The part of the motor cortex involved in essential tremor is uncertain. The current electrophysiological study is the first to assess corticomuscular coherence systematically. The study shows a dynamic nature of corticomuscular coherence and a possible influence of cognitive states. The results elucidate the involvement of the motor cortex in tremor and help interpret the varying results in the literature. In clinical practice, the findings may guide in standardizing tremor registration and its interpretation.
Keywords: essential tremor, EEG, EMG, coherence, pathophysiology, tremor registration
Abstract
Cortical involvement in essential tremor, an involuntary action tremor supposedly of subcortical origin, is uncertain. Conflicting results of corticomuscular coherence studies in essential tremor suggest an intermittent corticomuscular coupling. On the basis of the literature, we hypothesized that corticomuscular coupling is influenced by bilateral motor synchronization and “cognitive states” such as awareness of tremor. In the present study, we investigated 1) the existence of intermittent corticomuscular coherence (CMC) in essential tremor and 2) factors that influence CMC strength. In 18 essential tremor patients and 18 healthy controls, who mimicked tremor, we simultaneously recorded 64-channel EEG and 6-channel bipolar surface EMG from right and left wrist extensors and flexors. Right-sided (mimicked) hand tremor was recorded with and without a cognitive arithmetic task and with left-sided (mimicked) hand tremor. CMC values per task were compared within and between groups. Changes in CMC strength during tasks were calculated. Our main findings are 1) significant CMC around the (mimicked) tremor frequency across all tasks in both groups; 2) significant differences in CMC between unilateral tasks, with the highest values during the cognitive task only in the essential tremor group; and 3) significant fluctuations of CMC strength over time, independent of the tremor intensity, only in the essential tremor group. Our results suggest a limited role, and certainly not a continuous steering role, of sensorimotor cortical neurons in the generation of tremor. In clinical practice, these findings might help to standardize tremor registration and the interpretation of the analysis.
NEW & NOTEWORTHY The part of the motor cortex involved in essential tremor is uncertain. The current electrophysiological study is the first to assess corticomuscular coherence systematically. The study shows a dynamic nature of corticomuscular coherence and a possible influence of cognitive states. The results elucidate the involvement of the motor cortex in tremor and help interpret the varying results in the literature. In clinical practice, the findings may guide in standardizing tremor registration and its interpretation.
essential tremor is one of the most common movement disorders. However, its pathophysiology is still under debate (Hallett 2014). Pathological oscillations within the olivo-cerebello-thalamo-cortical network are believed to cause the 4- to 12-Hz bilateral postural tremor of the hands during action (Pedrosa et al. 2012; Raethjen et al. 2007). Within this tremor network, the cerebellum is considered to play a key role in the pathophysiology (Hopfner et al. 2016). More upstream, the role of the cortex is unclear. In the literature it ranges from no involvement to facilitating or even playing a role in tremor generation (Cerasa and Quattrone 2016; Hopfner et al. 2016; Sharifi et al. 2014). Corticomuscular coherence (CMC) reflects the coupling between sensorimotor cortical activity and tremor activity in muscles. It detects to which extent electroencephalography (EEG) and electromyography (EMG) signals have the same frequency and consistent phase difference. Although CMC analysis in essential tremor is thought to be well known, results of CMC studies are diverse, reporting significant CMC only in a selection of patients (Hellwig et al. 2001; Raethjen et al. 2007; Schnitzler et al. 2009) or suggesting the involvement of the cortex not to be robust within patients (Raethjen et al. 2007) (Table 1). Differences in patient selection, recording methods and analysis techniques, type of motor tasks, and possibly cognitive state (e.g., awareness of tremor) might (partly) explain the inconsistent results (Hellwig et al. 2003; Koller and Biary 1989). Bilateral tremor evoked by a bilateral motor task has been associated with a dynamic interhemispheric synchronization of cortical motor areas (Hellwig et al. 2003). A bilateral task might therefore influence CMC, which is not the case during a simple unilateral task. Alternatively, an interruption of CMC can indicate other, non-tremor-related, EEG oscillations overpowering pathological tremor oscillations. A better understanding of the influence of these factors on the cortical involvement in tremor mechanisms will form a basis for future electrophysiological studies on tremor generation and standardized tremor registration in essential tremor.
Table 1.
CMC studies in literature
| Reference | Study Type | No. of ET Subjects | Patient Selection | Significant CMC |
|---|---|---|---|---|
| Halliday et al. (2000) | MEG-EMG | 6 | Postural tremor; 4/6 drug resistant | No |
| Hellwig et al. (2001) | EEG-EMG | 7 | Postural tremor | 5 of 9 arms, some during CT |
| Hellwig et al. (2003) | EEG-EMG | 8 | Postural tremor | 42.6% contralateral, 21.6% bilateral |
| Raethjen et al. (2007) | EEG-EMG | 15 | ET criteria; 4/15 family history | Yes |
| Schnitzler et al. (2009) | MEG-EMG | 8 | Postural tremor | Yes |
| Muthuraman et al. (2012) | EEG-EMG | 10 | ET criteria | Yes |
CMC, corticomuscular coherence; CT, cognitive task; EEG, electroencephalography; EMG, electromyography; ET, essential tremor; MEG, magnetoencephalography.
We studied the effects of different motor tasks and different cognitive states on the possible intermittent trait of CMC in essential tremor. We studied a large homogeneous group of patients with propranolol-sensitive essential tremor. The findings were compared with those in age-matched healthy controls who mimicked tremor to correct for physiological effects of different tasks and the temporal trait of CMC. We expected to find CMC in essential tremor patients around the tremor frequency during unilateral tremor manifestation and hypothesized that its nature would be influenced by a bimanual motor task or a cognitive task.
MATERIALS AND METHODS
Participants.
