Abstract
Background: Average thyrotropin (TSH) levels are known to be higher in older adults when measured in cross-sectional populations. Possible etiologies include differential survival, neutral aging changes, or increased disease prevalence at older ages. This study aimed to elucidate the mechanisms underlying changing thyroid function during aging, and to determine the association of changes with survival, by analyzing the individual thyroid axis over time.
Methods: Individual patterns of changing TSH and free thyroxine (fT4) were determined in 640 participants in the Baltimore Longitudinal Study of Aging who had at least three measures of serum TSH and fT4, not on medications, over an average of seven years of follow-up. Participants with changing phenotypes were identified based on quintiles for both slopes. Those with alterations in primary thyroid gland function demonstrated intact negative feedback (rising TSH with declining fT4 or declining TSH with rising fT4). Other participants had a parallel rise or fall of TSH and fT4 levels, consistent with pituitary dysfunction. Predictors of phenotype were analyzed by logistic regression. Differential survival between thyroid aging phenotypes was analyzed using multivariate Cox regression.
Results: While the majority of participants at all ages had stable thyroid function, changes were more common among older adults, with 32.3% of those aged >80 years but only 9.5% of those aged <60 years demonstrating thyroid function changes in the highest and lowest quintiles. Regression to the mean accounts for some of the changes, for example increased baseline TSH was associated with a falling TSH pattern (odds ratio = 1.4 [confidence interval 1.1–1.7] per 1 mIU/L). Importantly, changing thyroid function in either the upper or lower quintiles of slope for TSH and fT4 was associated with increased risk of death compared to stable thyroid status (hazard ratio = 5.4 [confidence interval 3.1–9.5]).
Conclusions: Changing thyroid hormone function is increasingly common at older ages and is associated with decreased survival. Nonetheless, the tendency for abnormal thyroid function tests to resolve, along with altered pituitary responsiveness underlying some TSH elevations, suggests that an elevated TSH level should be not assumed to represent subclinical hypothyroidism in older adults. Thus, caution is appropriate when determining the need for thyroid hormone supplements in older adults.
Keywords: : thyroid function, aging, longitudinal study
Introduction
Multiple large population studies have demonstrated that mean circulating thyrotropin (TSH) levels are higher in older populations (1–3). Consequently, older patients are started on thyroid hormone therapy at higher rates than those who are younger (4,5). This practice assumes that modestly elevated TSH levels are undesirable in this group; balancing this against the risk of treatment is debated in the literature (6,7). Resolving the debate requires, in part, understanding whether TSH remains a reliable tool for the diagnosis of thyroid gland failure in the aging population.
When the hypothalamic–pituitary–thyroid axis is intact, TSH rises in response to thyroid hormone deficiency to increase thyroid hormone production. However, higher TSH levels during aging may reflect changes other than primary thyroid gland failure. For example, decreased TSH bioactivity would require greater TSH production to maintain stable thyroid hormone levels, with neutral metabolic effects. Alternatively, decreased pituitary responsiveness to thyroid hormone would lead to both higher TSH and thyroid hormone levels with potential peripheral metabolic over-activation. Additionally, changes in thyroid homeostasis might reflect appropriate adaptation to other morbidities, such as occurs with non-thyroidal illness—metabolic changes for which pharmacologic intervention has not been shown to be beneficial (8).
Understanding the physiology of aging with respect to the thyroid axis is essential to developing appropriate management guidelines for interpreting and acting upon thyroid function tests in older adults. Therefore, thyroid function has been analyzed over time in individual participants enrolled onto the Baltimore Longitudinal Study of Aging (BLSA). By evaluating the simultaneous longitudinal changes in TSH and thyroid hormone levels within individuals over an average of 7.7 years, five different age-related thyroid axis profiles have been identified. Moreover, how patterns of change in thyroid function correlate with survival in this healthy community-dwelling population of older adults has been determined.
