Muscle sensory neurons signal information necessary for controlling limb movements. The information encoded and transmitted by muscle proprioceptors to networks in the spinal cord is known in detail only for the cat, but differences in size and behavior of other species challenge the presumed generalizability. This report presents the first findings detailing specializations in mechanosensory signaling and intraspinal targets for functionally identified subtypes of muscle proprioceptors in the rat.
Keywords: species adaptation, primary afferent, spinal cord, muscle spindle, tendon organ
Abstract
The characteristic signaling and intraspinal projections of muscle proprioceptors best described in the cat are often generalized across mammalian species. However, species-dependent adaptations within this system seem necessary to accommodate asymmetric scaling of length, velocity, and force information required by the physics of movement. In the present study we report mechanosensory responses and intraspinal destinations of three classes of muscle proprioceptors. Proprioceptors from triceps surae muscles in adult female Wistar rats anesthetized with isoflurane were physiologically classified as muscle spindle group Ia or II or as tendon organ group Ib afferents, studied for their firing responses to passive-muscle stretch, and in some cases labeled and imaged for axon projections and varicosities in spinal segments. Afferent projections and the laminar distributions of provisional synapses in rats closely resembled those found in the cat. Afferent signaling of muscle kinematics was also similar to reports in the cat, but rat Ib afferents fired robustly during passive-muscle stretch and Ia afferents displayed an exaggerated dynamic response, even after locomotor scaling was accounted for. These differences in mechanosensory signaling by muscle proprioceptors may represent adaptations for movement control in different animal species.
NEW & NOTEWORTHY Muscle sensory neurons signal information necessary for controlling limb movements. The information encoded and transmitted by muscle proprioceptors to networks in the spinal cord is known in detail only for the cat, but differences in size and behavior of other species challenge the presumed generalizability. This report presents the first findings detailing specializations in mechanosensory signaling and intraspinal targets for functionally identified subtypes of muscle proprioceptors in the rat.
movements are guided through novel or dynamic environments by information about the kinematic and kinetic state of an animal’s body and limbs (Pearson 2004; Prochazka 1981; Prochazka and Ellaway 2012; Windhorst 2007). Muscle proprioceptors contribute by encoding various features of these state variables in sensory signals transmitted to neural circuits, including ones that rapidly adjust intended muscle activation patterns to accommodate perturbations in gait or posture (Nichols et al. 1999; Prochazka and Ellaway 2012). Whereas fundamental features and operations of these reflex circuits appear common to diverse terrestrial vertebrates (Goulding 2009; Grillner and Wallen 1985; Ijspeert 2008; Pearson 1993; Tresch et al. 2002), little is known about how these circuits are tuned to species differences in physical dimensions and behavior.
The most comprehensive understanding of spinal reflex pathways in adult terrestrial vertebrates derives from studies of cats (reviews: Baldissera et al. 1981; Jankowska 1992; Matthews 1972; Whelan 1996). Extensive study in cats includes reflex circuits activated by different classes of muscle proprioceptors, i.e., primary afferents supplying muscle spindle and tendon organ receptors. Muscle spindle afferents encode kinematic information, biased toward muscle velocity for group Ia afferents and muscle length for group II afferents (Houk et al. 1981; Matthews 1981a); group Ib afferents supplying tendon organs generate a kinetic signal most sensitive to active muscle force (Binder and Osborn 1985; Jami 1992). The intraspinal projections and synaptic terminations for each of the three afferent classes have been mapped in detail for cats, both anatomically (Brown 1981a; Fyffe 1979; Ishizuka et al. 1979) and physiologically (Baldissera et al. 1981; Jankowska 1992). All three afferent classes from multiple muscles converge on the deep dorsal horn in spinal segments, giving premotor interneurons direct access to both kinematic and kinetic information and the capacity to coordinate muscle activity throughout the limb based on a multimodal model of mechanical state (Jankowska and Edgley 2010). Only the muscle spindle afferents project into the ventral spinal gray matter, where they transmit kinematic information used by premotor interneurons and α-motoneurons to coordinate quick responses of muscle agonists and antagonists acting at single joints (Nichols et al. 1999). This simplified description characterizes some of the fundamental features of structural and functional organization by which muscle kinematics and kinetics are monitored and used to adjust intended movements in dynamic mechanical environments. Generalizability is supported by information less complete for other adult mammalian species, including humans (Burke et al. 1983), rats (Alvarez et al. 2011; Bullinger et al. 2011a), and mice (Arber 2012; Goulding 2009), and for muscle receptors in multiple species (Banks 2006; Banks et al. 2009).
The reflex model just described, despite its general similarity among several species, needs to accommodate constraints on movement imposed by differences in species geometry and size. A theoretical framework for comparing the mechanics of locomotion in animals of different size is provided by the dynamic similarity hypothesis (Alexander and Jayes 1983; Biewener 1989; McMahon et al. 1987). When animals walk with similar ratios of inertial to gravitational forces, i.e., similar Froude numbers, their limb movements can be equated by scaling the dynamic mechanical parameters of movement, including spatial, temporal, and kinetic parameters. Because scaling factors for limb length (L), velocity (V), and peak force (F) are unequal (Alexander and Jayes 1983), the ratio L:V:F should differ across the size spectrum of terrestrial vertebrates. This prediction and its supporting evidence (Alexander and Jayes 1983; Farley et al. 1993) suggest that for different-sized animals to move in dynamically similar fashion, they must develop adaptations somewhere in the reflex circuits that monitor and process kinematic and kinetic state parameters.
Attention to possible reflex adaptations gains importance as research on spinal reflex networks shifts focus to rodents. It is unlikely that the details of proprioceptor reflexes so well characterized in cats are suitable to achieve comparable control of dynamic movements in rats, for example, which are ~10× lighter in mass and 6× shorter in limb length. An additional difference arises with limb orientation, which is shown to affect control of movement (Jenkins 1971; McMahon 1985). Mammals greater than ~3 kg in mass tend to stand and move with proximal limb segments oriented more nearly vertical. Cats are the smallest of these so-called cursorial mammals. By contrast, rodents are classified as noncursorial for their tendency to stand and move with the humerus and femur more horizontal than vertical. These limb orientations may put limb extensor muscles under dissimilar strain and strain rates to constrain movement in ways that drive adaptations in proprioceptor reflexes (Donelan and Kram 2000; McMahon 1985).
Interest in possible species adaptations motivated us to more closely examine different classes of muscle proprioceptors and their reflex circuits in adult rats. We began by studying regional distributions of synapses made in spinal cord segments. Results indicated that laminar distribution of provisional synapses from group Ia, II, and Ib afferents was roughly the same for the rat as that established for the cat. Additionally, we examined a large sample of each afferent class in the rat for a variety of firing properties characterizing physiologically relevant responses to stretch of passive muscle. Population statistics for the three afferent classes in the rat produced a qualitative description of mechanosensory encoding for the rat that closely resembled that for the cat. However, encoding by rat afferents was distinguished by heightened signaling of dynamic muscle stretch by Ia afferents and by robust encoding of static stretch parameters by group Ib afferents approaching that for group II afferents. These distinguishing features suggest candidate species adaptations in proprioceptive function.
Portions of these results have been presented in abstract form (Gabriel et al. 2015).
METHODS
Animal Care
All procedures and experiments were approved by the Wright State University Institutional Animal Care and Use Committee. Adult female Wistar rats (250–300 g; Charles Rivers Laboratory, Wilmington, MA) were studied in terminal experiments only and were not subject to any other experimental procedures. All animals were housed in clean cages and provided food and water ad libitum in a temperature- and light-controlled environment in Wright State’s Laboratory Animal Resources facility.
Terminal Physiological Experiments
Experiments were designed to measure the firing of individual muscle afferents in response to muscle contraction and stretch in vivo with electrophysiological techniques and to label the central axonal projections and varicosities of these same afferents for later morphological analysis. At the conclusion of data collection, rats were killed by exsanguination preceded either by overdose with isoflurane inhalation (5%) in most cases or by Euthasol (50 mg/ml, ip injection) in nine rats studied for afferent morphology.
Anesthesia and monitoring of vital signs.
Rats were deeply anesthetized (complete absence of withdrawal reflex) by inhalation of isoflurane, initially in an induction chamber (5% in 100% O2) and for the remainder of the experiment via a tracheal cannula (1.5–2.5% in 100% O2). Surgical and recording preparation followed by data collection lasted for up to 15 h. Subcutaneous injections of lactated Ringer solution were given to support fluid levels and blood pressure. The rats’ respiratory rate, Pco2, and core temperature were monitored continuously; pulse rate and Po2 were monitored intermittently. Sustained variations in vital signs exceeding normal ranges (Lerche et al. 2000) were corrected by adjusting anesthesia (within the bounds of suppressing withdrawal reflex), and temperature was maintained (36–38°C) by adjusting heat sources (heated water pad and radiant heat).
Surgical preparation for data collection.
Anesthetized rats were dissected as described in earlier reports from this laboratory (e.g., Bullinger et al. 2011b; Haftel et al. 2004). Briefly, dorsal roots (lumbar L4–6), muscles, and nerves in the left hindlimb were prepared for stimulation and/or recording with the rat fixed in a rigid frame at the snout, vertebral bodies, distal tibia, and distal femur (knee angle 120°). Triceps surae muscles (lateral and medial gastrocnemii and soleus) were partially freed of surrounding connective tissue and marked for their resting length (Lr) at ankle angle 90° before their common Achilles tendon was severed at the calcaneus and tied directly to the lever arm of a force- and length-sensing servomotor (model 305B-LR; Aurora Scientific). Triceps surae nerves were loosely positioned in continuity on a unipolar silver stimulating electrode, and all other hindlimb nerves including common peroneal, sural, and posterior tibial nerves were crushed. Dorsal rootlets were carefully freed in continuity from overlying connective tissue and supported on bipolar hook electrodes positioned close to the rootlet’s entry into the dorsal spinal cord. Exposed tissues were covered with warm mineral oil in pools formed by attaching the edges of severed skin to the recording frame.
Electromechanical study of single-muscle afferents.