In this study, we recruited 18 patients with essential tremor (4 women; mean age 59.4 ± 17.0 yr) according to the criteria of the Tremor Investigation Group (Bain et al. 2000) and 18 age-matched healthy controls (8 women; mean age 57.1 ± 15.0 yr). All subjects were older than 18 yr and were right-handed. Patients had a positive family history and a positive response to propranolol and alcohol. Patients showed bilateral postural arm tremor and no other neurological disorders, especially no dystonia. Tremor medication was discontinued at least 3 days before the study. Patients were scored with the essential tremor rating assessment scale (TETRAS) by an experienced movement disorders neurologist (JDS), blinded for clinical details and study results, from a standardized video (Elble et al. 2012). The clinical characteristics of all essential tremor patients are summarized in Table 2. All subjects gave their written informed consent. The study was performed in accordance with the Declaration of Helsinki with the approval of the Medical Ethical Committee of the Academic Medical Center, Amsterdam, and conforms to the Dutch Act on Medical Research Involving Human Subjects (WMO) and the Standard EN ISO 14155:2011 “Clinical investigation of medical devices for human subjects – Good clinical practice.”
Table 2.
Clinical characteristics of essential tremor patients, all with positive family history
| Subject No. | Sex | Age, yr | Tremor Frequency, Hz | Tremor Onset Age, yr | TETRAS Score | Medication |
|---|---|---|---|---|---|---|
| 1 | F | 22.5 | 7.8 | 19 | 15 | |
| 2 | M | 26.4 | 7.8 | Childhood | 11 | Propranolol (3×80 mg) |
| 3 | M | 47.5 | 4.6 | 40 | 18 | Propranolol (2×80 mg) |
| 4 | M | 49.7 | 7.9 | Childhood | 13.5 | Propranolol (30 mg) |
| 5 | F | 50.0 | 6.6 | Childhood | 14 | Propranolol (3×40 mg), Primidone (2×12.5 mg) |
| 6 | M | 53.3 | 6.8 | 50 | 13 | |
| 7 | M | 53.4 | 6.3 | 16 | 11.5 | Propranolol (40 mg) |
| 8 | M | 55.1 | 7.8 | Childhood | 16.5 | Propranolol (3×10 mg) |
| 9 | M | 60.4 | 6.0 | 15 | 9 | Propranolol (80 mg) |
| 10 | M | 63.0 | 6.7 | 43 | 13 | Propranolol (40 mg if necessary) |
| 11 | M | 63.7 | 7.5 | 18 | 24 | Propranolol (240 mg), Primidone (125 mg) |
| 12 | M | 64.3 | 6.6 | 20 | 16 | Propranolol (2×40 mg) |
| 13 | M | 69.4 | 7.8 | 61 | 13.5 | |
| 14 | M | 72.1 | 6.2 | 60 | 19.5 | Propranolol (20 mg) |
| 15 | M | 72.5 | 5.3 | 13 | 28 | Propranolol retard (2×80 mg) |
| 16 | F | 80.8 | 5.8 | 60 | 31.5 | Primidone (250 mg), Propranolol (40 mg) |
| 17 | F | 81.4 | 5.1 | 20 | 34 | |
| 18 | M | 84.5 | 4.8 | 33 | 36 | Propranolol (3×80 mg) |
| Mean | 59.4 (±17.0) | 18.7 (±8.3) |
F, female; M, male; TETRAS, The Essential Tremor Rating Assessment Scale (OFF medication, maximum score 52). Medication was discontinued at least 3 days before the study.
Tasks.
Participants were asked to fix their eyes at one point at a distance of 2 m while sitting on a bed with supported back and head. Tasks included 1) keeping both arms outstretched (BAO), 2) keeping the right arm outstretched (RAO), and 3) keeping the right arm outstretched with a cognitive arithmetic task (CT). The cognitive task was a simple arithmetic operation of subtracting whole numbers starting with 100 minus 7. Healthy controls performed the same tasks but were asked to mimic the tremor by performing fast self-paced rhythmic movements with their hand(s). While arms were outstretched, the palm of the hand faced the floor. As a rule, we collected at least 2 min of data per task free from movement artifacts. For the cognitive task, which was most burdensome, an artifact-free data set of around 1 min was collected. To prevent fatigue, task periods of maximally 1 min were alternated with periods of rest. Two data sets were prepared: one with maximal length, to increase the signal-to-noise ratio, and one where all data sets were shortened according to the shortest data set (1 min), to rule out differences as a result of the data set length.
Recordings.
We simultaneously recorded 64-channel EEG with a cap and 6-channel bipolar surface EMG (Twente Medical Systems International, Oldenzaal, The Netherlands). The EMG electrodes were placed on the wrist flexor (m. flexor carpi radialis), on the wrist extensor (m. extensor carpi ulnaris), and on the thumb adductor (first dorsal interosseus). Horizontal and vertical eye movements were monitored with four extra electrodes. Heart rate was registered with an extra bipolar electrode. The sampling rate was 2,048 Hz for all channels.
Data preprocessing.
The EEG signals were filtered digitally offline in MATLAB (The MathWorks, Natick, MA) with a bandpass filter (1–250 Hz; 4th-order Butterworth, no phase shift). To identify focal patterns of specific cortical activity from the monopolar EEG derivations, we composed reference-free derivations according to the Hjorth method (Hjorth 1991). All data were downsampled to 512 Hz after filtering. Eye movement, heartbeat, muscle, and line noise artifacts were removed before the analysis with independent component analysis (ICA). With the use of the Infomax ICA algorithm, the data were disentangled in independent components that could be removed from the total signal by minimizing mutual information among the data (Delorme and Makeig 2004). In addition, the data were visually inspected to warrant comparable high-quality EEG for both groups.
EMG signals were filtered digitally offline in MATLAB environment on the basis of a common procedure for tremor with a bandpass filter (52–750 Hz; 8th-order Butterworth, no phase shift) and subsequently full-wave rectified to extract oscillatory envelope modulation from surface EMG (Timmer et al. 1998). The EMG channel with the best visible tremor peak in the power spectral density signal (PSD) estimated according to Welch’ s method was selected for further analysis.
