Abstract
Biomarkers for acute kidney injury have numerous potential roles to play both at the bedside and in the design and conduct of clinical trials. Given the heterogeneous nature of this disease and the difficulty, so far, in developing effective therapies, a strategy that deploys all of our available tools in the treatment and in study of treatments would seem prudent. In this review, we discuss how biomarkers will change the way we do we take care of patients with and do clinical trials in acute kidney injury and why, in fact, biomarkers are necessary.
Keywords: acute kidney injury, acute renal failure, biomarkers, clinical trial design, iagnostics, risk assessment
Biomarkers, substances that can be measured in a patient and whose concentration is indicative of disease presence, severity or outcome, can serve a variety of clinical purposes. They can be used for risk assessment, diagnosis, prognosis and for monitoring the clinical course or response to therapy. High expectations for existing biomarkers of acute kidney injury (AKI) have given way to much more modest views in the wake of many studies that have shown less than desirable characteristics. However, what has been lost in much of the current literature is what we can or should be expecting from biomarkers and how we can apply them for clinical practice or for clinical trials. In this review, we will discuss the current clinical environment in AKI and why we need biomarkers. We will discuss how biomarkers can be used and why we should expect that their performance will be limited by the nature of this complex disease. Finally, we will discuss how biomarkers will change the way we do clinical trials in AKI and why, in fact, they are necessary.
What is acute kidney injury?
First, let us consider what is AKI and why, given our ability to estimate glomerular filtration rate (GFR) with a variety of existing tools, we need biomarkers. Standard definitions for AKI (Table 1) have only been available for a relatively short time [1–3], and yet they have been almost universally integrated into clinical research. These definitions rely on functional assessments, surrogates if you will, for glomerular function – one aspect of kidney function as a whole. Serum creatinine (sCr), the most widely used marker of GFR, is a component of the definition and yet a single measurement of sCr itself is completely useless in differentiating acute from chronic kidney disease. Furthermore, while sCr is an adequate marker of GFR, sCr itself dose not correlate with hospital survival whether measured at the time of presentation [4] or the start of dialysis [5]. What predicts short- and long-term outcomes is the change in renal function and herein lies the problem. Changes in sCr require several hours to days before they reach a steady state following an injury to the kidney. Thus the change in sCr is an excellent tool for defining when a change in function has occurred but not particularly good for detecting that is occurring (or about to occur). Worse yet, serum creatinine is affected by changes in muscle mass, protein metabolism and tubular secretion so that its relationship with GFR can be complex. Finally, like all functional markers, creatinine may be both insensitive to and nonspecific for kidney damage.
Table 1.
Diagnostic criteria* and staging for acute kidney injury.
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | 1.5–1.9-times baseline OR ≥ 0.3 mg/dl (>26.5 µmol/l) increase* | <0.5 ml/kg/h for 6–12 h |
| 2 | 2.0–2.9-times baseline | <0.5 ml/kg/h for ≥12 h |
| 3 | 3.0-times baseline OR increase in serum creatinine to ≥4.0 mg/dl (353.6 µmol/l) OR initiation of renal replacement therapy OR, in patients <18 years, decrease in eGFR to <35 ml/min per 1.73 m2 | <0.3 ml/kg/h for ≥24 h OR Anuria for ≥12 h |
A diagnosis of acute kidney injury is based on a change in serum creatinine or urine output. For serum creatinine the increase should be at least 50% from baseline that is known or inferred to have occurred within 1 week or a documented increase in serum creatinine of at least 0.3mg/dl within 48 hours. Once the diagnosis of AKI is made, using either of the criteria, the patient is staged according to the worse criteria in each domain.
Reproduced with permission from [3].
