenveld 2007; Oostenveld et al. 2011). Briefly, this test 1) generates a randomized partition of comodulograms based on the comodulograms associated with the conditions being compared, 2) computes a statistic (sum of t values) of connected MI measurements (clusters on the space of frequencies for phase and amplitude) whose levels are above threshold (99.75 quantile), 3) repeats steps 1 and 2 1,000 times and creates a histogram of the statistic, and 4) calculates a P value based on the histogram and the statistics of the clusters computed with the comodolugrams from the studied conditions. The result of the tests we carried out is a comodulogram with the largest cluster whose P value is less than the prescribed significance level (P <P 0.01, Bonferroni corrected for four comparisons). This statistical approach tackles the problem of multiple comparisons across frequencies for phase and amplitude without making assumptions on where PAC measurements changed across conditions.
The cluster-based, nonparametric statistical tests indicate that there were significant differences between measurements in the normal and parkinsonian condition in the M1 of subject J (Fig. A1A) and GPe of subject K (Fig. A1B). There was no significant difference between measurements in the normal and parkinsonian condition in the M1 of subject K and GP of subject J (data not shown). The tests we performed also indicated that there were significant changes in PAC measurements between the awake and sleep states in subjects J and K in the normal (tests not shown) and parkinsonian conditions (Fig. A1, C and D). Furthermore, the clusters that exhibited significant differences across conditions overlapped with the regions of interest selected for the post hoc analysis and presented in results.
The results from the a priori statistical analysis verify the findings we presented in results on the relationship between PAC measurements and parkinsonism and vigilance.
Fig. A1.
Nonparametric cluster-based statistical test to compare PAC measurements between conditions. Presented are comodulograms, with the largest cluster exhibiting significant differences in PAC measurements. Comodulograms in A and B are for comparisons between the normal and parkinsonian conditions (awake state) of measurements in the M1 of subject J and GPe of subject K, respectively. Comodulograms in C and D are for comparisons between the awake and sleep states (parkinsonian condition) of measurements in the M1 of both subject J and subject K, respectively. The color scale represents t-statistic values used to create the clusters and compute significant differences with level P < 0.01.
REFERENCES
- Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery 71: 804–814, 2012. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, Singer W, Vicente R. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol 31: 51–61, 2015. doi: 10.1016/j.conb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Askenasy JJ. Sleep in Parkinson’s disease. Acta Neurol Scand 87: 167–170, 1993. doi: 10.1111/j.1600-0404.1993.tb04095.x. http://www.ncbi.nlm.nih.gov/pubmed/8475684. [DOI] [PubMed] [Google Scholar]
- Askenasy JJ, Yahr MD. Parkinsonian tremor loses its alternating aspect during non-REM sleep and is inhibited by REM sleep. J Neurol Neurosurg Psychiatry 53: 749–753, 1990. doi: 10.1136/jnnp.53.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat : A MATLAB toolbox for circular statistics. J Stat Softw 31: 1–21, 2009. doi: 10.18637/jss.v031.i10. [DOI] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520, 1994. [DOI] [PubMed] [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. J Neurosci Methods 192: 146–151, 2010. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord 18: 357–363, 2003. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 21: 1033–1038, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol 116: 2510–2519, 2005. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain 125: 1235–1246, 2002. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Cole SR, van der Meij R, Peterson EJ, de Hemptinne C, Starr PA, Voytek B. Nonsinusoidal beta oscillations reflect cortical pathophysiology in Parkinson’s disease. J Neurosci 37: 4830–4840, 2017. doi: 10.1523/JNEUROSCI.2208-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly AT, Jensen AL, Bello EM, Netoff TI, Baker KB, Johnson MD, Vitek JL. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity. J Neurosci 35: 6231–6240, 2015. doi: 10.1523/JNEUROSCI.4137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci USA 110: 4780–4785, 2013. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, Starr PA. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 18: 779–786, 2015. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N, Hatsopoulos NG, Haga ZD, Parker RA, Greger B, Halgren E, Cash SS, Destexhe A. Avalanche analysis from multielectrode ensemble recordings in cat, monkey, and human cerebral cortex during wakefulness and sleep. Front Physiol 3: 302, 2012. doi: 10.3389/fphys.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiol Dis 68: 156–166, 2014. doi: 10.1016/j.nbd.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder CM, Vitek JL. The motor thalamus: alteration of neuronal activity in the Parkinsonian state. In: Basal Ganglia and Thalamus in Health and Movement Disorders. Boston, MA: Springer, p. 257–265, 2001. [Google Scholar]
- Foffani G, Ardolino G, Rampini P, Tamma F, Caputo E, Egidi M, Cerutti S, Barbieri S, Priori A. Physiological recordings from electrodes implanted in the basal ganglia for deep brain stimulation in Parkinson’s disease. the relevance of fast subthalamic rhythms. Acta Neurochir Suppl (Wien) 93: 97–99, 2005. doi: 10.1007/3-211-27577-0_16. [DOI] [PubMed] [Google Scholar]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E, Moxon KA, Cerutti S, Barbieri S. 300-Hz subthalamic oscillations in Parkinson’s disease. Brain 126: 2153–2163, 2003. doi: 10.1093/brain/awg229. [DOI] [PubMed] [Google Scholar]