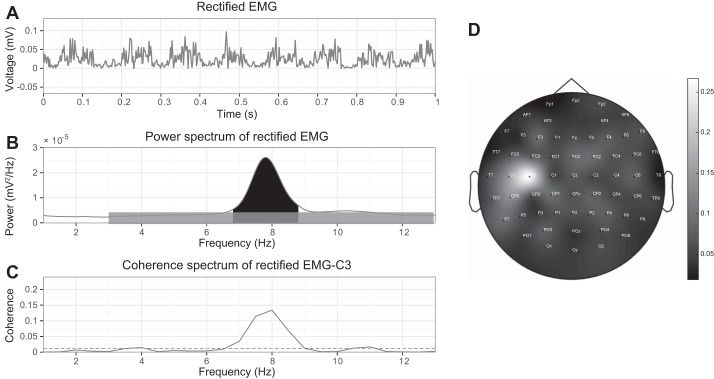
Coherence around the tremor frequency necessitates the presence of tremor power in EMG. Because CMC might negatively be influenced by superimposed noise in the frequency band of interest, we were interested in the tremor power as well as the noise level in the EMG around frequencies of interest. To investigate tremor severity on the basis of EMG recordings, we chose to use tremor signal-to-noise ratio (SNR). The tremor SNR reflects tremor severity while accounting for noise in the same frequency band. It was defined as the ratio between the signal of interest (in this case, the tremor power) and the underlying noise power in the PSD (Fig. 1 and Eq. 1) (Hellwig et al. 2001; Timmer et al. 1996):
| (1) |
where EMGpxx(f) is the power at each frequency bin and tf is the (mimicked) tremor frequency. Tremor power was defined as the area under the PSD curve at the tremor peak tremor frequency ±1 Hz, divided by the bandwidth (2 Hz). The noise power in the frequency band of interest was calculated using the median of the noise powers between 3 and 13 Hz that led to a stable noise estimation. The median and this specific frequency band were chosen to minimize an influence of the tremor peak and possible second harmonic peaks on noise power calculation. The variance of tremor power and noise were calculated separately to appoint the variance of tremor SNR to either variable. We chose to use SNR over a normalized tremor peak to distinguish the difference between healthy controls and essential tremor patients. If intermittencies in the CMC would occur simultaneously with a drop in SNR, this could indicate that the intermittencies are caused by a loss in tremor power or increase in EMG noise.
Fig. 1.
Representative examples in an essential tremor patient with right arm outstretched, inducing tremor, and recordings from right wrist extensor muscles and EEG showing 1-s filtered and rectified EMG revealing a tremor pattern (A) and a power spectrum of 3 min of rectified EMG (B). Tremor SNR is defined as the ratio between the tremor peak (black) and noise (gray). C: coherence between cortex (C3) and EMG with horizontal dashed line indicating the confidence level. D: topographic distribution of the CMC in a 2-dimensional circular view between right-sided wrist extensor muscle around tremor frequency (6.8–8.8 Hz) and cortical Hjorth derivations, using biharmonic spline interpolation for smoothing. The maximum CMC was predominantly found in the contralateral left sensorimotor cortex.
Corticomuscular coherence analyses.
The EEG and EMG recordings were segmented into nonoverlapping 2-s epochs. For each epoch, the Fourier transform was computed using a Hanning window; these were subsequently averaged, and coherence was calculated (frequency resolution 0.5 Hz) with the use of the NeuroSpec toolbox (http://www.neurospec.org/) (Halliday et al. 1995). Coherences between rectified EMG signals of the arm muscles and the EEG signals (Hjorth derivations) over the sensorimotor areas (CP3, C3, FC3) were calculated around the tremor frequency (3–13 Hz). The EEG channel that revealed the strongest coherence over the sensorimotor cortex in combination with the selected contralateral EMG (best visible tremor peak in PSD) was used for further analyses. The confidence limit for statistical significance of coherence was set at 95% and was determined by the number of epochs used for the spectral estimation (Halliday et al. 1995). The CMC value, ranging from 0 to 1, expresses the co-occurrence of oscillations for each frequency over time. We visually controlled for inappropriate spatially widespread coherence patterns due to tremor contamination to the EEG signals. We estimated the corticomuscular phase to assign the time delay between the corticomuscular coupling and provide information on directionality. Phase was defined as the argument of the cross spectrum. The phase estimation was only performed if there were at least four adjacent significant data points (2 Hz) at which coherence was significant and regression of the slope was reliable (Hamon and Hannan 1974; Timmer et al. 2000).
Corticomuscular coherence between groups and within tasks.
To statistically compare CMC between the three different tasks, we performed repeated-measures ANOVA on the CMC values between groups and within tasks (α = 0.05). An important issue is that statistical comparison of coherences is troublesome with unequal sample sizes because these are biased depending on the sample size. For this reason, the comparison of CMC values between tasks was performed after every task was shortened to the length of the shortest task, which resulted in a data set of 1 min. Before performing statistical testing, we performed a variance-stabilizing transformation [z = atanh(√coherence)] (Rosenberg et al. 1989). A difference in CMC over the three tasks (main effect for the task) was tested. Also, the CMC values between the two groups (main effect for the group) was statistically compared. Finally, we tested whether the change in CMC over the three tasks was different for the two groups (interaction effect). In case of significant within-subject main effects, we conducted post hoc pairwise comparison.
Corticomuscular coherence over time.
To capture coherence variations over time, we applied a 30-s moving window with 90% overlap (Raethjen et al. 2007). Within this window, we performed the same CMC analyses as described above, dealing with equal-size windows over which CMC was calculated. This method, to determine the intermittent behavior of CMC, was simulated by Raethjen et al. (2007) and shown to be reliable. To confirm the correctness of the dynamic CMC methodology, we investigated the difference of the coherence values over time for both essential tremor patients and healthy controls. Healthy controls performing purposefully induced movements, likely to be driven via the motor cortex, were expected to reveal high CMC values as appeared in previous studies (Muthuraman et al. 2012). Therefore, CMC values in healthy controls were expected not to fall below a statistically significant confidence level.