Materials and Methods
The BLSA is a long-term study of aging, begun in 1958, with ongoing, rolling enrollment that recruits healthy volunteers living independently in the community (9). There are 1483 participants, with thyroid function tests drawn at least once between January 1, 2003, and March 31, 2015, and extensive health data, as previously characterized (5). For this study, the thyroid phenotype was characterized in a cohort of 640 participants with at least three measures of serum TSH and fT4 performed off interfering medications (thyroid hormone preparations, antithyroid medications, oral glucocorticoids within three months, lithium, estrogenic compounds, anti-estrogenic therapies, amiodarone, carbamazepine, phenobarbital, or phenytoin). Individual visits were excluded for subjects with transient therapies, while all later visits were excluded for those on continuous therapy, such as new thyroid hormone treatment.
Thyroid testing is performed for the BLSA in Clinical Laboratory Improvement Amendments–certified laboratories (5). Because assays have been updated several times (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy), all values were normalized to the current assays (in use since 2011) using assay-specific means and standard deviations derived from the full BLSA cohort of those aged <65 years not on interfering medications, since this most closely matched the reference population used by the laboratories, which were not able to supply us with the original data.
Study baseline was defined as the first visit for which either TSH or fT4 was available. TSH measures are available for 99.6%, and fT4 is available for 99.3% of the visits conducted with the phenotyping cohort. Free triiodothyronine (fT3) is available for 81.4% of visits because it was not included in the study protocol until 2005. Thyroid autoantibodies are not available.
Demographic variables have <5% missing values. Questionnaires about social and medical history and physical function were initiated in 2005. Baseline smoking status and high school graduation information could be obtained for the vast majority of participants based on the data obtained at later visits, which included quit dates for former smokers. Baseline values of self-rated health and walking ability are missing in up to 35%, specifically in those with baseline visits before 2005, which restricted the use of these variables in subsequent analysis. Deaths were confirmed through December 2014 using the SSI administration registry.
Aging thyroid phenotype was characterized by first performing linear regression of TSH and fT4 separately with age to generate slope estimates for annual change. Sensitivity analysis performed using log-transformed TSH values did not provide a better fit for the regression analyses and did not alter the distribution of thyroid function aging patterns. Therefore, the TSH values with their natural units are reported. Four phenotypes were then defined based on the combination of changes in TSH and fT4 either in anti-parallel or parallel directions, reflecting thyroid and pituitary derived change patterns, respectively. Quintiles for the slopes were used in defining the phenotypes to maximize the specificity of the phenotype while preserving sufficient numbers for analysis in the subgroups. Because of the smaller population with fT3 values and a lack of correlation between fT4 and fT3, including fT3 in the phenotyping definitions prevented meaningful analysis of predictive variables and survival.
Multinomial regression modeling was performed to examine the relationships between phenotype and other participant characteristics. The most parsimonious model was built by stepwise exclusion. Survival analysis was performed with Cox regression to examine the relationship between thyroid function aging patterns and other participant characteristics with survival within the phenotyping cohort.
Results
Study population characteristics
A total of 1294 participants in the BLSA had at least one TSH since 2003 and were not on interfering medications at the start of observation. Of these, 640 had at least three measures of serum TSH and fT4 off such medications and thus were included in the phenotyping cohort.
The BLSA population is highly educated and in good health, as demonstrated by the high proportion who report very good to excellent self-rated health and the mean gait speed; these values do not differ for the phenotyping cohort (Table 1). The phenotyping cohort is slightly older, and has a higher proportion of non-white individuals compared to those with shorter follow-up. Baseline thyroid function was essentially similar. Thyroid hormone therapy was initiated during follow-up in 4–5% of participants for both the phenotyping cohort and the BLSA overall. Subject visits were censored when participants started thyroid hormone or any other potentially interfering medication. Since longitudinal follow-up of at least three visits was required, this subset had a higher rate of active participation than the full study: 82% remained active in the phenotyping cohort as of March 31, 2015; 10% were deceased; and 8% had lost contact, failed to complete the most recent visit, or had withdrawn, compared to 75%, 13%, and 12%, respectively, among all those with a visit since 2003.
Table 1.