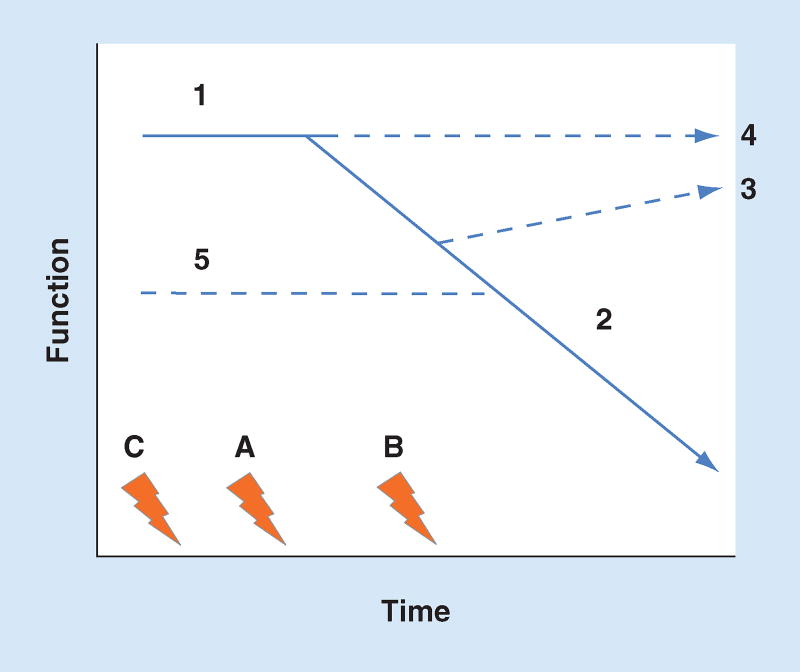
Figure 1 shows the relation between function and time in the setting of a set of hypothetical insults (A–C). In the most straightforward scenario, insult A occurs in the setting of normal function (1) and following the injury function declines (2) eventually reaching a new steady state. In this scenario, biomarkers that are sensitive for damage at insult (A) will predict the change in function when measured any time after the start of vector (2). However, function may recover (3) and thus if it were assessed too late, the decline (and the damage) could be missed. This is a fairly typical problem for biomarkers – when to assess the change in function. If it is assessed too early or too late, it may not be detected. However, this is not the most challenging problem. In the setting of normal function (1), an insult (A) may not be sufficiently severe to produce a change in renal function and it may appear as though no damage has occurred (4). When assessing AKI by examining function, the scenario in which insult A is followed by functional trajectory 4, the damage biomarker will be viewed as a false positive even when injury (damage) has actually occurred. The functional reserve of the kidney ensures that this scenario will occur frequently. A normal human can lose a kidney, in other words, one half of all nephrons, and no change in sCr will occur. Meanwhile, the same relative magnitude of damage (half the functioning nephrons lost) will always result in a change in function when baseline function is already impaired (line 5). This disparity between damage and function makes it very difficult to assess AKI and therefore very difficult to assess the potential of biomarkers. Also shown in the figure, is when function declines in response to an extra-renal process (e.g., shock) and only later does insult (B) occur. If a biomarker were to be measured prior to insult (B), the change in function (2) would prompt a false-negative determination when in fact the marker was working perfectly. Finally, many patients may have multiple insults (A–C) occurring over time. Some may be ‘silent’ (C), others may occur after (B) the initial insult. In some cases, only after multiple insults will function decline.
Figure 1. Shown is the relation between function and time in the setting of a set of hypothetical insults (A–C).
In the most straightforward scenario, insult A occurs in the setting of normal function (1) and following the insult function declines (2). Function may also recover (3) or there may be discernible change (4). Baseline function may already be impaired (line 5). Functional decline may also be due to an extrarenal process (e.g., shock) and only later does damage (B) occur. Finally, many patients may have multiple insults (A–C) occurring over time. Some may be ‘silent’ (C), others may occur after (B) the initial insult.
Given the significant limitations of sCr noted above, the criteria for defining AKI also includes urine output (UO). UO will often decrease before sCr increases making it a more time-sensitive marker of GFR. However, UO is not nearly as specific for GFR. While it is true that in the absence of obstruction if there is no UO, there can be no GFR, however not all reductions in UO signal AKI. Sustained oliguria is invariably associated with AKI but then, the timeliness of UO as an early indicator is less valuable.
Regardless of the measure one uses to assess function, the fact remains that functional change is neither necessary nor sufficient to define AKI as it is occurring. Over time a persistent functional change can be used to infer damage but acutely, the change may be purely functional. Inversely, the absence of functional change cannot be used to exclude damage, especially in previously healthy individuals with normal renal reserve. This reality creates an ‘upper limit’ for observed sensitivity and specificity for damage biomarkers such that clinical applications will almost certainly require tradeoffs. Said another way, a given biomarker (cutoff) may be sensitive or specific for AKI but not both; or separate cutoffs for high sensitivity and high specificity will be required. The clinical use of such markers will therefore require an understanding of the limitations implicit in their performance characteristics.