Intermittency of the CMC was quantified by using the percentage of epochs that showed significant coherence. We used between 16 and 76 epochs from each recording depending on the quality of the data and length of the recording. Intermittency was defined as the percentage of the total amount of epochs that showed significant coherences in the tremor frequency band over the selected central electrode during the entire measurement.
Intermittent tremor activity is one of the main factors that could explain CMC variation over time. With the help of a linear regression analysis, we investigated the amount of variation in z-transformed CMC explained by variation in tremor SNR. For this, we pooled all epochs of the three tasks regardless of the functional tasks. We performed a linear mixed effects analysis of the relationship between CMC and SNR with the use of R (R Core Team 2016) and lme4 (Bates et al. 2012). Linear mixed-effects model was appropriate in our case because it can describe the relationship between SNR and CMC, accounting for individual distribution of individual participants. As fixed effects, we entered tremor SNR into the model. As random effects, we accounted for individual by-participant variation in intercepts. P values were obtained by likelihood ratio tests of the full model with the effect in question against the model without the effect in question.
RESULTS
The participants did not experience difficulties in the execution of the tasks. The length of measurements varied between 1 and 3 min. After artifact removal EEG quality in both groups was similar. No data sets had to be removed, and therefore we used the total amount of epochs derived from each task. For both groups, we used 16 epochs for the cognitive tasks. On the remaining 2 tasks, we used 76 epochs and 46 epochs in the essential tremor group and the healthy controls, respectively. For statistical comparison of CMC values between tasks, equal sizes of 1-min recordings (16 epochs) were selected. The EEG-EMG results of the included subjects are summarized in Table 3. The spectral analysis of the surface EMG revealed the (mimicked) tremor frequency in patients (Fig. 1) and healthy controls. The (mimicked) tremor frequency did not differ significantly between tasks. Tremor frequency was constant within the essential tremor group during and between tasks. Healthy controls only showed a slight decrease in tremor frequency during the cognitive task. In essential tremor, 10 patients revealed the best distinguishing tremor peak, derived from the PSD, in the electrode placed on the wrist extensor. For six participants, the electrode placed on the thumb adductor showed the best tremor peak, and for two patients, this was the electrode placed on the wrist flexor. In healthy controls, the tremor peak was best detectable first in the electrode placed on the wrist extensor (n = 14) and second on the wrist flexor and thumb adductor (both n = 2). The selection of EMG channel and the EEG electrode did not systematically differ between groups or conditions. Tremor SNR in patients was significantly lower compared with the strong voluntary tremor-like movements in healthy controls (2-tailed 1-sample t-test, P < 0.05; Table 3). Tasks did not have a significant effect on tremor SNR. EMG noise levels did not differ significantly between groups. The amount of variation of the tremor power in both groups was significantly greater than the amount of variation in noise, indicating that the variability in tremor SNR is predominantly caused by variability in tremor power.
Table 3.
EEG-EMG group results
| Measure | Task | Essential Tremor | Healthy Controls |
|---|---|---|---|
| Tremor frequency, Hz | BAO | 6.6 (1.2) | 5.3 (1.2)* |
| RAO | 6.5 (1.1) | 5.3 (1.2)* | |
| CT | 6.6 (1.2) | 5.0 (1.1)* | |
| Tremor SNR | BAO | 2.7 (0.9–19.7) | 8.4 (2.2–29.0) |
| RAO | 3.2 (1.0–23.2) | 7.9 (1.6–27.2) | |
| CT | 2.6 (1.0–20.7) | 8.4 (2.1–21.5) | |
| Epochs with significant CMC, % | BAO | 31 (15–100) | 100 (43–100)* |
| RAO | 35 (10–86) | 100 (43–100)* | |
| CT | 59 (13–100) | 100 (77–100)* |
BAO, both arms outstretched; CL, confidence level; CMC, corticomuscular coherence; CT cognitive task; RAO right arm outstretched; SNR, signal-to-noise ratio. Continuous data are either means (SD) or meadian (range).
P < 0.05; statistical significance between groups was assessed with Student’s t-test or the Mann-Whitney U-test.
Corticomuscular coherence.
In all essential tremor patients, the CMC between the selected contralateral EEG electrode covering the sensorimotor areas (C3, FC3, or PC3) and selected arm muscle (Fig. 1) was significant around tremor frequency during one or more tasks. All healthy controls mimicking tremor revealed significant CMCs around the mimicked tremor frequency during every task. There was a substantial main effect for CMC between groups [F(1,34) = 25.49, P < 0.05, partial η2 = 0.43]. CMC in healthy controls was significantly stronger than in essential tremor patients. Phase analyses did not yield a reliable delay between cortex and muscles in both groups because of the narrow significant frequency band.
Effect of tasks on corticomuscular coherence.
As shown in Fig. 2, the mean CMC values of the three different tasks were found to be slightly different. A repeated-measures ANOVA was conducted to assess the difference in CMC of essential tremor and mimicked tremor in healthy controls, and the impact of the various tasks. Mauchly’s test indicated the assumption of sphericity not to be violated, and therefore the repeated-measures ANOVA did not require any degrees of freedom corrections. However, more stringent corrections did not change any significant results. There was a moderate main effect for the task [F(2,68) = 3.33, P < 0.05, partial η2 = 0.09] with mainly the essential tremor group influenced by the tasks (Fig. 2). This indicated a significant difference in coherence across the different tasks. Post hoc t-tests revealed that, in essential tremor, the cognitive task showed significantly stronger CMC compared with the unilateral motor task (P = 0.03). Furthermore, there was no interaction effect between the groups and the different tasks.
Fig. 2.