Comparison of Cohort Characteristics
| BLSA, n = 1294 | Phenotyping, n = 640 | p-Valuea | |
|---|---|---|---|
| Male | 52.9% | 53.9% | NS |
| White | 67.9% | 65.2% | <0.05 |
| Baseline age (years) | 64.1 | 66.3 | <0.01 |
| Baseline TSH (mIU/L) | 2.6 | 2.6 | NS |
| Baseline fT4 (ng/dL) | 0.97 | 0.97 | NS |
| Baseline fT3 (pg/mL) | 3.08 | 3.01 | <0.05 |
| Education (high school grad) | 99.2% | 99.4% | NS |
| Smoking (within 10 years) | 5.2% | 5.4% | NS |
| Walking index | 8.4 | 8.4 | NS |
| Self-rated health (SF-12) | 1.9 | 1.9 | NS |
| Later levothyroxine useb | 5.0% | 3.8% | NS |
| Body mass index | 27.1 | 26.9 | NS |
| Active cohort | 75% | 82% | <0.01 |
| Deceased | 13% | 10% | |
| Lost to follow-up | 12% | 8% |
p-Values are derived using the chi-square test for categorical variables or two-sided t-test for continuous variables between included and excluded participants.
Participants can initiate thyroid hormone during observation; visits were censored once on therapy.
BLSA, Baltimore Longitudinal Study of Aging; TSH, thyrotropin; fT4, free thyroxine; fT3, free triiodothyronine; NS, not significant.
Population-based assessment of TSH
When analyzed in cross-section, using baseline measurements for all participants not on thyroid hormone or interfering medications, the average TSH was higher and more normally distributed among the oldest compared to youngest participant age brackets (Supplementary Fig. S1), consistent with other cross-sectional studies of TSH (1–3). Mean TSH at baseline was progressively higher with age, from 2.4 mIU/L in those <60 years old (n = 464) to 2.6 mIU/L in those 60–69 years old (n = 291), 2.7 mIU/L in those 70–79 yeard old (n = 327), and 3.2 mIU/L in those >79 years old (n = 206). The progression of mean TSH is not linear but largely accrues to the oldest age group. In parallel, the shape of the distribution shifts was significantly different by analysis of variance (ANOVA; p < 0.010), such that the 97.5th percentile increases from 5.5 mIU/L in those <60 years old up to 7.0 mIU/L among those aged ≥80 years old.
Mean fT4 did not differ by age: the youngest and oldest age groups had mean fT4 levels of 0.98 ng/dL and 0.99 ng/dL, respectively. In contrast, there was a significant negative correlation between mean fT3 and age (p < 0.01), with a mean fT3 of 3.2 pg/dL and 2.9 pg/dL in the youngest and oldest groups, respectively. Restricting the analyses to the phenotyping cohort yielded essentially identical results (data not shown).
Natural history of thyroid function
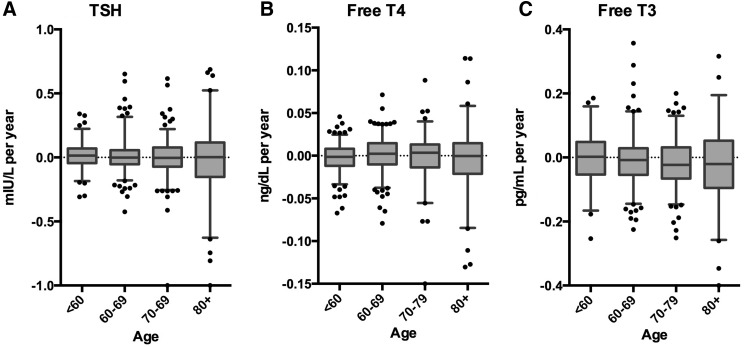
Individual estimates of the change in TSH, fT4, and fT3 over time were generated using linear regression for all BLSA participants in the phenotyping cohort (n = 640 for TSH and fT4; n = 474 for fT3). The mean individual changes in TSH, fT4, and fT3 levels over time were close to zero for all three hormones (Fig. 1). Furthermore, mean changes in TSH (0.006 mIU/L/year), fT4 (−0.001 ng/dL/year), and fT3 (−0.01 pg/mL/year) did not differ by age using one-way ANOVA. The fact that the mean changes are modest is consistent with the lack of improved fit for log transformation overall. However, as illustrated in Figure 1, the magnitude of the change in thyroid function is highly variable, and the variability is significantly greater in the oldest age group (p < 0.001 using Bartlett's test for equal variance).