Figure 1A illustrates the experimental configuration for data collection. Dorsal rootlets positioned in continuity on bipolar recording electrodes were selected for sampling afferents when they produced robust action potential activity in response to both electrical stimulation of triceps surae nerves and stretch of triceps surae muscles. Individual axons penetrated in these rootlets by glass micropipettes (∼15 MΩ filled with 2 M K+-acetate) were selected for study when electrical stimulation of triceps surae nerves produced orthodromic action potentials that were readily resolvable and had conduction delay of <3 ms. Afferent firing was recorded as described below, first to classify muscle afferents on the basis of their responses to artificial stimuli, e.g., muscle twitch contraction and vibration, and second to characterize the afferents’ sensory encoding of physiologically meaningful mechanical events.
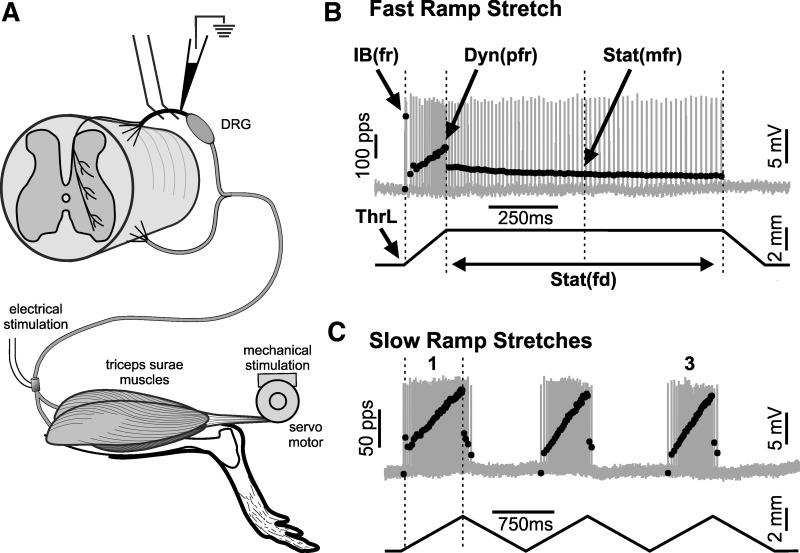
Fig. 1.
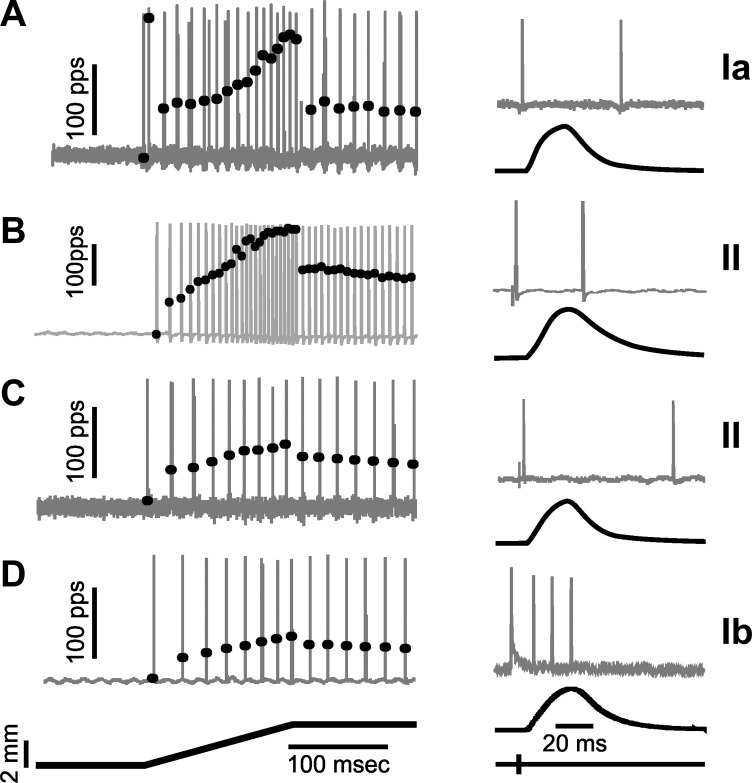
Mechanosensory signaling by muscle proprioceptors. A: key features of in vivo recording paradigm: attachment of triceps surae muscles through Achilles tendon to motor operating in length servo mode to produce controlled muscle stretch; electrode on triceps surae nerve for electrical stimulation of orthodromic action potentials and isometric muscle twitches; glass micropipette penetrating single afferent axon in dorsal root (rostral to dorsal root ganglion) for recording action potentials and labeling axon with Neurobiotin. B and C: action potentials (gray traces) with superimposed instantaneous firing rates (black dots) responding to different waveforms of 3-mm muscle length changes (black traces). B: ramp-hold-release stretch with relatively fast ramp velocity (bottom trace; 20 mm/s). C: 3 successive triangular stretches at slow ramp velocity (bottom trace; 4 mm/s, 3 mm). 1 and 3, 1st and 3rd slow triangular stretches, respectively. Measurements identified (B) instantaneous firing rates (fr) for initial burst IB(fr) and for peak dynamic Dyn(pfr) and mid-static firing rate Stat(mfr); static firing duration Stat(fd); length threshold (ThrL).
Afferents were classified on the basis of binary scoring of three criteria (Table 1). Afferents that fired during the rising phase of isometric twitch force (evoked by nerve stimulation with electrical pulses 0.04-ms duration at 1 pps) were designated group Ib tendon organ afferents (see Fig. 2), while those that did not were labeled muscle spindle afferents (see Figs. 3 and 4). Muscle spindle afferents designated Ia were distinguished from group II by firing with virtually perfect entrainment to 1-s bouts of high-frequency, small-amplitude vibration (100–333 Hz, 80 µm) and by responding with an initial burst of high-frequency firing (>100 pps) at the onset of muscle stretch (Fig. 3). These same criteria distinguish analogous afferent groups in the cat (Matthews 1972).
Table 1.
Afferent group classification by binary scoring for 3 criteria
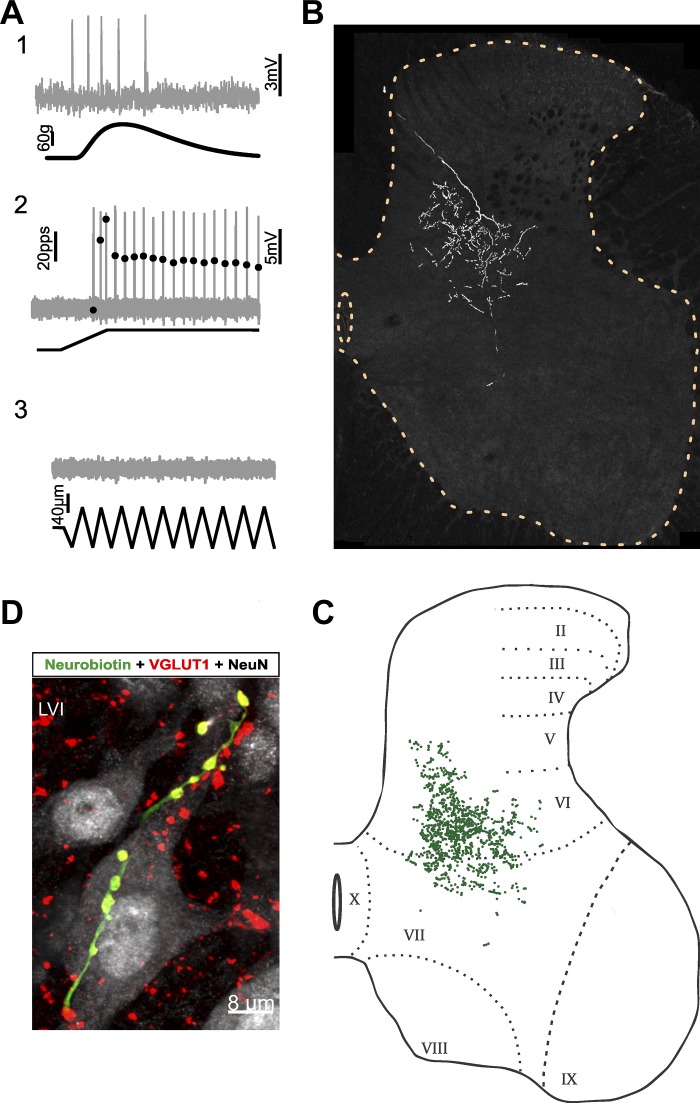
Fig. 2.
Group Ib axon collaterals and varicosities (afferent 7 in Fig. 5, Tables 2 and 3) A: group Ib classification: action potentials (gray traces) recorded during mechanical stimulation of triceps surae muscles (black traces). A1: firing accelerated during the rising phase of isometric twitch contraction. A2: absence of high-frequency initial bursting at the onset of fast muscle stretch. A3: failure to fire during high-frequency muscle vibration (≥100 Hz). B: axon collateral distribution: Neurobiotin-filled afferent collaterals in sequential tissue sections projected in 2 dimensions and superimposed in relation to the gray matter borders and the central canal (dashed yellow lines, L4/L5) to create the appearance of “looking through” several tissue sections. Collateral branches roughly spanned medial lamina V/VI and VII. C: each green dot represents a Neurobiotin-labeled varicosity (1,087/1,167 varicosities stained positive for VGLUT1 ir). Greatest density in medial LVI. D: postsynaptic contacts: examples of double-labeled varicosities aligned with soma and dendrites of postsynaptic neurons (gray, NeuN).