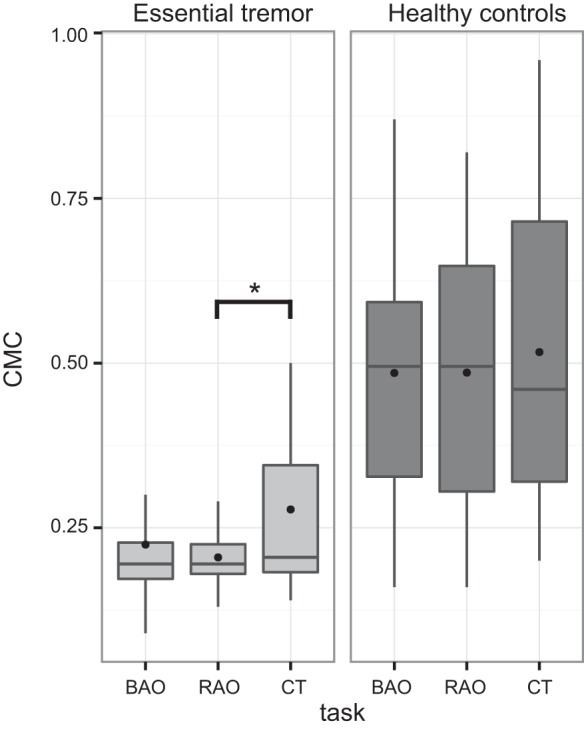
Box plot of z-transformed CMC calculated during 1-min recordings, depicting the spread (without showing the outliers), mean (filled circle), and median (line), with group differences between essential tremor and healthy controls during 1) both arms outstretched (BAO), 2) right arm outstretched (RAO), and 3) cognitive task (CT) and a between-task difference in the essential tremor group between RAO and CT (*P < 0.05).
Intermittent behavior of corticomuscular coherence.
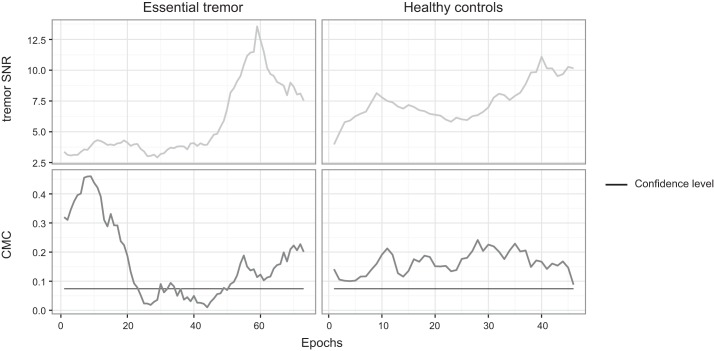
The dynamic analysis showed that the CMC in both groups varied during one recording. In healthy controls, having purposefully induced movements, the corticomuscular coherence values varied over time but remained relatively high throughout the entire recording and did not fall below the confidence level. This indicated that the methodology used for dynamic corticomuscular coherence analysis was valid.
In the essential tremor group, the median percentages of epochs showing significant CMC indicated intermittent CMC in all tasks (Table 2; example Fig. 3). For example, during the unilateral measurement, 35% (median) of the epochs revealed significant CMC values in the essential tremor group. Coherence levels frequently dropped below the confidence level regardless of task. Thus the intermittency analyses in essential tremor indicated that coherence did not persist during the entire measurement.
Fig. 3.
Dynamic CMC in one essential tremor patient while the right arm was outstretched during a 3-min measurement (left) and in one healthy control during a 2-min measurement (right). Top graphs show that the tremor SNR during each segment in both cases is clearly detectable (SNR > 2.5). In bottom graphs, the CMC of each 30-s window (2-s steps) is depicted with its confidence level. From these graphs it can be appreciated that the CMC drop below the confidence level in essential tremor is not due to insufficient tremor SNR. At the same time, high CMC values can be reached without very high tremor SNR (essential tremor, first epochs) and that very high tremor SNR does not guarantee high CMC values (essential tremor, last epochs).
To investigate the relationship between CMC and tremor SNR, the assumptions for linear regression had to be met. The analysis of residuals confirmed the assumption of linearity. In healthy controls, the linear regression model with the independent variable tremor SNR and the dependent variable CMC showed a reasonable relationship [χ2(1) = 112.6, P < 0.001 with R2 = 0.54]. In essential tremor, the linear regression model was valid, but the outcomes indicate that the prediction of CMC based on SNR is poor [χ2(1) = 92.1, P < 0.001 with R2 = 0.37]. Therefore, only 37% of the variation in CMC variation can be explained by variation in SNR. Also, when CMC values are approached not as continuous values but as dichotomous, both significant and not significant epochs show high and low tremor SNR (Fig. 4). In other terms, the intermittent phenomenon of CMC, present only in the essential tremor group, cannot be explained by an accompanied rise or drop in tremor SNR (Figs. 3 and 4).
Fig. 4.
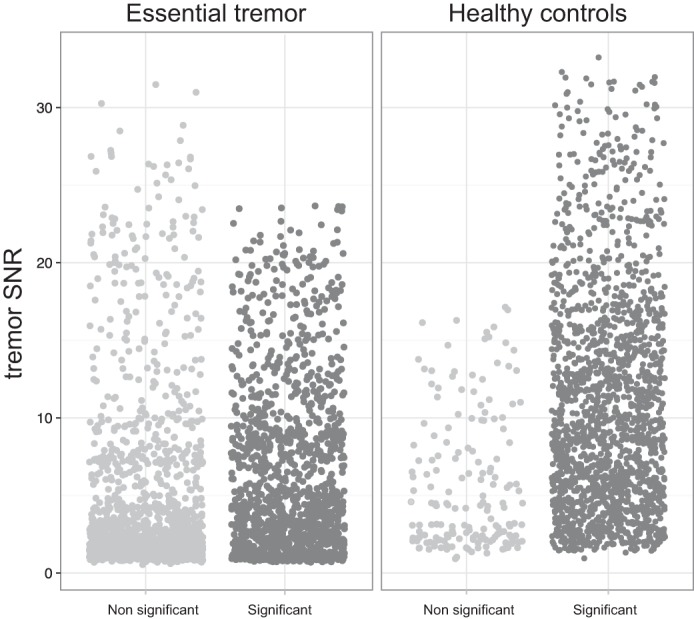
Jittered plot of all individual epochs of every subject grouped as not significant and significant CMC epochs. The relationship between the tremor SNR is set against the CMC significance (>0.073). The data at right illustrate that in essential tremor, both significant and not significant epochs show high and low tremor SNR.