Clinical assessment for AKI
Although the criteria for AKI have been adopted into clinical practice by many, and while this adoption is recommended by some [3], it has not been without controversy [6,7]. The primary focus of criticism has been around the concern that functional criteria for AKI are too liberal [8,9]. Conversely setting the threshold high enough for functional criteria to be specific, means they become extremely insensitive and/or delay diagnosis to the point of being unworkable for clinical purposes.
A similar problem exists for early chronic kidney disease (CKD). The definition for CKD requires a 90-day duration to establish chronicity [10]. However, this arbitrary cutoff of 90 days for diagnosis of CKD should by no means trivialize persistent renal dysfunction following AKI just because 90 days has not been reached. To address the issue of the ‘black hole’ between AKI and CKD, the KDIGO AKI Workgroup proposed the concept of Acute Kidney Disease (AKD) [3]. AKD, defined as a GFR <60 ml/min/1.73 m2 or evidence of structural kidney damage for less than 3 months, provides an operationally integrated bridge between AKI and CKD. The AKD concept, which incorporates the concept of partial renal recovery, might also help raise awareness and engender the necessary clinical mechanisms to follow AKI survivors for progression to CKD, which has been recently highlighted as a missed opportunity for adequate transitions of care [11,12].
All of this, makes even modestly accurate biomarkers potentially useful. Consider the following example. A 68-year-old patient with underlying chronic disease (CKD, cardiovascular disease and diabetes) presents with a clinical diagnosis of pneumonia. She had an sCr of 1.6 mg/dl 5 months prior and now it is 1.8. She has only made 30 ml of urine in the 4 h she has been in the emergency department but her mucus membranes are dry and IV fluids have been started. She is given IV antibiotics as well but her WBC count and lactate are within normal limits and she is normotensive and oxygen saturation is 93% on room air. Her pulse rate is 88 but she does take a β-blocker. Clearly she is at high risk for AKI given both susceptibilities (advanced age and chronic disease) as well as an important exposure (pneumonia). However, what is the probability that she will develop AKI in the next 12–24 h? In a large epidemiologic study of patients with community-acquired pneumonia the event rate for AKI was 34% [13]. Even in the subgroup of patients not admitted to the ICU, the AKI event rate was 25%. However, more than half of these patients had AKI on presentation and a small number developed AKI late leaving about 5–10% of patients, like the one described above, developing AKI in the next 12–24 h after initial presentation. Obviously if 1–2 in 20 patients like this will develop a condition that decreases their chances of survival to less than half and doubles their hospital costs, it would be important to avoid missing them. Conversely if only 1–2 in 20 develop AKI, it is difficult to apply time-consuming and potentially resource-intensive interventions to all such patients. Clinical judgment cannot solve this problem. Although clinical risk prediction models have shown performance as high as an area under the receiver operating characteristic curve (AUC) of 0.81–0.84 [14,15], these models require several variables and are not intuitive. Neither have they been validated in subsequent cohorts and therefore their performance is likely overestimated. However, whether one is using a clinical risk prediction model or a biomarker panel or both, the sensitivity and specificity will not both be greater than 90% so the clinician using the test will need to understand how it may aid in, but not replace, clinical decision making.