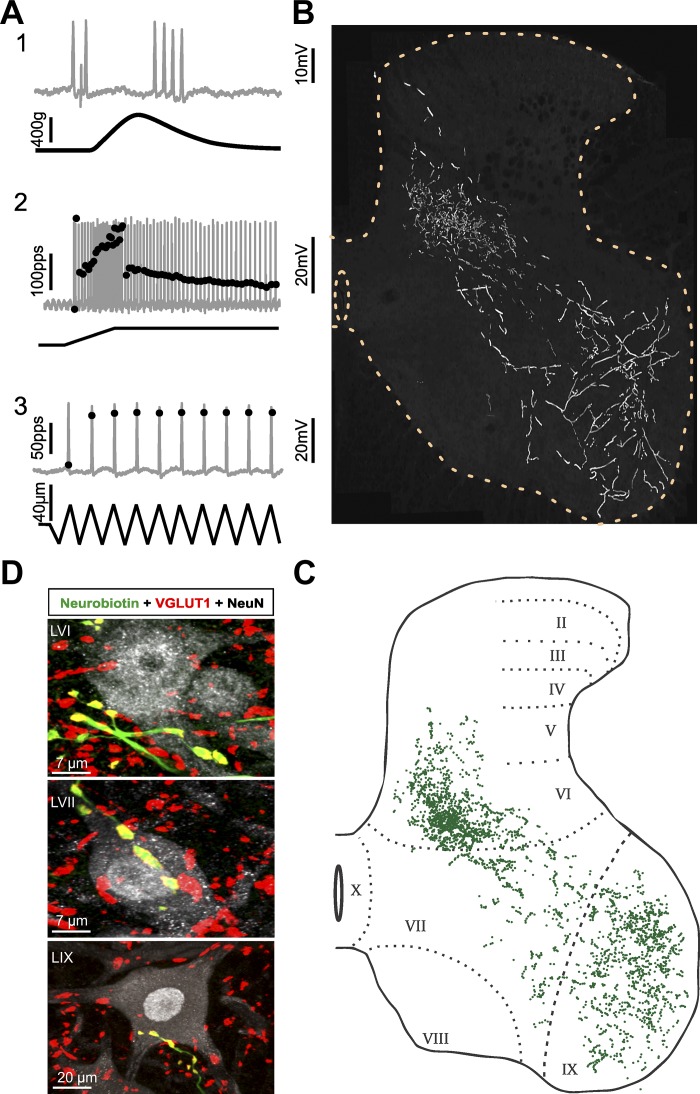
Fig. 3.
Group Ia axon collaterals and varicosities (afferent 1 in Fig. 5, Tables 2 and 3; same format as Fig. 2). A: group Ia classification. A1: firing interrupted during the rising phase of isometric twitch contraction. A2: high-frequency initial bursting at the onset of fast muscle stretch. A3: perfect firing entrainment to high-frequency muscle vibration. B: collateral branches: dense in LV/VI, sparse in LVII, and expansive in LIX. C: each green dot represents a Neurobiotin-labeled varicosity (all varicosities, n = 2,926, stained positive for VGLUT1 ir). D: postsynaptic contacts: examples of double-labeled varicosities aligned with soma and dendrites of postsynaptic neurons in 3 laminar territories.
Fig. 4.
Group II axon collaterals and varicosities (afferent 4 in Fig. 5, Tables 2 and 3; same format as Fig. 2). A: group II classification. A1: firing interrupted during the rising phase of isometric twitch contraction. A2: absence of high-frequency initial bursting at the onset of fast muscle stretch. A3: incomplete entrainment of firing to high-frequency muscle vibration. B: collateral branches: relatively dense in medial LV/VI and dorsolateral LIX, sparse in LVII. C: each green dot represents a Neurobiotin-labeled varicosity (all varicosities, n = 1,836, stained positive for VGLUT1 ir). D: postsynaptic contacts: examples of double-labeled varicosities aligned with soma and dendrites of postsynaptic neurons in 3 laminar territories.
Two stretch paradigms (Fig. 1, B and C) were used to characterize the firing responses of afferents to physiologically relevant mechanical stimuli. Stretches were delivered by the servo motor to triceps surae muscles, which were not engaged in active contraction, i.e., passive as they are when stretched, for example, during the swing phase in walking gait (Thota et al. 2005). Ramp-hold-release stretches tested afferent encoding of both dynamic (ramp and release phases) and static (hold phase) muscle length (e.g., Vincent et al. 2016); successive triplets of triangular stretch tested activity-dependent encoding in dynamic stretch (Haftel et al. 2004) and provided an additional, slower velocity of ramp stretch. In both paradigms, triceps surae muscles were stretched by 3 mm from Lr, which represented 7% of the muscle + tendon length (MTL) measured here as 44 mm from origin to insertion of rat gastrocnemius muscles. Dynamic stretch was applied at constant velocities at 20 mm/s or 47% MTL/s in fast stretch trials (Fig. 1B) and at 4 mm/s or 9% MTL/s in slower stretches (Fig. 1C). These amplitudes and rates of stretch expressed as %MTL fall within values that are expected for animals engaged in normal activities including locomotion (Gillis and Biewener 2001) and that have been applied in studying muscle stretch encoding by afferents in adult cats (Matthews 1972, 1981a).
Intra-axonal records of action potentials together with records of muscle length and force were digitized (20 kHz) and were monitored online and stored on computer for later analysis, both with Spike2 software. Figure 1B identifies some of the parameters measured to characterize mechanosensory encoding of passive-muscle stretch for ramp-hold-release stretch, including 1) muscle length at firing initiation, i.e., threshold (ThrL); 2) peak instantaneous firing rate of high-frequency initial burst firing [IB(fr)]; 3) peak dynamic firing rate occurring at the peak of ramp stretch [Dyn(pfr)]; 4) static firing rate at the midpoint of the hold phase of stretch [Stat(mfr)] and static firing duration [Stat(fd)], measured as the duration of continuous repetitive firing during stretch hold phase; and 5) dynamic index (DI) calculated as Dyn(pfr) – Stat(mfr). Figure 1C illustrates firing responses to three successive triangular stretches. ThrL and Dyn(prf), together with other parameters defined below, were measured from the first and third triangles.
Morphological Study of Single-Muscle Afferents
Intra-axonal labeling.
After their physiological characterization, the axons of nine different afferents classified as group Ia, II, or Ib (3 each; 1 per rat) were injected with 10% Neurobiotin (Vector Laboratories, Burlingame, CA) in Tris buffer (0.1 M Tris·OH, 1 M potassium acetate, pH 7.6) through the recording micropipette located ~1 mm from the dorsal root entry zone. The best labeling of the axons’ intraspinal projections and collaterals was obtained when the positive current pulses (400-ms duration, at 2 Hz and 5- to 15-nA amplitude) used to inject label were applied for >12 min with membrane potential greater than −45 mV. After label injection, rats were maintained under deep anesthesia to allow anterograde labeling for a minimum of 6 h before being overdosed with Euthasol (50 mg/ml ip). Animals were then immediately perfused transcardially with ice-cold vascular rinse (0.01 M phosphate buffer with 0.8% NaCl, 0.025% KCl, and 0.05% NaHCO3, pH 7.4) followed by room temperature fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4). The lumbar spinal cord was quickly dissected and postfixed overnight in the same fixative at 4°C. The following day, spinal tissue was transferred to 0.01 M PBS and processed for post hoc immunohistochemistry as described below.
Immunohistochemistry.
Transverse sections (75 µm thick) of spinal cord segments L4–S1 were cut on a vibrating microtome and collected in 0.01 M phosphate buffer with 0.9% NaCl (PBS, pH 7.4) for free-floating immunohistochemistry. Tissue sections were first incubated in blocking buffer (5% normal donkey serum diluted in 0.01 M PBS containing 0.1% Triton X-100) for 30–60 min. Neurobiotin was revealed by incubating the sections for 2–3 h at room temperature in blocking buffer containing a 1:100 dilution of streptavidin conjugated to Alexa Fluor 488 (Life Technologies). Sections were subsequently incubated overnight at 4°C in blocking buffer containing an antibody recognizing the vesicular glutamate transporter 1 isoform (VGLUT1; Synaptic Systems, Goettingen, Germany; guinea pig, dilution 1:2,000) and an antibody against neuronal nuclear protein (NeuN; Chemicon, Temecula, CA; mouse, 1:1,000 dilution). The specificity and sensitivity of the primary antibodies in this study have been amply characterized for their specific labeling patterns in the spinal cord (Alvarez et al. 2011; Rotterman et al. 2014). After overnight incubation, the sections were washed in PBS and immunoreactive sites were revealed with species-specific secondary antibodies raised in donkey and conjugated to Cy3 or Cy5 (Jackson Immunoresearch, West Grove, PA) diluted 1:50 in PBS-0.1% Triton X-100, pH 7.4. After a 2- to 3-h incubation period in secondary antibodies, the sections were thoroughly washed in PBS, mounted on gelatin-coated slides, and coverslipped with Vectashield (Vector Laboratories).
Confocal microscopy and quantitative analysis.
Spinal cord sections were excited sequentially with argon, krypton, and HeNe lasers to visualize streptavidin-Alexa Fluor 488, NeuN-Cy3, and VGLUT1-Cy5 conjugates, respectively. Z-axis stack images were obtained on an Olympus Fluoview 1000 confocal microscope (Center Valley, PA) with a ×10 objective at 1.0-µm Z-steps, a ×20 objective at 0.65-µm Z-steps, and a ×60 oil immersion objective at 0.5-µm Z-steps using 1.0–3.0 digital zoom. Stacks were quantitatively analyzed with Fluoview software (Olympus) for Neurobiotin-filled structures including axons and varicosities. Stacks in which immunoreactivity (ir) was strong for axons or varicosities (fluorescence intensity exceeding background by at least 2-fold) were analyzed. Varicosities were morphologically identified as discreet axonal swellings, either en passant or terminal. Stacks not meeting the intensity criterion were discarded, and their removal may have resulted in underrepresentation of the full rostral-caudal extent of the filled afferents. Laminar boundaries were estimated for each individual transverse section. This process has been amply characterized in the rat by Molander et al. (1984).
Varicosities were examined for colocalization with VGLUT1 ir, to give added support for their provisional identification as synaptic boutons (Alvarez et al. 2011; Hughes et al. 2004). While VGLUT1 was strongly expressed (2× background intensity) by all morphologically designated vesicles in afferents 1–4, it was weak or absent in some varicosities that plainly belonged to Neurobiotin-labeled collaterals of afferents 5–9 (see Table 2). Further analysis with Amaris software allowing for a three-dimensional rotation of the stacks demonstrated that the absence of VGLUT1 resulted from incomplete penetration of the antibody throughout the tissue section. We consistently observed that Neurobiotin-labeled varicosities stained positive for VGLUT1 if positioned within the VGLUT1 penetration zone, while varicosities staining negative were always found outside that zone. These observations led us to greater confidence in identifying provisional synapses by morphological designation of varicosities rather than by VGLUT1 ir, which, as a result of incomplete staining, would have underestimated synapse number. Thus we relied on morphological designation of varicosities to describe their number, distribution, and density, following the approach of earlier studies of muscle proprioceptors in the cat (Brown and Fyffe 1978; Burke and Glenn 1996; Ishizuka et al. 1979), and we provide added support for provisional designation of synapses with VGLUT1 ir in most cases (see Table 2).
Table 2.