DISCUSSION
In the present study, CMC analyses in a homogenous essential tremor group showed an intermittent coupling of the motor cortex with the tremulous muscle: CMC intermittency. We also found a moderate effect of cognitive load on the overall strength of CMC, but the task did not have an effect on the intermittency. CMC strengths depend on the power at the tremor frequency compared with additional power such as noise in EMG and EEG and the stability of phase difference between these signals. We have shown that variation in tremor SNR is mostly due to a variation of the tremor power peak, concluding that tremor SNR truly reflects tremor severity and not noise variations. These results may explain the diversity of outcomes in previous studies. Furthermore, with the use of the linear regression model, we pointed out a relatively poor underlying relationship between CMC and tremor SNR in essential tremor. CMC is barely dependent on tremor power measured by EMG. Instead, because of the continuous tremor as reflected in EMG power, CMC may be dependent on variations of another influencing factor, i.e., significant variation of phase-locked tremor power over the sensorimotor cortex. Because EEG measures were thoroughly inspected after artifact removal, we are confident that the quality remained the same during measurements and between groups. Note that cortical EEG activity associated with tremor is immersed in the total dynamic EEG activity, and power variation in the tremor frequency is not solely due to variation in tremor amplitude.
Interference of nontremor networks, an effect of cognitive load.
A cognitive arithmetic task during a unilateral motor task induces a cognitive load, and we found it to strengthen the CMC in only essential tremor patient, compared with that during a simple unilateral task. This suggests that the CMC of actual tremor is open to influence from other networks or activities in the brain. Presumably, the cognitive task, not focusing on the tremor, induces more tremor oscillations or less tremor inhibition, strengthening the corticomuscular coupling. There may be an active central inhibition mechanism that is less accurate during distraction, which primarily aims to actively dampen the pathological oscillations elsewhere in the tremor network, or possibly to counteract the tremulous movements, resulting in a diminished CMC. Thus a second stressor lowers CMC inhibition. Alternatively, subcortical regions that are involved in emotional processing can influence the coupling between tremor subnetworks, including the reticular formation, associated with arousal. The noradrenergic locus coeruleus in the brain stem, which has been associated with tremor (Isaias et al. 2012), is known to be involved in emotion and attention.
Intermittent behavior of corticomuscular coherence.
In addition to interference of functional tasks on CMC, our results point to variation over time in essential tremor and not in healthy controls. A previous study initiated an effort to investigate the dynamics of CMC (Raethjen et al. 2007). We progressed on their work by specifically investigating the dynamics in different conditions with accurate simultaneous tremor registration. Our results, although indicating a significant CMC, do not point to a robust coupling in essential tremor. A temporary drop of CMC below the confidence level, or intermittency, occurred despite the presence of sufficient EMG power around the frequency of interest during all tasks. Moreover, the observed intermittency did not co-occur with variation in tremor SNR. The intermittent CMC, despite evident tremulous movements, can theoretically be explained either by a transient absence of phase-locked EEG power in the frequency band of interest or by the EEG power of interest being overpowered or interrupted, causing phase shifts by oscillations of nontremor networks. EEG power resulting from other tasks might, therefore, influence CMC intermittency. However, the bilateral task, associated with a dynamic interhemispheric synchronization of cortical sensorimotor areas (Hellwig et al. 2003), had no effect on CMC. The execution of a bilateral motor task could theoretically have enforced cortical tremor activity and thereby diminished intermittency. However, in the present study, it did not affect the intermittency of CMC. Intermittent behavior of the CMC in essential tremor was observed in all three tasks. Also, during cognitive loading, a task where the ability to suppress tremor might be prevented at most according to our hypothesis, the intermittency during the total recording was still present. The intermittency seems therefore not to be merely attributed to differences in tasks but may be an actual phenomenon in essential tremor.
Involvement of the motor cortex in the essential tremor network.
An explanation for tremor in essential tremor is the oscillating network hypothesis, which implies one or multiple interacting oscillators in the tremor network causing excessive neuronal oscillations throughout the whole motor circuit. It is difficult to interpret the cortical involvement established by CMC. The contribution of the sensorimotor cortex in the generation of tremor has been a topic of discussion in imaging studies. Some appoint a minor cortical involvement in essential tremor (Gallea et al. 2015; Halliday et al. 2000; Pedrosa et al. 2014), whereas others argue major cortical involvement in tremor generation (Govindan et al. 2006; Schelter et al. 2006). Intervention studies assign an important role to the primary motor cortex in tremor by influencing tremor amplitude through transcranial magnetic stimulation (TMS) of the motor cortex (Britton et al. 1992; Hellriegel et al. 2012; Pascual-Leone et al. 1994). However, stimulation of other entries in the tremor network can influence tremor amplitude, as well. Tremor amplitude is also susceptible to cerebellar stimulation and peripheral limb perturbation (Elble et al. 1992; Lee and Stein 1981). Animal studies investigating the role of the primary motor cortex in physiological tremor determined a reciprocally coupled network between the periphery and the primary motor cortex; however, in these studies, other brain areas such as the deep cerebellar nuclei and reticular formation were also attributed to tremor generation. Also, dysregulated spinal interneurons appear to admit tremulous activity (Williams et al. 2009, 2010). Therefore, in our opinion, these studies do not prove a cortical drive or cortical generation of tremor. In another study, somatosensory feedback mechanisms were pointed out to be of great importance, demonstrating tremor onset before thalamomuscular coherence (Pedrosa et al. 2014). In line with the intermittent nature of our EEG-EMG coupling, the per-operative study by Pedrosa et al. (2014) also determined a variable thalamomuscular coupling. Altogether, these findings point to a cortical involvement in essential tremor that is not likely to be a continuous source of tremor generation. In the tremor network, connected with other regions, e.g., thalamus and cerebellum, the sensorimotor cortical areas might sustain or facilitate the pathological oscillations, or react as compensation. Regarding the origin of tremor and output of pathological oscillatory activity, intermittent CMC despite continuous tremor raises the assumption of alternative pathways involved, such as the bulbospinal pathway. Our findings in essential tremor are consistent with the hypothesis of a cerebral-spinal tremor output not necessarily involving the sensorimotor cortex actively, which might be a topic of further research.