Application of AKI biomarkers: clinical practice
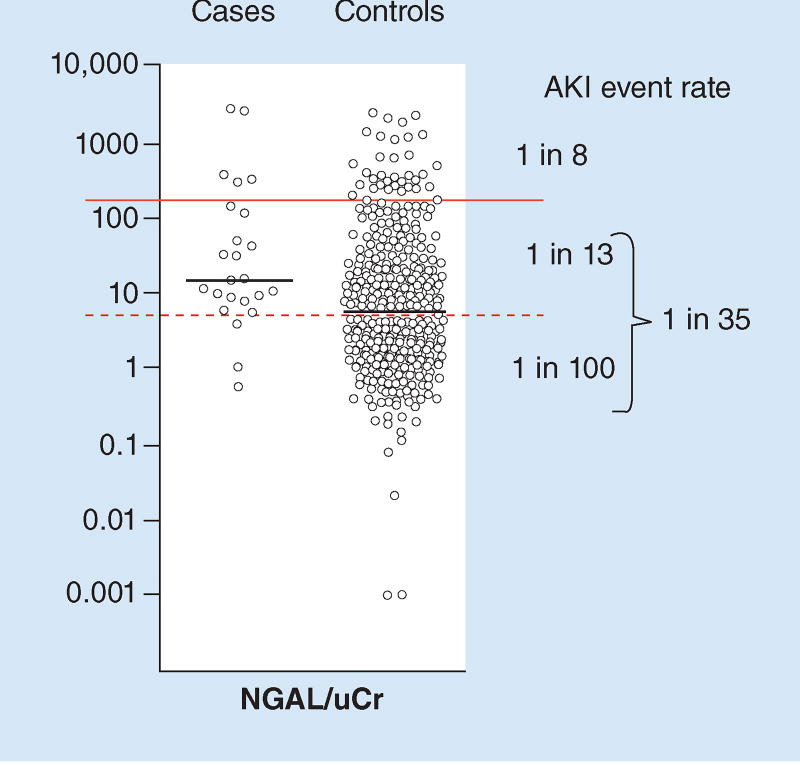
Let us consider a study by Endre and colleagues that found an AKI event rate of 5% within 24 h – comparable to the scenario described above [16]. Although this study found one of the poorest performing receiver-operating characteristic curves for one of the best studied biomarkers for AKI, urine neutrophil gelatinase associated lipocalin (uNGAL) (AUC = 0.68 for AKI at 24 h) patients developing AKI still tended to have higher values than patients that did not (Figure 2). Indeed, although overall 1 in 20 patients developed AKI, a cutoff can be found that separates the relative risk for AKI into a high risk group (1 in 8) and a low risk group (1 in 35) – a more than fourfold difference in risk. Indeed, we can even apply two cutoffs to separate the low risk group into a moderate risk (1 in 13) and very low risk subgroup (1 in 100). Table 2 provides some clinical decisions based on these three categories of risk for the patient described above. Further discussion on clinical applications can be found in recent reviews [17,18].
Figure 2. Results for urine neutrophil gelatinase associated lipocalin normalized by urine creatinine (uCr) in cases with acute kidney injury (AKI) versus controls without.
The solid line separates the cohort into two risk groups (high: 1 in 8 and low: 1 in 35). The dashed line further separates the low risk group into moderate risk (1 in 13) and very low risk (1 in 100). NGAL: Neutrophil gelatinase associated lipocalin; uCr: Urine creatinine Adapted with permission from [16].
Table 2.
Hypothetical clinical decision-making based on biomarker results.
| Decision type | Very low risk 1 in 100 | Moderate risk 1 in 13 | High risk 1 in 8 |
|---|---|---|---|
| Admission | Discharge or admit to low intensity unit | Admit to low intensity unit | Admit to ICU or high intensity unit |
| Monitoring: urine output sCr | No catheter, routine I/O daily | No catheter, strict I/O daily | Foley catheter q.12h I/O |
| Medications | No change | Avoid nephrotoxins | Consult pharmacist on dosing and selection |
| Subspecialty consultation | No | No | Yes |
Application of AKI biomarkers: clinical trials
A similar logic can be applied for the application of biomarkers in clinical research. Biomarkers can serve multiple purposes in clinical trials (Table 3). Each of these applications has tradeoffs (pros and cons) that are somewhat specific to the particular use. For example when used for enrichment (to exclude low-risk cases), the performance of a biomarker could have the following results. Let us consider a drug that is being studied to prevent AKI and to find a 20% relative risk reduction. Without the biomarker, let us say that the inclusion/exclusion criteria result in an event rate of 30% (i.e., a higher risk group than in our clinical example). A 20% relative risk reduction would mean a decrease to 24% (a 6% absolute risk reduction). A clinical trial designed to find this difference would require more than 3000 patients. However if some of the patients enrolled had virtually no risk and if we could use a biomarker (or another method) to exclude these patients we could enrich the population so that the event rate would increase. If we could enrich, for example, to a 50% event rate a 20% relative risk reduction would be to a 40% event rate so we would now have a 10% absolute risk reduction. A trial to find this difference could be nearly four-times smaller, requiring roughly 780 patients! Even when biomarkers perform ‘poorly’ as in the study by Endre discussed above, the effect they have on enrichment can be dramatic. Going from 1 in 35 to 1 in 8 is more than a fourfold increase in risk. If we were to use such a marker to separate the overall population with an event rate of 30% into a 12% low risk and 48% high risk (a fourfold difference), we could exclude the lower risk group and achieve the baseline event rate approaching 50%. Note this enrichment was achieved with a one of the lowest reported estimations of uNGAL performance (when applied to a higher baseline