Varicosity counts
| Ia |
II |
Ib |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lamina | Afferent 1 | Afferent 2 | Afferent 3 | Afferent 4 | Afferent 5 | Afferent 6 | Afferent 7 | Afferent 8 | Afferent 9 |
| LV/VI | 1,405 | 277 | 537 | 854 | 912 (463) | 539 (216) | 1,101 (80) | 848 (111) | 542 (312) |
| LVII | 344 | 170 | 310 | 307 | 212 (76) | 98 (17) | 66 | 128 | 31 (20) |
| LIX | 1,177 | 730 | 859 | 675 | 119 (10) | 332 (16) | 0 | 0 | 0 |
| No. of sections | 59 | 35 | 42 | 27 | 20 | 23 | 25 | 22 | 16 |
| First to last collateral, mm | 4.9 | 4.2 | 4.8 | 3.8 | 2.9 | 4.0 | 5.6 | 5.5 | 2.6 |
Values are numbers of Neurobiotin-filled varicosities for each of 9 afferents classified as group Ia, II, or Ib. Table identifies counts taken in identified laminae from the number and distance of tissue sections spanning the first to last ones containing Neurobiotin-labeled collateral axons. All varicosities expressed VGLUT1 ir except numbers in parentheses (see methods). Afferent number identity (1–9) is retained throughout this report (text, Table 3, Figs. 2, 3, and 4).
Figure composition and qualitative analysis.
Microscopic images were prepared with ImagePro Plus software (Media Cybernetics, Silver Spring, MD), and CorelDRAW and/or Corel PHOTO-PAINT (version X5) were used to create the figures. Sequential optical sections were manually aligned in relation to the borders of the gray matter and central canal and then compiled and collapsed into single two-dimensional images. Although no corrective scaling was applied to account for transitions in segmental morphology of the spinal gray matter, structures of labeled afferents in individual sections were well represented by the aggregate.
Statistical Analyses
Parameters characterizing the firing responses of afferents sampled from multiple rats and pooled into groups classified as Ia, II, or Ib were tested for statistically significant differences with one-way ANOVA and Tukey post hoc analysis. α for statistical tests was set to 0.01 in recognition of the large sample size and to reduce false discovery associated with multiple ANOVAs.
Linear discriminant analysis (LDA) was used to provide supervised dimensionality reduction for the multiple features of afferent firing responses to mechanosensory stimuli, in an attempt to find a linear combination of features that separated and characterized the three classes of afferents. Data were first de-meaned, normalized to unit standard deviation, and tested by one-way multivariate ANOVA. The derived covariance matrix was then normalized by within-group, pooled covariance. The eigenvectors of that modified covariance matrix defined two canonical variables that characterized and separated the three classes of afferents identified a priori as Ia, II, and Ib. LDA was performed in R (R Development Core Team 2016).
RESULTS
Firing responses to mechanical stimuli were recorded in vivo from 298 individual afferents supplying triceps surae muscles in 47 normal adult female Wistar rats. Afferents were classified by criteria described in methods (see Table 1) as muscle spindle group Ia (n = 144) or group II (n = 62) or as tendon organ group Ib (n = 58). Thirty-four muscle afferents could not be classified and were excluded from most analyses. Physiological classification, which remains the only means to date for distinguishing muscle proprioceptors, supported the two major goals of this study. One was to determine for the first time in adult rat the intrasegmental distribution of axon collaterals and varicosities for all three afferent classes within spinal cord segments. The other goal was to extend information about mechanical signaling by group Ia, II, and Ib afferents in rats.
Afferent Classification and Distribution in Spinal Laminae
Figures 2, 3, and 4 illustrate the firing responses used to classify afferents, together with the spatial distribution of their axon collaterals and varicosities in spinal cord segments.
Tendon organ group Ib afferent.
group ib classification.
Records in Fig. 2A show that the labeled afferent met the classification criterion for group Ib afferents by firing during isometric twitch contractions (Fig. 2A1). Secondary confirmation was obtained from the absence of both a high-frequency burst of firing at stretch onset (Fig. 2A2) and firing during muscle vibration (Fig. 2A3). The afferent fired during passive stretch, as did all 58 Ib afferents sampled here. Figure 2A2 shows that firing accelerated during the ramp, adapted rapidly in the transition to static stretch, and persisted during static stretch.
group ib axon branches and varicosities.
Along its ventrolateral trajectory through the dorsal horn, this labeled Ib axon distributed dense arborizations centered in the medial to central portion of lamina (L)VI (Fig. 2B). The boundaries were extended by some branches projecting short distances dorsomedially into LV and ventrally into LVII. Few collateral branches extended into the lateral portion of LVI. Projections into LVII were primarily located along the LVI/LVII border, with the exception of a single branch with one to six varicosities penetrating deeper into LVII. No axon branches extended into LIX.
Varicosities had their greatest concentration in the medial half of LVI (Fig. 2C), where they were found in close apposition to soma and proximal dendrites of small (<10 µm) and medium-sized (10 µm < x < 25 µm) neurons (Fig. 2D). Varicosities appeared to contact medium-sized, sometimes spindle-shaped neurons in the dorsal most portions of LVII, but more ventrally varicosities were observed in apposition to dendrites but not soma.
Muscle spindle group Ia afferent.
group ia classification.
This labeled afferent was designated a muscle spindle afferent because it stopped firing during the rising phase of isometric twitch force (Fig. 3A1); it was subclassified as group Ia because its firing was perfectly entrained to high-frequency muscle vibration (Fig. 3A3) and responded with a high-frequency initial burst at the onset of ramp stretch (Fig. 3A2). Spindle afferents that entrained to vibration but failed to demonstrate an initial burst (n = 25) were excluded from analysis. In response to ramp-and-hold stretch (Fig. 3A2), the afferent fired with a pattern stereotypical for Ia afferents sampled here: firing began with two rapid spikes, fell to substantially lower rates in transition from dynamic to static stretch, and persisted but with distinct rate accommodation during the hold phase of stretch.
group ia axon branches and varicosities.
The distribution of axon collaterals and varicosities (all colabeled with VGLUT1 ir) shown for the Ia afferent in Fig. 3 closely resembled that for all Ia afferents studied here and described previously in adult rat (Alvarez et al. 2011). Along its diagonal path from dorsal horn entry to the ventral horn, the Ia axon shown in Fig. 3B formed dense arborizations in LVI. Collaterals reaching the lateral third of LVI were less developed and occurred sparsely in comparison to the complex arborizations in the medial two-thirds of LVI. Axon branches exhibited relatively sparse collaterals as they passed through LVII in divergent but parallel routes (Fig. 3B), a pattern previously reported in rats (Watson and Bazzaz 2001) and in cats (Brown and Fyffe 1978). Terminal collateral branches spread widely throughout LIX.
The Ia collateral axons produced aggregations of varicosities that were dense in LV/VI and LIX but relatively sparse in LVII (Fig. 3C). Sample images of the varicosities colabeled with VGLUT1 ir and their apparent synaptic connections with neurons in all three laminae are shown in Fig. 3D. In LV/LVI, VGLUT1 varicosities appeared to contact soma and proximal dendrites of small/medium-sized neurons, while in LVII contacts formed with medium-sized and sometimes spindle-shaped neurons. Varicosities distributed widely throughout LIX, but contacts with soma and proximal dendrites of presumed α-motoneurons (diameter ≥ 30 µm) were restricted to the dorsolateral region of LIX known to contain triceps surae motor pools (Molander et al. 1984; Nicolopoulos-Stournaras and Iles 1983; Swett et al. 1986).
Muscle spindle group II afferent.
group ii classification.
The afferent examined in Fig. 4 met criteria for group II muscle spindle afferents by exhibiting a pause in firing during muscle twitch (Fig. 4A1), incomplete entrainment to muscle vibration (Fig. 4A3), and no high-frequency initial burst at ramp stretch onset (Fig. 4A2). Spindle afferents that did not entrain to vibration but did display an initial burst (n = 9) were excluded from analysis. Figure 4A2 shows that this afferent responded to passive stretch with modest acceleration during ramp, slight deceleration in transition to hold, and little accommodation throughout hold.
group ii axon branches and varicosities.
The branching pattern of collateral axons for this group II afferent in Fig. 4B resembled that just described for the Ia afferent (cf. Fig. 3B). Axons penetrated the dorsal horn and ramified extensively in a territory focused in the medial two-thirds of the LVI. Small branches with limited contacts extended dorsally into LIV. No branches were observed extending into lateral LVI, in contrast with description of group II collateral projections in the cat (Fyffe 1979). Axon branches progressed obliquely through the intermediate zone, but in comparison with Ia afferents these branches were noticeably thinner and in LIX they covered a smaller territory restricted dorsolaterally in the region of the triceps surae motor pool. In contrast with the cat (Jankowska and Edgley 2010), there was no evidence of collateral projections into LVIII. In comparison with Ia and Ib afferents, the sample of three group II afferents exhibited the greatest variability in the complexity of their terminal arborizations and number of varicosities. Arborization patterns in LV/VI, LVII, and LIX varied from highly developed and extensive (Fig. 4B) to weakly developed with simple branching (afferent 5, data not shown).
Figure 4C shows examples of varicosities expressing VGLUT1 ir in apparent synaptic contact with neurons in three laminae. Varicosities associated with soma and proximal dendrites on small and medium-sized neurons in LVI, on medium-sized neurons in LVII, and on large neurons in the dorsolateral ventral horn (Fig. 4D). Group II varicosities rarely contacted soma or proximal dendrites in the ventral portion of LIX.