FIG. 1.
Change in thyroid function over time for healthy aging individuals. Slopes representing the change in TSH (A) free T4 (B) and free T3 (C) are derived by linear regression for each individual in the phenotyping cohort. All values were normalized to the current assay (see methods). The distribution of these slopes is graphed according to initial age category. Boxes represent median and 25th and 75th percentiles; whiskers represent the 5th and 95th percentiles; dots are individual outliers. TSH, thyrotropin.
To reconcile the incrementally higher mean TSH observed in older populations, as above, with the relative stability of individual TSH values, the subset of 70 year olds who aged into their 80s without being treated with thyroid hormone was examined. Of the 179 with a starting age between 70 and 79 years, 118 had a last study observation at an age >80 years. These participants had a mean baseline TSH of 2.8 mIU/L at average age of 75.7 years, which was unchanged at 2.8 mIU/L at the end of observation, with an average age of 83.7 years and 8.2 years of follow-up. This stands in contrast to participants initially enrolled age >80 years (average age 84.6 years; n = 99) in whom the TSH was 3.3 mIU/L at baseline. In this older group, mean TSH also did not increase during observation, remaining at 3.2 mIU/L after an average of 4.9 years of follow-up (average age 89.3 years). This finding reached significance (p = 0.03) and suggests a survival bias impacting on selection, as discussed below.
In order to characterize the variability in thyroid function changes further, the cohort was compared across quintiles for slope. Mean initial TSH declined from 3.6 mIU/L in the first quintile (with declining slope) to 2.7 mIU/L in the fifth quintile (with increasing slope). The annual percent change in TSH ranged from −5.9% in the first quintile to 0.2% in the third and 11.4% in the fifth quintile. Thirty-nine percent of those in the fifth quintile had at least one TSH above the reference range, while 18% of those in the first quintile and only 7% of those in the third quintile ever had an elevated TSH (p < 0.001). Eight subjects had TSH levels below the reference rangem and these were distributed across the slope quintiles. Race/ethnicity and sex were not different across slope quintiles. Older ages are overrepresented in the first and fifth quintiles, with 34% and 29% of those aged ≥80 years old in the first and fifth quintiles for TSH slope, respectively, while only 18% of those <60 years old were in these quintiles (p < 0.001). Similarly, for fT4 slope, 54% of those aged 80 years old were in the first and fifth quintiles combined versus 28% of those <60 years old.
Thyroid aging phenotypes
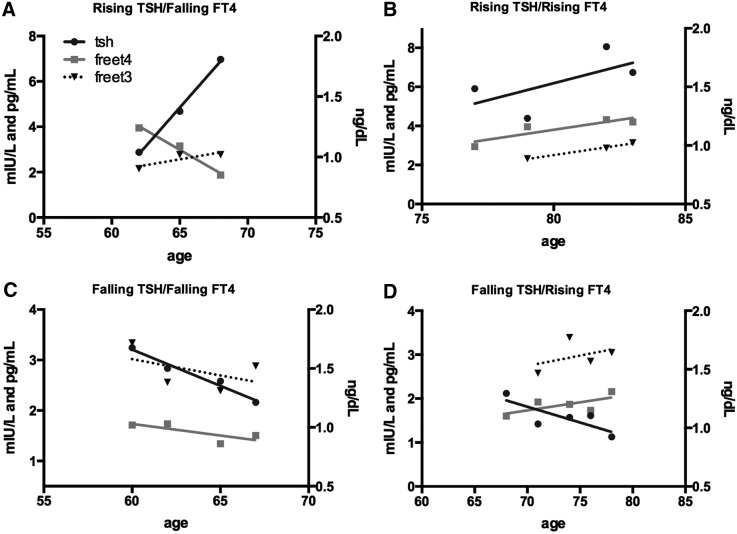
It was hypothesized that there are four possible patterns that deviate from stable thyroid function. Increasing or decreasing fT4 with a negatively correlated change in TSH would suggest thyroid gland disease, while positively correlated TSH and fT4 changes would reflect altered hypothalamic–pituitary (central) alterations. All four patterns were observed in individuals, as illustrated with the examples provided in Figure 2.
FIG. 2.