event rate). Most studies have found significantly greater discrimination and thus the potential for far greater enrichment [19]. There are disadvantages of this approach as well. Use of a biomarker in the way described above will invariably result in a limiting of the patient population that the drug is indicated for. In the example above, cutting the target population in half may not be desirable and patients with a 12% risk of AKI might also benefit, particularly if the therapy has few adverse effects. Finally, a study design employing a biomarker for patient selection could result in regulatory agencies requiring use of the biomarker in the product labeling.
Table 3.
Roles for biomarkers in clinical trials.
| Application | Pros | Cons |
|---|---|---|
| Entry criteria (inclusion or exclusion) | Increases absolute differences and statistical power. Reduces harm in prevention trials | Limit target population. Could necessitate biomarker for use |
| – Enrichment | Exclude low-risk patients | |
| – Narrow for effect | Exclude cases where drug cannot help (e.g., wrong etiology, injury already occurred). Also increases relative differences | |
| Adjudication of end points | ||
| – Increase end point detection | Increases number of events in placebo arm. Increases absolute differences and statistical power | Increases number of events in treatment arm |
| – Improve adjudication accuracy | Increases likelihood of finding effect | Requires acceptance of the biomarker, influenced by marker accuracy |
Other scenarios are also shown in Table 3. Biomarkers may be used to adjudicate endpoints. Increasing endpoints by using sensitive biomarkers to define the endpoint will increase events and increase statistical power. However, in order for the biomarker to be used in this way, it would need to be accepted in its own right – something that has been slow in coming for AKI biomarkers.
Conclusion & future perspective
New biomarkers for AKI are being developed and some include concepts that even transcend the function/damage paradigm discussed above. For example, urinary tissue inhibitor of metalloproteinases-2 (TIMP2) and insulin-like growth factor-binding protein 7 (IGFBP7), have recently been reported [14] and subsequently validated [20] for risk assessment for AKI in critically ill patients. Indeed, they have become the first AKI biomarkers to be approved by the US FDA. Importantly, both TIMP-2 and IGFBP7 may increase in response to a wide variety of insults (inflammation, oxidative stress, ultraviolet radiation, drugs and toxins) [21–23]. This may help explain why they correspond to risk for AKI, a syndrome known for its multiple etiologies even in the same patient. However, these insults may not actually destroy cells and these molecules appear to be able to signal in autocrine and paracrine fashions [23,24] thus behaving more like an ‘alarm’ spreading to adjacent cells. In terms of timing, this signal could be represent the earliest point of cellular stress. Biomarkers that can detect cellular stress (or conversely cell health) may be more useful than markers of damage or cell death. However, a note of caution is also required because cellular stress or even temporary functional change may not lead to long-term disability and are not themselves patient-centered outcomes.
Finally, as we better understand the processes whereby the cells are injured and attempt to protect themselves, there exists the possibility that we will be able to develop ‘theragnostics’ for the kidney. A theragnostic is a tool that can be used to guide therapy. For example if a stress marker is high and/or a health marker is low but the markers move in opposite directions when therapy is applied, one might be able to use them not only for diagnosis but to titrate therapy. Another obvious approach is to develop diagnostics and therapeutics together – for example replacing a protective substance or blocking a harmful molecule based on measuring its concentration. Note this approach will benefit from all of the advantages but suffer from the limitations of the enrichment strategy discussed above.