Regional Distribution and Density of Afferent Varicosities
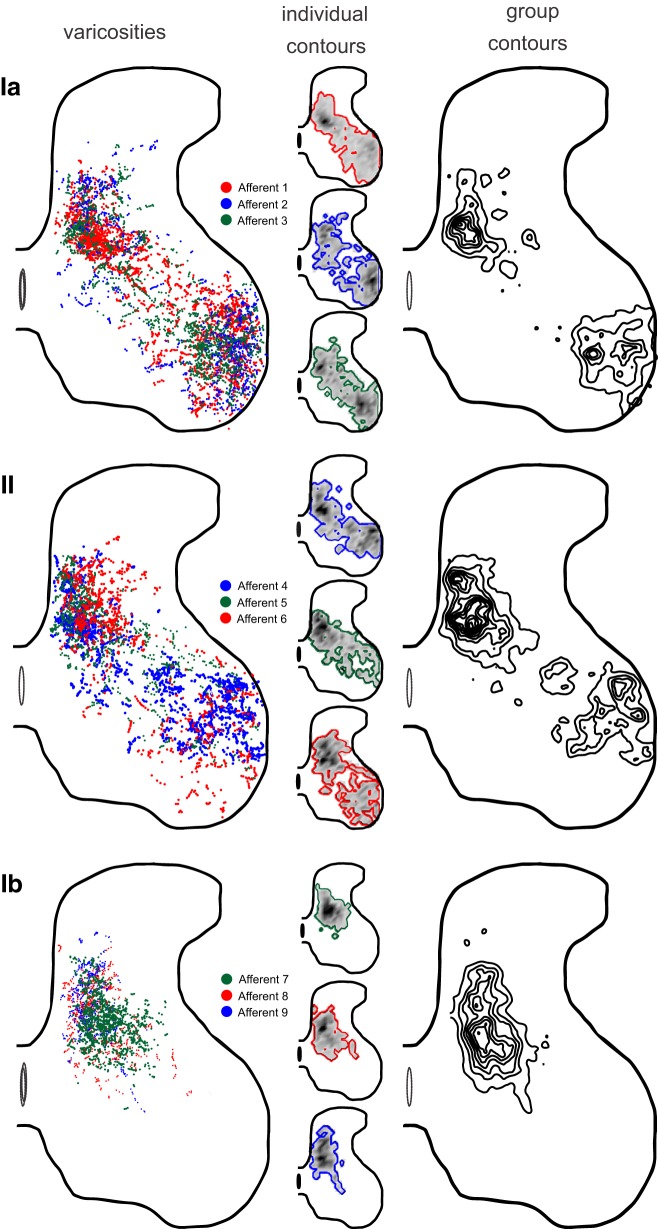
Figure 5 variously illustrates the territories of varicosities sampled from three afferents of each class. Figure 5, left, shows the locations of all identified varicosities, demonstrating approximate superposition of varicosities belonging to different afferents of the same class. All three labeled group Ia afferents aggregated varicosities densely in two locations, LV/VI and LIX, spanned by more sparsely distributed varicosities in LVII; group II afferents distributed synaptic varicosities in a similar pattern, although with lower density in LIX. Group Ib afferents were uniformly confined to LV–LVII. Figure 5, center, separates data from the nine individual afferents to illustrate the territory and density of varicosities for each. The fine-grained detail of each map was unique; however, the spatial distributions of varicosities belonging to afferents of the same class were broadly similar. Figure 5, right, presents contour maps that represent both territory and relative density of varicosities for the three afferent classes. These maps emphasize the intense convergence of varicosities belonging to individual afferents of all classes in the deep dorsal horn. The maps also accentuate the rank ordering of varicosity density in the ventral horn, which was highest for group Ia, modest for group II, and absent for group Ib afferents.
Fig. 5.
Distribution of afferent varicosities. Columns illustrate different views of the spatial distributions for varicosities from group Ia, II, and Ib afferents segregated in rows. Left: dots representing Neurobiotin-filled varicosities superimposed and color coded for afferent number. Two-dimensional images were constructed from sequential tissue sections oriented with respect to the spinal gray matter border and central canal in L4/5, with no attempt to demarcate lamina or shifting boundaries of gray matter from L6 to L3. Center: territory contour and density (grayscale intensity coded) of varicosities for each afferent. Right: contour maps of varicosities combined across all 3 afferents for each afferent class. Contour maps were created by calculating the density of varicosities and outlining areas above a threshold.
The laminar weighting of varicosities is compared in Fig. 6 and in Table 2. These data quantify the trends shown in Fig. 5 for the weighting of varicosities across laminae. Group Ib afferents distributed the majority of their varicosities (>85%) to LV/VI and none in LIX. Spindle afferents produced >80% of their varicosities in LV/VI and IX combined; group II afferents were more heavily represented in LV/VI, while group Ia afferents were biased toward LIX. All three afferent groups contributed relatively few varicosities, ≤20% to LVII. Figure 6 includes comparable data available only for group Ia afferents from earlier studies in rat (Alvarez et al. 2011) and cat (Ishizuka et al. 1979). Comparison of these data suggests a bias in the rat toward a lower proportion of Ia varicosities in LIX than in the cat.
Fig. 6.
Proportions of varicosities in target laminae from 3 classes of muscle proprioceptors: distribution of varicosities in each of 3 laminar regions expressed as % of total number of varicosities per individual afferent (see Table 2) classified as group Ia, II, or Ib in the present study (circles); for rat Ia afferents reported by Alvarez et al. (2011); and for cat Ia afferents reported in Ishizuka et al. (1979).
Despite these group trends, Fig. 6 shows wide variability in the laminar weighting of varicosities. The sets of both Ia and II afferents varied by 20% or more in LV/VI and LIX. Within-group variability was also noted in the firing properties of the nine labeled afferents (Table 3). For example, afferent 1 had a length threshold that exceeded the other two Ia afferents by 10-fold, and it exhibited an appreciably lower peak dynamic firing rate, properties more characteristic of group II. Among the three labeled group II afferents, afferent 4 had total varicosity count and dynamic and static firing rates more similar to the typical group Ia than group II afferents. This was also the only group II afferent with projections into the deep ventral horn (Fig. 5). These and other comparisons in Table 3 demonstrate that, just as for the proportion of afferent varicosities per lamina, firing responses to mechanical stimulation can vary widely among afferents belonging to the same class, to the extent of overlapping across class. No noteworthy population correlations were detected between variance in synaptic weighting and sensory encoding.
Table 3.
Firing response properties of Neurobiotin-labeled afferents
| Ia |
II |
Ib |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Afferent 1 | Afferent 2 | Afferent 3 | Afferent 4 | Afferent 5 | Afferent 6 | Afferent 7 | Afferent 8 | Afferent 9 | |
| Conduction delay, ms | 1.5 | 1.5 | 1.6 | 2.7 | 1.6 | 1.9 | 1.6 | 1.7 | 1.8 |
| ThrL, mm | 0.4 | 0.03 | 0.04 | 0.1 | 0.2 | 0.1 | 2.3 | 1.0 | 1.0 |
| Dynamic firing | |||||||||
| Fast ramp | |||||||||
| Dyn(pfr), pps | 167 | 238 | 249 | 157 | 128 | 128 | 54 | 119 | 94 |
| Slope, fr/mm | 77 | −0.6 | 46 | 24 | 23 | 32 | 46 | 100 | 36 |
| No. of spikes | 14 | 33 | 34 | 20 | 16 | 14 | 3 | 6 | 7 |
| Slow ramp | |||||||||
| Dyn(pfr1), pps | 99 | 160 | 185 | 127 | 92 | 95 | 61 | 124 | 65 |
| Slope, fr/mm | 69 | −0.6 | 31 | 23 | 27 | 31 | 74 | 94 | 44 |
| No. of spikes | 32 | 98 | 94 | 71 | 53 | 48 | 13 | 35 | 16 |
| Static firing | |||||||||
| Stat(mfr), pps | 14 | 85 | 49 | 101 | 69 | 68 | 27 | 45 | 53 |
| Stat(fd), ms | 921 | 995 | 780 | 1,000 | 1,000 | 996 | 996 | 741 | 998 |
| No. of spikes | 18 | 96 | 94 | 105 | 73 | 70 | 26 | 35 | 55 |
| Dynamic index | 153 | 154 | 200 | 56 | 59 | 60 | 28 | 119 | 41 |
Mechanosensory Encoding
Population statistics presented in Table 4 resolved tendencies in mechanosensory encoding expected for group Ia, II, and Ib afferents (Matthews 1963, 1972). For example, mean values for measures of dynamic firing, e.g., Dyn(pfr) and DI, decreased progressively from groups Ia to II to Ib. However, the firing responses within groups were highly variable, and the group differences were often less than one standard deviation. High variability in measures of static encoding, e.g., Stat(mfr), among individual afferents obscured any differences among the three afferent groups. Figure 7 illustrates the broad spectra of firing behaviors represented by two group II afferents, one with firing responses resembling Ia afferents and the other firing like a Ib afferent.
Table 4.
Population statistics for 3 classes of muscle proprioceptors
| Ia |
II |
Ib |
Unclassified |
|||||
|---|---|---|---|---|---|---|---|---|
| Means ± SD min-max | n | Means ± SD min-max | n | Means ± SD min-max | n | Means ± SD min-max | n | |
| Conduction delay, ms* | 1.5 ± 0.2(a) | 144 | 1.8 ± 0.4(b) | 62 | 1.6 ± 0.1(a) | 58 | 1.6 ± 0.2(a) | 34 |
| 1.2–2.1 | 1.3–3.0 | 1.3–1.8 | 1.4–2.2 | |||||
| ThrL, mm* | 0.2 ± 0.2(a) | 140 | 0.6 ± 0.6(b) | 61 | 0.9 ± 0.8(c) | 56 | 0.8 ± 0.7(bc) | 34 |
| 0.01–1.0 | 0.0–2.0 | 0.0–2.9 | 0.0–2.7 | |||||
| Dynamic firing | ||||||||
| Fast ramp | ||||||||
| Dyn(pfr), pps* | 176.4 ± 53.2(a) | 144 | 105.4 ± 48.9(b) | 62 | 66.9 ± 26.3(c) | 58 | 137.1 ± 31.4(d) | 34 |
| 48.1–330.7 | 29.5–234.7 | 26.5–132.7 | 56.8–184.37 | |||||
| Slope, fr/mm | 34.8 ± 23.1(a) | 120 | 38.0 ± 24.3(a) | 54 | 30.2 ± 25.0(a) | 47 | 60.65 ± 28.7(b) | 34 |
| −45.7 to 108.9 | 4.6–115.0 | 0.0–123.4 | 19.16–131.8 | |||||
| No. of spikes* | 19.6 ± 7.9(a) | 144 | 10.7 ± 5.4(b) | 62 | 6.3 ± 2.7 | 58 | 11.7 ± 4.6(b) | 34 |
| 7–48 | 3–25 | 1–14 | 4–23 | |||||
| Slow ramp | ||||||||
| Dyn(pfr1), pps* | 124.4 ± 54.1(a) | 129 | 78.2 ± 34.4(b) | 52 | 55.8 ± 20.1(b) | 48 | 87.5 ± 32.7(b) | 24 |
| 42.8–378.8 | 24.8–163.4 | 23.9–124.4 | 41.4–157.2 | |||||
| Slope, fr/mm | 28.5 ± 12.8(a) | 129 | 23.3 ± 11.6 | 51 | 28.1 ± 37.5 | 47 | 33.0 ± 12.7 | 23 |
| −18.6 to 68.3 | 4.7–62.7 | 0.1–217.5 | 12.2–62.6 | |||||
| No. of spikes* | 56.7 ± 30.0(a) | 129 | 35.4 ± 21.4(b) | 52 | 16.8 ± 8.5(c) | 48 | 33.7 ± 21.7(bc) | 24 |
| 2–196 | 6–80 | 5–35 | 4–97 | |||||
| Static responsiveness | ||||||||
| Stat(mfr), pps | 30.1 ± 48.4 | 138 | 43.3 ± 33.2 | 62 | 28.6 ± 19.6 | 58 | 29.6 ± 29.2 | 34 |
| 0.2–165.0 | 0.2–130.2 | 0.2–75.2 | 0.2–100.4 | |||||
| Stat(fd), ms | 831.0 ± 269.1 | 142 | 929.5 ± 196.4 | 62 | 923.0 ± 186.3 | 58 | 861.0 ± 296.4 | 33 |
| 0.5–1,000.0 | 79.2–1,000.0 | 76.6–1,000.0 | 76.6–1,000.0 | |||||
| No. of spikes | 45.3 ± 33.0 | 143 | 52.7 ± 35.3 | 62 | 38.0 ± 15.5 | 58 | 40.4 ± 27.26 | 34 |
| 0–199 | 1–152 | 2–84 | 0–109 | |||||
| Dynamic index* | 137.9 ± 48.4(a) | 143 | 54.2 ± 32.7(b) | 62 | 30.4 ± 21.0(c) | 58 | 97.8 ± 34.3(d) | 34 |
| 8.1–273.6 | 7.2–119.6 | 4.2–118.8 | 11.4–166.5 | |||||
Firing properties are reported as means ± SD for n afferents followed by range from minimum to maximum value.