- Gerber EM, Sadeh B, Ward A, Knight RT, Deouell LY. Non-sinusoidal activity can produce cross-frequency coupling in cortical signals in the absence of functional interaction between neural sources. PLoS One 11: e0167351, 2016. doi: 10.1371/journal.pone.0167351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Exp Neurol 226: 120–127, 2010. doi: 10.1016/j.expneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AB, Wilson D, Shinn M, Moehlis J, Netoff TI. Phasic burst stimulation: a closed-loop approach to tuning deep brain stimulation parameters for Parkinson’s disease. PLOS Comput Biol 12: e1005011, 2016. doi: 10.1371/journal.pcbi.1005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh KC, Robinson EL, Fuller CA. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep 31: 1239–1250, 2008. [PMC free article] [PubMed] [Google Scholar]
- Iber C. The AASM Manual for the Scoring of Sleep and Associated Events: rules,Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- Jensen O, Spaak E, Park H. Discriminating valid from spurious indices of phase-amplitude coupling. eNeuro 3: 3, 2017. doi: 10.1523/ENEURO.0334-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB, Vitek JL. Closed-loop deep brain stimulation effects on Parkinsonian motor symptoms in a non-human primate - is beta enough? Brain Stimulat 9: 892–896, 2016. doi: 10.1016/j.brs.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Swann N, De Hemptinne C, Miocinovic S, Miller A, Starr PA, Carmena JM. Neurofeedback control in parkinsonian patients using electrocortigraphy signals accessed wirelessly with a chronic, fully implanted device. IEEE Trans Neural Syst Rehabil Eng, 2016. doi: 10.1109/TNSRE.2016.2597243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Tort ABL, Kopell NJ. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods 170: 352–357, 2008. doi: 10.1016/j.jneumeth.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci 23: 1956–1960, 2006. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider G-H, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 215: 380–387, 2009. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci 1265: 9–24, 2012. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, FitzGerald J, Green AL, Aziz TZ, Brown P. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: 449–457, 2013. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci 30: 6667–6677, 2010. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Soldevilla D, Ter Huurne N, Oostenveld R. Neuronal oscillations with non-sinusoidal morphology produce spurious phase-to-amplitude coupling and directionality. Front Comput Neurosci 10: 87, 2016. doi: 10.3389/fncom.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Maynard EM, Nordhausen CT, Normann RA. The Utah intracortical Electrode Array: a recording structure for potential brain-computer interfaces. Electroencephalogr Clin Neurophysiol 102: 228–239, 1997. doi: 10.1016/S0013-4694(96)95176-0. [DOI] [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: The Basal Ganglia II. Advances in Behavioral Biology, edited by Carpenter MB, and Jayaraman A. Boston, MA: Springer, 1987, p. 415–427. [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl (Wien) 97: 561–567, 2007. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Degen K, Schneider G-H, Brücke C, Huebl J, Brown P, Kühn AA. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord 31: 1748–1751, 2016. doi: 10.1002/mds.26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson’s disease. Neurology 57: 1392–1396, 2001. doi: 10.1212/WNL.57.8.1392. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wojtecki L, Schnitzler A. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol 229: 324–331, 2011. doi: 10.1016/j.expneurol.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Rossi L, Marceglia S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 245: 77–86, 2013. doi: 10.1016/j.expneurol.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: National Institute of Neurological Diseases and Blindness, Neurological Information Network, 1968. [Google Scholar]
- Rosa M, Arlotti M, Ardolino G, Cogiamanian F, Marceglia S, Di Fonzo A, Cortese F, Rampini PM, Priori A. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord 30: 1003–1005, 2015. doi: 10.1002/mds.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, Sassi M, Scelzo E, Barbieri S, Priori A. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson’s disease in hyperacute and chronic phases. Neurosignals 19: 151–162, 2011. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng 20: 413–422, 1992. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- Sherman MA, Lee S, Law R, Haegens S, Thorn CA. Hämäläinen MS, Moore CI, Jones SR. Neural mechanisms of transient neocortical beta rhythms: converging evidence from humans, computational modeling, monkeys, and mice. Proc Natl Acad Sci USA 113: E4885–E4894, 2016. doi: 10.1073/pnas.1604135113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Ostrem JL, Galifianakis NB, Luciano MS, Wang SS, Ziman N, Taylor R, Starr PA. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson’s disease. J Neurosurg 1–12, 2017. doi: 10.3171/2016.11.JNS161162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson’s disease: a community-based study. Mov Disord 14: 922–927, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Tort ABL, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104: 1195–1210, 2010. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokos C, Hu X, Pouratian N. 200–300Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s Disease. Neurobiol Dis 54: 464–474, 2013. doi: 10.1016/j.nbd.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, de Hemptinne C, Miocinovic S, Qasim SE, Miller AM, Ostrem JL, Galifianakis NB, San Luciano M, Starr PA. Subthalamic local field potentials in Parkinson’s disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol Dis 89: 213–222, 2016. doi: 10.1016/j.nbd.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol 96: 3248–3256, 2006. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Yang AI, Vanegas N, Lungu C, Zaghloul KA. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson’s disease. J Neurosci 34: 12816–12827, 2014. doi: 10.1523/JNEUROSCI.1895-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.