In summary, biomarkers have numerous potential roles to play both at the bedside and in the design and conduct of clinical trials. Given the heterogeneous nature of AKI and the difficulty, so far, in developing effective therapies, a strategy that deploys all of our available tools in the treatment and in study of treatments would seem prudent.
Executive summary.
What is acute kidney injury?
Functional changes are neither necessary nor sufficient to define acute kidney injury (AKI) as it is occurring.
Clinical assessment for AKI
AKI decreases chances of survival and doubles hospital costs – yet only a small fraction of those at risk for AKI develop it. Thus it is difficult to apply time-consuming and potentially resource-intensive interventions to all such patients.
Application of AKI biomarkers
Biomarkers can be used to enrich populations so as to focus resources or clinical investigation on those most likely to benefit.
Future perspective
In the future, new markers of cell stress may alter the way we think about clinical tests – risk assessment, not just diagnosis.
Acknowledgments
JA Kellum has received grants and served as a paid consultant to multiple companies involved in developing biomarkers for acute kidney injury, including Abbott, Alere, Astute and Roche. He has received royalties through the University of Pittsburgh for biomarker technology licensed to Astute Medical. P Devarajan has received royalties through Cincinnati Children’s Hospital for biomarker technology licensed to Abbott and Alere.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Clin. Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.KDIGO AKI Workgroup. Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012. 2012;2:1–141. First ever multidisciplinary international clinical practice guideline for acute kidney injury (AKI). [Google Scholar]
- 4.Bell M, Granath F, Mårtensson J, et al. Cystatin C is correlated with mortality in patients with and without acute kidney injury. Nephrol. Dial. Transplant. 2009;24(10):3096–3102. doi: 10.1093/ndt/gfp196. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 2002;13(5):1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 6.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am. J. Kidney Dis. 2013;61(5):649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 7.The Ad-Hoc Working Group of ERBP. Fliser D, Laville M, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines on Acute Kidney Injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol. Dial. Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ralib AM, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit. Care. 2013;17(3):R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin. J. Am. Soc. Nephrol. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann. Intern. Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 11•.Goldstein SL, Jaber BL, Faubel S, Chawla LS. Acute Kidney Injury Advisory Group of American Society of Nephrology. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin. J. Am. Soc. Nephrol. 2013;8(3):476–483. doi: 10.2215/CJN.12101112. Report from the American Society of Nephrology of long-term follow-up for patients sustaining an episode of AKI. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein SL, Chawla L, Ronco C, Kellum JA. Renal recovery. Crit Care. 2014;18(1):301. doi: 10.1186/cc13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535. doi: 10.1038/ki.2009.502. Large observational study of AKI in patients with non-severe sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care. 2013;17(1):R25. doi: 10.1186/cc12503. Discovery validation study of cell cycle arrest biomarkers in human AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr KF, Meisner A, Thiessen-Philbrook H, Coca SG, Parikh CR. Developing risk prediction models for kidney injury and assessing incremental value for novel biomarkers. Clin. J. Am. Soc. Nephrol. 2014;9(8):1488–1496. doi: 10.2215/CJN.10351013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79(10):1119–1130. doi: 10.1038/ki.2010.555. Large clinical study comparing multiple different biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 2008;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann. Clin. Biochem. 2014;51(3):335–351. doi: 10.1177/0004563214521795. Careful review of the current literature on neutrophil gelatinase-associated lipocalin as a biomarker of AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am. J. Respir. Crit. Care Med. 2014;189(8):932–939. doi: 10.1164/rccm.201401-0077OC. Multicenter validation study of cell cycle arrest biomarkers using clinical adjudication for AKI. [DOI] [PubMed] [Google Scholar]
- 21.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int. 2009;76(6):604–613. doi: 10.1038/ki.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Seo D-W, Li H, Qu C-K, et al. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J. Biol. Chem. 2006;281(6):3711–3721. doi: 10.1074/jbc.M509932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo S, Liu C, Wang J, et al. IGFBP-rP1 induces p21 expression through a p53-independent pathway, leading to cellular senescence of MCF-7 breast cancer cells. J. Cancer Res. Clin. Oncol. 2012;138(6):1045–1055. doi: 10.1007/s00432-012-1153-y. [DOI] [PubMed] [Google Scholar]