Significant difference among afferents by ANOVA (P < 0.01); entries with different letter codes are significantly different by Tukey least significant difference (P < 0.01).
Fig. 7.
Similarity in firing responses of different classes of muscle proprioceptors. Examples are shown for 1 group II afferent (B) with firing properties resembling those of a representative Ia afferent (A) and for another group II afferent (C) that fired similarly to a typical Ib afferent (D). Right: action potential firing (gray traces) during isometric twitches (black traces) distinguish spindle afferents (group Ia and II) from the tendon organ afferent (group Ib). Left: action potentials in gray traces superimposed by black dots representing instantaneous firing rates in response to fast muscle stretch (ramp-hold-release 3 mm, 20 mm/s).
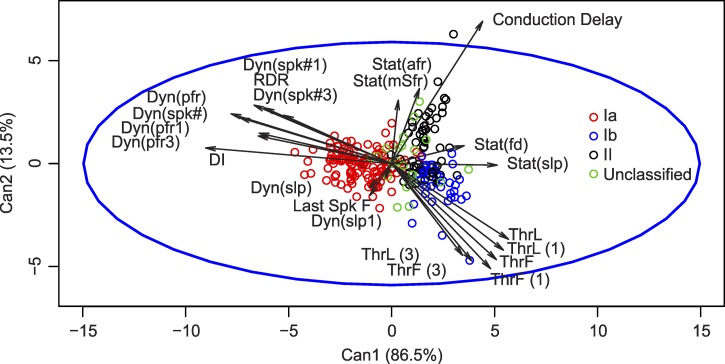
Because single measures of afferent firing provided little insight into the unique information encoded by individual afferents, we applied linear discriminant analysis (see methods) using all response properties to provide a more holistic account of the physiologically relevant information encoded by afferents. Discriminant analysis was performed on 212 of 264 classified afferents for which all 22 properties of mechanosensory encoding were measured (118 Ia, 44 Ib, and 50 II). Analysis yielded two canonical variables (Fig. 8) that allowed a posteriori classification with overall 88% accuracy (Ia: 96%, Ib: 91%, II: 68%). Of the misclassified Ib afferents, all were classified as group II. Of the misclassified group II afferents, 19% were classified as Ia and 81% as Ib. This analysis indicates that although there is substantial separation in the parameter spaces occupied by the three afferent groups, those spaces are not completely disjoint, i.e., the overlap seen in individual parameter distributions was maintained in the higher-dimensional parameter space.
Fig. 8.
Canonical variable mapping of muscle proprioceptors. Linear discriminant analysis determined canonical variables that accounted for 86.5% (Can1), leaving 13.5% (Can2) of population variance (see methods). Vectors represent the correlation between individual parameters and the canonical variables. Points represent individual afferents classified by color; unclassified afferents are shown but were not used in formulating canonical variables. Parameter abbreviations (see also Fig. 1 and methods): firing threshold (Thr) as the time (ThrT), length (ThrL), or force (ThrF) measured for the first stretch-evoked action potential; peak instantaneous rate of high-frequency initial burst firing (IBfr); peak dynamic firing rate Dyn(pfr) occurring at peak stretch; static firing rate at midpoint of hold phase Stat(mfr) and static firing duration Stat(fd); dynamic index (DI) = Dyn(pfr) − Stat(mfr); additional measures during ramp (Dyn) and static (Stat) muscle stretch included numbers of spikes [Dyn(spk#) and Stat(spk#)], average firing rates [Stat(afr)], and slope of linear regression between instantaneous firing rate vs. muscle length [Dyn(slp)] and [Stat(slp)]. Parameters designated with (1) or (3) were measured from the 1st and 3rd slow triangular stretches, respectively (cf. Fig. 1); reduced dynamic response was calculated as Dyn(Spk1) − Dyn(spk3); see Haftel et al. (2004).
Correlations between the canonical variables and their components shown in Fig. 8 clustered mainly around three groups that seem to represent dynamic response, static response, and threshold or sensitivity. Although all parameters contained some degree of unique information (no one parameter was perfectly correlated with any other), clustered parameters reflected substantial redundant information, such as similar measures, e.g., Dyn(pfr), or derived measures, like DI, calculated as the difference between Dyn(pfr) and Stat(mfr).
Because 20-dimensional spaces are difficult to visualize, we chose parameters representative of velocity, length, and sensitivity signals from the groups of canonical correlates to evaluate differential encoding of these parameters in rat afferents (Fig. 9). Dyn(pfr) was used to represent afferent encoding of muscle stretch velocity, Stat(mfr) for muscle length, and ThrL for stretch sensitivity. Viewed in this space, group Ia afferents were distinguishable by their dynamic (velocity) and sensitivity (threshold) signaling but not by their static (length) response, which overlaps considerably among all three afferent classes. Group II and Ib afferents exhibited substantial overlap in all three planes. In sum, Fig. 9 shows that even when comparison was based on vectors that strongly capture canonical separation among afferent groups, mechanosensory signaling by individual afferents belonging to different classes could be ambiguous.
Fig. 9.
Selected canonical parameters characterizing afferent types. Surface projections generated from group covariance matrices represent 1 standard deviation (SD) from group means, with ellipses projected onto corresponding planes. Surfaces were constructed from all classified afferents (n = 212); SDs in the 2-dimensional planar projections ideally enclose 46% (68%2) of data points, and SDs in the 3-dimensional ellipsoids enclose 31% (68%3) of the data. Despite the separation of afferent classes emphasized by this statistical depiction, the figure shows considerable overlap, especially among group II and Ib afferents.
γ-Motoneuron Influence
The firing responses of muscle spindle afferents are regulated by γ-motoneuron input to spindle receptors (Matthews 1981b), making it important to assess the influence of this input on the present data sample. We assume that the influence on spindle afferent responses was minimal, because all experiments were performed on rats deeply anesthetized by isoflurane. To test that assumption, we examined the responses of 21 group Ia afferents in three rats with γ-motoneuron input to spindles eliminated by sectioning ventral roots during the recording sessions. In these experiments we selected Ia afferents for study because their firing is very responsive to γ-motoneuron drive of muscle spindles (Matthews 1981b). In support of our assumption, the firing response parameters measured from Ia afferents in isoflurane-anesthetized rats were not significantly different (ANOVA with Tukey P > 0.05) for those with ventral roots cut vs. intact. In rats with ventral roots cut, for example, Ia afferent mean ± SD (range) for Dyn(pfr), Stat(mfr), and DI were 140 ± 73 (75–250), 26 ± 20 (1 ± 69), and 114 ± 67 (7–227), respectively. Corresponding values for peak dynamic firing and dynamic index seen in Table 4 for rats with intact ventral roots were nominally but not significantly greater. We conclude, therefore, that gamma efferent input had little effect on our findings.
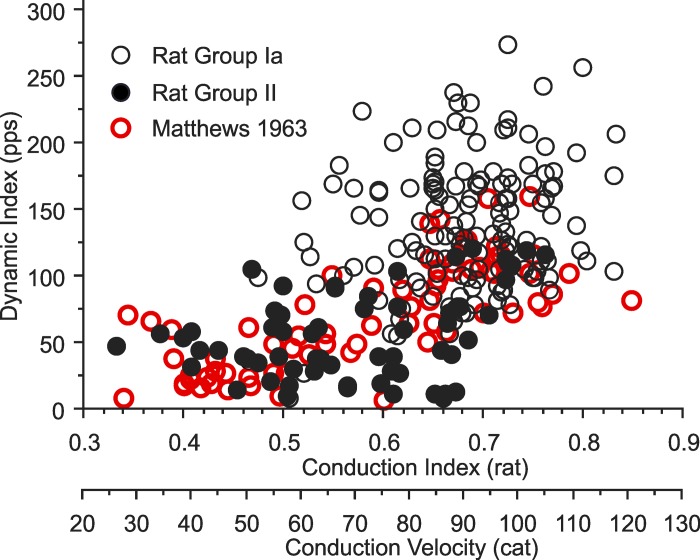
Comparison of Rat vs. Cat Dynamic Index
Published data for cat made it possible to compare dynamic index for reasonably sized data samples from both rat and cat. Matthews (1963, Fig. 12A) reports dynamic index for group Ia and II afferents supplying the deefferented soleus muscle in cat for strain and strain rate matched to that applied to triceps surae muscles here for rat, 7% and 47%/s, respectively. The overlay of these two data sets is shown in Fig. 10. This comparison demonstrates species-similar correlations between dynamic index and axon conduction speed, suggesting a similar underlying continuum across group Ia and II afferents. By contrast, Fig. 10 also illustrates a prominent difference in the two data sets. The population of rat Ia afferents exhibits a conspicuous upward shift of dynamic index, which is considered in Interspecies Scaling Factors in Sensory Feedback Control of Movement in relation to differences in size and motor behavior of rats vs. cats.