Limitations.
We acknowledge some limitations of our study. First, the cerebral central sensorimotor areas are determined on the basis of the positioning of the electrodes on the EEG cap. We applied an Hjorth montage to detect local changes in cortical brain activity. Still, we note that anatomic variations cannot be excluded, which could complicate an accurate localization of primary motor cortex (Koessler et al. 2007). To overcome this limitation, we investigated the maximum CMC over the central area covering more than one electrode. Second, the cognitive task was most burdensome for the participants and therefore has, on average, the shortest measurement duration. Statistical comparison of CMC between tasks was performed with equal 1-min recordings to ensure statistical validity. However, especially because CMC is subject to variability, longer recordings are recommended for a more thorough investigation. An alternative analysis, investigating our other outcome measures besides CMC, in which every task was shortened to the length of the shortest task led to the same results that we report presently. Finally, in our study, analyses of the phase spectrum did not show any consistent pattern because of the narrow significant frequency band. Only in cases where the coupling is an expression of a linear causality within sufficient adjacent frequencies, the interpretation of the phase spectrum is valid. For directionality between cortex and periphery (tremor), we suggest using methods other than conventional Fourier analysis to study essential tremor, such as Granger causality.
Conclusion.
With the use of the well-established coherence method, we studied a large homogenous essential tremor group. From the results of our study, we conclude that CMC in essential tremor is intermittent and possibly subject to different functional tasks. The CMC intermittency itself, however, is task independent. On this foundation, other techniques can be used to help us understand the underlying mechanism of tremor and the cortical involvement in tremor. In clinical practice, these findings may help to standardize tremor registration and the interpretation of the analysis. Although it may be a matter of definition, we argue that the cortex is not involved in the active generation of the tremor without detracting from its involvement in the tremor-generating network.
GRANTS
The project was funded by NeuroSIPE, Technology Foundation STW.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., F.L., T.H., L.J.B., and A.-F.v.R. conceived and designed research; S.S. and F.L. performed experiments; S.S. and J.D.S. analyzed data; S.S., R.V., L.J.B., and A.-F.v.R. interpreted results of experiments; S.S. prepared figures; S.S. drafted manuscript; S.S., F.L., R.V., T.H., J.D.S., L.J.B., and A.-F.v.R. edited and revised manuscript; S.S., F.L., R.V., T.H., J.D.S., L.J.B., and A.-F.v.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Part of the content of this manuscript was presented as a poster at the International Congress of Clinical Neurophysiology, Berlin, Germany, March 2014.
REFERENCES
- Bain P, Brin M, Deuschl G, Elble R, Jankovic J, Findley L, Koller WC, Pahwa R. Criteria for the diagnosis of essential tremor. Neurology 54, Suppl 4: S7, 2000. [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. “Resetting” of postural tremors at the wrist with mechanical stretches in Parkinson’s disease, essential tremor, and normal subjects mimicking tremor. Ann Neurol 31: 507–514, 1992. doi: 10.1002/ana.410310508. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Quattrone A. Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum 15: 263–275, 2016. doi: 10.1007/s12311-015-0739-8. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos JL, Lewitt P, Lyons K, Ondo W, Pahwa R, Sethi K, Stover N, Tarsy D, Testa C, Tintner R, Watts R, Zesiewicz T. Reliability of a new scale for essential tremor. Mov Disord 27: 1567–1569, 2012. doi: 10.1002/mds.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble RJ, Higgins C, Hughes L. Phase resetting and frequency entrainment of essential tremor. Exp Neurol 116: 355–361, 1992. doi: 10.1016/0014-4886(92)90014-H. [DOI] [PubMed] [Google Scholar]
- Gallea C, Popa T, García-Lorenzo D, Valabregue R, Legrand AP, Marais L, Degos B, Hubsch C, Fernández-Vidal S, Bardinet E, Roze E, Lehéricy S, Vidailhet M, Meunier S. Intrinsic signature of essential tremor in the cerebello-frontal network. Brain 138: 2920–2933, 2015. doi: 10.1093/brain/awv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RB, Raethjen J, Arning K, Kopper F, Deuschl G. Time delay and partial coherence analyses to identify cortical connectivities. Biol Cybern 94: 262–275, 2006. doi: 10.1007/s00422-005-0045-5. [DOI] [PubMed] [Google Scholar]
- Hallett M. Tremor: pathophysiology. Parkinsonism Relat Disord 20, Suppl 1: S118–S122, 2014. doi: 10.1016/S1353-8020(13)70029-4. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Shahani U, Russell AJ, Rosenberg JR. Coherence between low-frequency activation of the motor cortex and tremor in patients with essential tremor. Lancet 355: 1149–1153, 2000. doi: 10.1016/S0140-6736(00)02064-X. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995. doi: 10.1016/S0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hamon BV, Hannan EJ. Spectral estimation of time delay for dispersive and non-dispersive systems. Appl Stat 23: 134–142, 1974. doi: 10.2307/2346994. [DOI] [Google Scholar]