Fig. 10.
Dynamic index vs. speed of axon conduction for rat and cat spindle afferents. Each symbol is taken from single afferents sampled from rat triceps surae muscles in the present study, with conduction index calculated as the reciprocal of conduction delay, and from cat soleus muscles reported in Matthews (1963), with conduction speed in m/s and group Ia > 72 m/s < group II.
DISCUSSION
The distributions of axon collaterals and their provisional synapses in spinal laminae are shown here for all three classes of muscle proprioceptors for the first time in an adult rodent. We found that physiologically classified group Ia, II, and Ib afferents projected to nearly the same territories in hindlimb spinal segments known in cat. The encoding of physiologically relevant parameters of muscle stretch characterized by population statistics and discriminant analysis also aligned with customary afferent classification, as it does in the cat. Nonetheless, mechanosensory encoding by muscle afferents in the rat exhibited unique features. The firing responses of Ib afferents were robust within the physiological range of passive muscle stretch, and in some cases similar to responses generated by individual group II afferents. Also notable was the heightened signaling of dynamic muscle stretch by Ia afferents, which for rats was disproportionately greater than for cats, even when accounting for species differences in the physics of movement.
Axon Collaterals and Varicosities Distributed by Muscle Proprioceptor Class
In spinal segments containing triceps surae motor pools, the spatial distributions of axon collaterals and varicosities in Rexed laminae were broadly the same for group Ia, II, and Ib afferents in the rat as reported for the cat (cf. Brown 1981a, 1981b; Brown and Fyffe 1978, 1979; Ishizuka et al. 1979). All three classes of muscle proprioceptors converged in the medial half of the deep dorsal horn (LV, LV1, dorsal LVII), while only the muscle spindle afferents projected deep into LVII and into motor nuclei in LIX. A similar pattern is apparent in the adult mouse (Fukuhara et al. 2013; Leslie et al. 2011), although definitive data are not available for the three afferent classes. Comparability in these cases supports generalizability of the basic pattern established in the cat, suggesting that intraspinal axon projections and synaptic distribution are roughly the same for muscle proprioceptors in these and possibly other mammals. While the cytoarchitectonics of spinal segments as well as motor pool organization are similar among these species (Molander et al. 1989; Rexed 1954), it remains to be determined whether muscle proprioceptors synapse with the same first-order interneurons and contribute equally to the same spinal networks in cats and rodents. Information available for the molecular identity of premotor interneurons and their precursors in adult mammals is restricted to the mouse (Goulding 2009), while details about the physiological profile of individual spinal interneurons exist only for the cat (Edgley and Jankowska 1987; Jankowska 2001). It remains unknown, therefore, whether the spinal reflex networks receiving input from muscle proprioceptors are assembled from analogous neurons across mammalian species. Although proprioceptor input to deep dorsal horn interneurons, for example, appears to play a similar role in coordinating limb muscles in cats (Jankowska and Edgley 2010) and mice (Levine et al. 2014), the possibility of species specialization in the underlying networks has not been tested.
Some features of laminar distribution of spindle afferents in rats may represent species specialization, although differences from cats may be more apparent than real, as a result of variability in individual afferents coupled with the small number of afferents that have been sampled both in cats and in rats. In contrast with cats, group Ia and II varicosities in LVII were sparsely distributed rather than concentrated adjacent to triceps surae motor pools in dorsolateral LIX. Additionally, group Ia varicosities appeared in greater density ventral to triceps surae motor pools, where they contacted soma and proximal dendrites of neurons smaller than α-motoneurons as well as dendrites possibly belonging to the expansive arbors of triceps surae motoneurons. One further possible distinction from the cat is the absence of group II collaterals or varicosities in LVIII. This distinction might suggest species differences in spinal coordination of right-left hindlimb movements, since commissural premotor interneurons reside in LVIII (Jankowska and Edgley 2010). This possibility will require further study, however, because, just as we show for the rat, group II afferent terminations in LVIII have not been demonstrated morphologically in the cat (Fyffe 1979, Brown 1981a), even though their presence is verified electrophysiologically by field potentials in cats (Edgley et al. 2003).
Mechanosensory Signaling by Muscle Proprioceptors
The profile of firing responses by muscle proprioceptors reported for rats here and previously (Bullinger et al. 2011b; De-Doncker et al. 2003; Haftel et al. 2004; Leslie 1973; Lewin and McMahon 1991; Vincent et al. 2016) resembled that reported for cats (Matthews 1972, 1981a). Muscle afferents were generally classifiable as group Ia, II, or Ib as they are in cat, on the basis of their responses to artificial stimulation of muscle, i.e., electrically evoked isometric twitch and high-frequency vibration. Population statistics and canonical analyses of behaviorally relevant firing properties aligned with these classes to yield qualitatively similar descriptions of muscle proprioceptors in the two species. By that description, dynamic muscle kinematics were generally represented by group Ia afferents, static muscle kinematics by group II afferents, and higher stretch threshold by group Ib afferents.
Mechanosensory distinctions among afferent classes that were recognizable in aggregate were much less evident for individual afferents. Quantitative variation for single parameters were distributed as a continuum, with considerable intermingling among different individuals from different afferent classes. Although it is difficult to find thorough representation of population variability in the cat literature, it does seem that muscle afferents in rats are more ambiguous than in cats in signaling kinematic and kinetic parameters (Houk et al. 1981; Matthews 1963). It is possible, however, for rats as in cats, that γ-motoneuron tuning of muscle spindles acts to regulate distinctions in signaling by different afferent classes (Ellaway et al. 2015; Matthews 1972).
Two features of mechanosensory signaling by muscle proprioceptors in rats stood out in contrast with cats. One difference was the robust firing of Ib afferents in response to stretching passive muscle in the physiological range. While Ib firing rates were much lower than those observed during active muscle contraction, the signal for passive muscle stretch for Ib afferents approached that of group II afferents (e.g., Fig. 7). These observations appear in contrast with the absence of Ib firing to physiologically relevant stretch of passive muscle in cat (Houk et al. 1981), although this point has been debated (Jami 1992). Our findings in rats suggest that group Ib and II afferents fire together, for example, when noncontracting triceps surae muscles lengthen during the swing phase of a step cycle. In this way, Ib afferents would act to reinforce the signal for passive muscle stretch sent to deep dorsal horn. For rats, therefore, Ib afferents may play a dual role by representing both passive and active muscle tension. A second distinction in mechanosensory signaling in rat was the exaggerated encoding of dynamic muscle stretch (see Fig. 10). Next, we consider this distinction in relation to species differences in size and behavior.
Interspecies Scaling Factors in Sensory Feedback Control of Movement
We expect that the general behavior and control of locomotion is similar across species, in the sense that quadrupeds are capable of walk, trot, and run gaits with analogous kinematics. However, geometrical and physical constraints require that the descriptors of similar gaits be adjusted for different body sizes, raising the questions of whether and where these adjustments are mirrored in the sensory system. The dynamic similarity hypothesis (Alexander and Jayes 1983) claims that animals will use similar gaits characterized by the ratio of kinetic to gravitational energy (Froude number). It predicts that stride length and muscle length should scale linearly with hip height, so rat and cat muscles should experience similar strain (~25%; Burkholder and Lieber 2001). Stride frequency, i.e., speed of locomotion, should scale with L−½(2). Relative to a cat with 22-cm hip height at midstance, the muscles of a rat having 3.7-cm hip height at midstance should strain ~150% faster.
In assessing scaling of proprioceptor feedback, we were particularly interested in gain of dynamic signaling for group Ia afferents, canonically described as more velocity sensitive, and group II afferents, canonically described as more length sensitive. The fast-ramp parameters used in these experiments in rats represented 7% strain at 47%/s strain rate, which are values similar to those measured during swing phase in slow walking (Hodson-Tole and Wakeling 2010). Using dynamic similarity to scale these stretch parameters to the cat yielded equivalent values of 7% and 20%/s, for which we estimated the firing responses of cat afferents from Matthews (1963), using his Figure 10. At these dynamically similar velocities, dynamic index for cat Ia afferents is ~100 pps vs. 25 for group II, a 4-fold ratio, moderately greater than the 2.6-fold ratio (140 vs. 54) that we find in rats. In comparison, the static response (SFR) following a 7% stretch in cats is 67 pps for Ia and 53 pps for II, whereas we find 30 pps (Ia) and 44 pps (II) in rats. The rat spindle afferents, particularly the Ia afferents, have lower SFR than the cat. The ratio DI/SFR, i.e., dynamic sensitivity, is 4.5 in rat Ia and 1.5 in cat Ia, while these ratios are 0.8 and 0.5 in group II. Thus the relative gain of dynamic information from Ia afferents, even under dynamically similar conditions, is much higher in the rat, i.e., the scaling of neural dynamic sensitivity for rat exceeds the kinematic predictions of dynamic similarity.
It is inappropriate to infer an allometric relationship from just two example species, but the exaggerated dynamic sensitivity of rat Ia afferents may indicate that scaling within the sensory and control system is subject to constraints beyond stress, strain, and their rates of change. Those constraints would more likely be constraints on the physics and scaling of the neurons themselves and the networks they form (MacGregor and Tajchman 1988). Scaling in the nervous system occurs through increase not in cell size but instead in cell number, which leads to a potentially exponential expansion in interneuronal connectivity and complexity of neural networks. Scaling laws within the nervous system may be more dependent on network connectivity than on Newtonian mechanics. We suggest the possibility that a small animal with few afferents may need greater gain to convey the same information as larger animals having more afferents (Banks 2006).
Comparison of walking in rats and cats may not be appropriate, as rats prefer brief bursts of high-speed locomotion whereas cats prefer sustained, low-speed locomotion. Given free access to running wheels, rats choose bouts of 5–10 m at 1–2 m/s, depending on resistance applied to the wheel (Legerlotz et al. 2008), corresponding to fast gallop, and their preferred speed for overground locomotion is 1.4–1.7 m/s (Perry et al. 1988). The preferred speed of cats for overground locomotion is 0.7–1.4 m/s (Blaszczyk and Loeb 1993), corresponding to a slow walk. According to Gillis and Biewener (2001) in the rat and to Goslow et al. (1973) in the cat, the swing phase of locomotion, which the present experiments were chosen to represent, changes little as speed and gait progress from slow walk to fast gallop. The rat’s exaggerated dynamic sensitivity seems to parallel its preference for faster gaits, suggesting that dynamic sensitivity scales with locomotor behavior. More accurate estimates of musculoskeletal dynamics under preferred locomotion conditions would provide a better framework for comparison of the afferent responses.
Summary and Conclusions
The findings presented here show that conventional classes of muscle proprioceptors in rats distributed information to segmental spinal laminae closely, though maybe not exactly, analogous to cats. Specializations were observed in rats for sensory encoding of mechanical variables, consistent with the idea that CNS processing of kinetic and kinematic state variables requires adaptation to species differences in physical dimensions and behavior. Further study will be required to determine whether heightened firing responses found here for group Ia and Ib afferents in the rat actually reflect species-dependent tuning of kinematic and kinetic state variables. We expect that species adaptations will also be found in the spinal network computations that rely on information from muscle proprioceptors to control movement.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant P01 NS-057228.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.V., H.M.G., P.N., and T.C.C. conceived and designed research; J.A.V., H.M.G., A.S.D., and P.N. performed experiments; J.A.V., H.M.G., A.S.D., P.N., T.J.B., and T.C.C. analyzed data; J.A.V., H.M.G., A.S.D., P.N., R.E.F., T.J.B., and T.C.C. interpreted results of experiments; J.A.V., H.M.G., P.N., and T.C.C. prepared figures; J.A.V., T.J.B., and T.C.C. drafted manuscript; J.A.V., H.M.G., A.S.D., P.N., R.E.F., T.J.B., and T.C.C. edited and revised manuscript; J.A.V., H.M.G., A.S.D., P.N., R.E.F., T.J.B., and T.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Lori Goss for technical assistance and to Dario Carrasco, Francisco Alvarez, Travis Rotterman, and Nicolas Housley for consultation and critical review of the manuscript.
REFERENCES
- Alexander RM, Jayes AS. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J Zool 201: 135–152, 1983. [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. doi: 10.1152/jn.01095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron 74: 975–989, 2012. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M.. Integration in spinal neuronal systems. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am Physiol Soc, 1981, sect. 1, vol. II, p. 509–595. [Google Scholar]
- Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat 208: 753–768, 2006. doi: 10.1111/j.1469-7580.2006.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Hulliger M, Saed HH, Stacey MJ. A comparative analysis of the encapsulated end-organs of mammalian skeletal muscles and of their sensory nerve endings. J Anat 214: 859–887, 2009. doi: 10.1111/j.1469-7580.2009.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA. Scaling body support in mammals: limb posture and muscle mechanics. Science 245: 45–48, 1989. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- Binder MD, Osborn CE. Interactions between motor units and Golgi tendon organs in the tibialis posterior muscle of the cat. J Physiol 364: 199–215, 1985. doi: 10.1113/jphysiol.1985.sp015739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczyk J, Loeb GE. Why cats pace on the treadmill. Physiol Behav 53: 501–507, 1993. doi: 10.1016/0031-9384(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord. Berlin: Springer, 1981a. doi: 10.1007/978-1-4471-1305-8 [DOI] [Google Scholar]
- Brown AG. The spinocervical tract. Prog Neurobiol 17: 59–96, 1981b. doi: 10.1016/0301-0082(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol 274: 111–127, 1978. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol 296: 215–226, 1979. doi: 10.1113/jphysiol.1979.sp013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011a. doi: 10.1152/jn.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Wang Q, Rich MM, Cope TC. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. J Neurophysiol 106: 704–709, 2011b. doi: 10.1152/jn.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol 372: 465–485, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol 204: 1529–1536, 2001. [DOI] [PubMed] [Google Scholar]
- De-Doncker L, Picquet F, Petit J, Falempin M. Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J Neurophysiol 89: 442–449, 2003. doi: 10.1152/jn.00153.2002. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R. Exploring dynamic similarity in human running using simulated reduced gravity. J Exp Biol 203: 2405–2415, 2000. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol 389: 647–674, 1987. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from feline group II muscle afferents. J Physiol 552: 961–974, 2003. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat 227: 157–166, 2015. doi: 10.1111/joa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley CT, Glasheen J, McMahon TA. Running springs: speed and animal size. J Exp Biol 185: 71–86, 1993. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, Yoshida Y. Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep 5: 748–758, 2013. doi: 10.1016/j.celrep.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. The morphology of group II muscle afferent fibre collaterals J Physiol 296: 39P–40P, 1979. [PubMed] [Google Scholar]
- Gabriel HM, Vincent JA, Nardelli P, Deardorff AS, Fyffe RE, Cope TC. Muscle proprioceptors and their central connections in the adult rodent. Abstract presented at the Society for Neuroscience 2015 Annual Meeting San Diego, CA: 2015. [Google Scholar]
- Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J Exp Biol 204: 2717–2731, 2001. [DOI] [PubMed] [Google Scholar]
- Goslow GE Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8: 233–261, 1985. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol 91: 2164–2171, 2004. doi: 10.1152/jn.01147.2003. [DOI] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. The influence of strain and activation on the locomotor function of rat ankle extensor muscles. J Exp Biol 213: 318–330, 2010. doi: 10.1242/jeb.031872. [DOI] [PubMed] [Google Scholar]
- Houk JC, Rymer WZ, Crago PE. Dependence of dynamic response of spindle receptors on muscle length and velocity. J Neurophysiol 46: 143–166, 1981. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res 1017: 69–76, 2004. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Ijspeert AJ. Central pattern generators for locomotion control in animals and robots: a review. Neural Netw 21: 642–653, 2008. doi: 10.1016/j.neunet.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Mannen H, Hongo T, Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol 186: 189–211, 1979. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72: 623–666, 1992. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533: 31–40, 2001. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins FA., Jr Limb posture and locomotion in the Virginia opossum (Didelphis marsupialis) and in other non-cursorial mammals. J Zool 165: 303–315, 1971. doi: 10.1111/j.1469-7998.1971.tb02189.x. [DOI] [Google Scholar]
- Legerlotz K, Elliott B, Guillemin B, Smith HK. Voluntary resistance running wheel activity pattern and skeletal muscle growth in rats. Exp Physiol 93: 754–762, 2008. doi: 10.1113/expphysiol.2007.041244. [DOI] [PubMed] [Google Scholar]
- Lerche P, Muir WW III, Bednarski RM. Nonrebreathing anesthetic systems in small animal practice. J Am Vet Med Assoc 217: 493–497, 2000. doi: 10.2460/javma.2000.217.493. [DOI] [PubMed] [Google Scholar]
- Leslie GC. Vibration sensitivity of muscle spindle endings in a rat hindlimb muscle and its relationship to conduction velocity. J Physiol 230: 45P–46P, 1973. [PubMed] [Google Scholar]
- Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y. Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development 138: 4085–4095, 2011. doi: 10.1242/dev.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, Pfaff SL. Identification of a cellular node for motor control pathways. Nat Neurosci 17: 586–593, 2014. doi: 10.1038/nn.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skeletal muscle in adult rats. J Neurophysiol 66: 1218–1231, 1991. [DOI] [PubMed] [Google Scholar]
- MacGregor RJ, Tajchman G. Theory of dynamic similarity in neuronal systems. J Neurophysiol 60: 751–768, 1988. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol 320: 1–30, 1981a. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. The response of de-efferented muscle spindle receptors to stretching at different velocities. J Physiol 168: 660–678, 1963. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Mammalian Muscle Receptors and Their Central Actions. Baltimore, MD: Williams and Wilkins, 1972, p. 1–59. [Google Scholar]
- Matthews PB. Muscle spindles: their messages and their fusimotor supply. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am Physiol Soc, 1981b, sect. 1, vol. II, p. 189–208. [Google Scholar]
- McMahon TA. The role of compliance in mammalian running gaits. J Exp Biol 115: 263–282, 1985. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Valiant G, Frederick EC. Groucho running. J Appl Physiol (1985) 62: 2326–2337, 1987. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol 230: 133–141, 1984. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Rivero-Melian C, Grant G. Cytoarchitectonic organization of the spinal cord in the rat. II. The cervical and upper thoracic cord. J Comp Neurol 289: 375–385, 1989. doi: 10.1002/cne.902890303. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Lin DC, Huyghues-Despointes CM. The role of musculoskeletal mechanics in motor coordination. Prog Brain Res 123: 369–378, 1999. doi: 10.1016/S0079-6123(08)62871-X. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol 217: 75–85, 1983. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16: 265–297, 1993. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Perry AK, Blickhan R, Biewener AA, Heglund NC, Taylor CR. Preferred speeds in terrestrial vertebrates: are they equivalent? J Exp Biol 137: 207–219, 1988. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Muscle spindle function during normal movement. Int Rev Physiol 25: 47–90, 1981. [PubMed] [Google Scholar]
- Prochazka A, Ellaway P. Sensory systems in the control of movement. Compr Physiol 2: 2615–2627, 2012. [DOI] [PubMed] [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2016. [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379, 1954. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci 34: 3475–3492, 2014. doi: 10.1523/JNEUROSCI.4768-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, Wikholm RP, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol 93: 227–252, 1986. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma 22: 442–465, 2005. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, d’Avella A, Bizzi E. Coordination and localization in spinal motor systems. Brain Res Brain Res Rev 40: 66–79, 2002. [DOI] [PubMed] [Google Scholar]
- Vincent JA, Nardelli P, Gabriel HM, Deardorff AS, Cope TC. Complex impairment of IA muscle proprioceptors following traumatic or neurotoxic injury. J Anat 227: 221–230, 2015. doi: 10.1111/joa.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Wieczerzak KB, Gabriel HM, Nardelli P, Rich MM, Cope TC. A novel path to chronic proprioceptive disability with oxaliplatin: distortion of sensory encoding. Neurobiol Dis 95: 54–65, 2016. (Erratum. Neurobiol Dis 98: 158, 2017). doi: 10.1016/j.nbd.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AH, Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol 433: 335–348, 2001. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515, 1996. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73: 155–202, 2007. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]