- Hellriegel H, Schulz EM, Siebner HR, Deuschl G, Raethjen JH. Continuous theta-burst stimulation of the primary motor cortex in essential tremor. Clin Neurophysiol 123: 1010–1015, 2012. doi: 10.1016/j.clinph.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Hellwig B, Häussler S, Schelter B, Lauk M, Guschlbauer B, Timmer J, Lücking CH. Tremor-correlated cortical activity in essential tremor. Lancet 357: 519–523, 2001. doi: 10.1016/S0140-6736(00)04044-7. [DOI] [PubMed] [Google Scholar]
- Hellwig B, Schelter B, Guschlbauer B, Timmer J, Lücking CH. Dynamic synchronisation of central oscillators in essential tremor. Clin Neurophysiol 114: 1462–1467, 2003. doi: 10.1016/S1388-2457(03)00116-0. [DOI] [PubMed] [Google Scholar]
- Hjorth B. Principles for transformation of scalp EEG from potential field into source distribution. J Clin Neurophysiol 8: 391–396, 1991. doi: 10.1097/00004691-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Hopfner F, Haubenberger D, Galpern WR, Gwinn K, Van’t Veer A, White S, Bhatia K, Adler CH, Eidelberg D, Ondo W, Stebbins GT, Tanner CM, Helmich RC, Lenz FA, Sillitoe RV, Vaillancourt D, Vitek JL, Louis ED, Shill HA, Frosch MP, Foroud T, Kuhlenbäumer G, Singleton A, Testa CM, Hallett M, Elble R, Deuschl G. Knowledge gaps and research recommendations for essential tremor. Parkinsonism Relat Disord 33: 27–35, 2016. doi: 10.1016/j.parkreldis.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Marzegan A, Pezzoli G, Marotta G, Canesi M, Biella GE, Volkmann J, Cavallari P. A role for locus coeruleus in Parkinson tremor. Front Hum Neurosci 5: 179, 2012. doi: 10.3389/fnhum.2011.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koessler L, Maillard L, Benhadid A, Vignal JP, Braun M, Vespignani H. Spatial localization of EEG electrodes. Neurophysiol Clin 37: 97–102, 2007. doi: 10.1016/j.neucli.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Koller WC, Biary NM. Volitional control of involuntary movements. Mov Disord 4: 153–156, 1989. doi: 10.1002/mds.870040207. [DOI] [PubMed] [Google Scholar]
- Lee RG, Stein RB. Resetting of tremor by mechanical perturbations: a comparison of essential tremor and parkinsonian tremor. Ann Neurol 10: 523–531, 1981. doi: 10.1002/ana.410100606. [DOI] [PubMed] [Google Scholar]
- Muthuraman M, Heute U, Arning K, Anwar AR, Elble R, Deuschl G, Raethjen J. Oscillating central motor networks in pathological tremors and voluntary movements. What makes the difference? Neuroimage 60: 1331–1339, 2012. doi: 10.1016/j.neuroimage.2012.01.088. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Toro C, Wassermann EM, Hallett M. Resetting of essential tremor and postural tremor in Parkinson’s disease with transcranial magnetic stimulation. Muscle Nerve 17: 800–807, 1994. doi: 10.1002/mus.880170716. [DOI] [PubMed] [Google Scholar]
- Pedrosa DJ, Quatuor EL, Reck C, Pauls KA, Huber CA, Visser-Vandewalle V, Timmermann L. Thalamomuscular coherence in essential tremor: hen or egg in the emergence of tremor? J Neurosci 34: 14475–14483, 2014. doi: 10.1523/JNEUROSCI.0087-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa DJ, Reck C, Florin E, Pauls KA, Maarouf M, Wojtecki L, Dafsari HS, Sturm V, Schnitzler A, Fink GR, Timmermann L. Essential tremor and tremor in Parkinson’s disease are associated with distinct ‘tremor clusters’ in the ventral thalamus. Exp Neurol 237: 435–443, 2012. doi: 10.1016/j.expneurol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. https://www.R-project.org/. [Google Scholar]
- Raethjen J, Govindan RB, Kopper F, Muthuraman M, Deuschl G. Cortical involvement in the generation of essential tremor. J Neurophysiol 97: 3219–3228, 2007. doi: 10.1152/jn.00477.2006. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Schelter B, Winterhalder M, Maiwald T, Brandt A, Schad A, Schulze-Bonhage A, Timmer J. Testing statistical significance of multivariate time series analysis techniques for epileptic seizure prediction. Chaos 16: 013108, 2006. doi: 10.1063/1.2137623. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord 24: 1629–1635, 2009. doi: 10.1002/mds.22633. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Nederveen AJ, Booij J, van Rootselaar AF. Neuroimaging essentials in essential tremor: a systematic review. Neuroimage Clin 5: 217–231, 2014. doi: 10.1016/j.nicl.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer J, Lauk M, Deuschl G. Quantitative analysis of tremor time series. Electroencephalogr Clin Neurophysiol 101: 461–468, 1996. doi: 10.1016/0924-980X(96)94658-5. [DOI] [PubMed] [Google Scholar]
- Timmer J, Lauk M, Häussler S, Radt V, Köster B, Hellwig B, Guschlbauer B, Lücking CH, Eichler M, Deuschl G. Cross-spectral analysis of tremor time series. Int J Bifurcat Chaos 10: 2595–2610, 2000. doi: 10.1142/S0218127400001663. [DOI] [Google Scholar]
- Timmer J, Lauk M, Pfleger W, Deuschl G. Cross-spectral analysis of physiological tremor and muscle activity. I. Theory and application to unsynchronized electromyogram. Biol Cybern 78: 349–357, 1998. doi: 10.1007/s004220050439. [DOI] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Coherence between motor cortical activity and peripheral discontinuities during slow finger movements. J Neurophysiol 102: 1296–1309, 2009. doi: 10.1152/jn.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Spinal interneuron circuits reduce approximately 10-Hz movement discontinuities by phase cancellation. Proc Natl Acad Sci USA 107: 11098–11103, 2010. doi: 10.1073/pnas.0913373107. [DOI] [PMC free article] [PubMed] [Google Scholar]