Individual examples of thyroid aging phenotypes. The patterns of change in thyroid function over time at various ages can be classified into groups, depending on the movement of TSH and the correlating movement of fT4. In (A) and (B), the change in TSH is opposite to that in fT4, consistent with changes in primary thyroid gland function, while in (C) and (D), the changes occur in parallel, suggesting a central origin from pituitary changes.
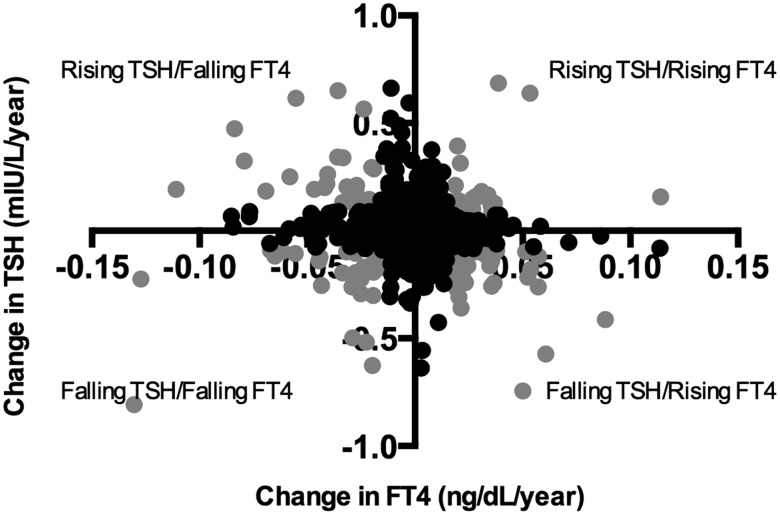
An operational definition was constructed of the four phenotypic groups using a combination of the first and fifth quintiles of slope for each variable (TSH and fT4), as illustrated in Figure 3. This is needed, since movement in either direction might be significant in the correct context, and therefore the slope is not expected to be a continuous predictor of outcome. The reference group included the 528 participants with one or both variables in the middle three quintiles by slope. This definition assigned 30 subjects to the rising TSH/falling fT4, 32 to the falling TSH/rising fT4, 21 to the rising TSH/rising fT4, and 29 to the falling TSH/falling fT4 phenotypes. As with the TSH quintiles, the combination was skewed by age group such that only 9.5% of those <60 years old are characterized as having changing thyroid function by this definition compared to 32% of those >80 years old.
FIG. 3.
Relationship between changing thyroid axis hormones. The change in TSH versus the change in fT4 is graphed for all participants in the phenotyping cohort (n = 640). The change phenotypes were defined based on top and bottom quintiles for both slopes, in four combinations, and are denoted by gray circles.
Predictors of phenotype
Increasing age was strongly associated with a greater likelihood of changing thyroid function tests. Taking all change patterns together, 32.3% of those >80 years met the criteria for change, while only 9.5% of those aged <60 years did so. Age was associated with an odds ratio (OR) of 1.5 [confidence interval (CI) 0.8–3.0], 2.6 [CI 1.4–5.0], and 4.2 [CI 2.1–8.5], respectively, for each decade in age (60–69, 70–79, and ≥80) compared to those <60 years old. The odds of a change phenotype was 2.5 times greater for smokers (OR = 2.6 [CI 1.1–5.9]). Higher initial TSH also predicted a change phenotype (OR = 1.4 [CI 1.2–1.6]).
Because the various change phenotypes reflect four different alterations in thyroid axis function, multinomial logistic regression was used to examine the predictors of each pattern separately, including baseline thyroid function, age, race/ethnicity, sex, and smoking status (Table 2). Age remained a strong predictor of change for primary patterns of altered thyroid function, with an OR of 8.5 [CI 1.7–43.2] for rising TSH/falling fT4 and an OR of 6.6 [CI 1.6–26.6] for falling TSH/rising fT4 in those >80 years old compared to those <60 years old. Rising TSH/rising fT4 also significantly increased among the oldest age group (OR = 4.9 [CI 1.2–19.7]), while there was no significant association with age for falling TSH/falling fT4.
Table 2.
Multinomial Analysis of Phenotype Predictors
| Rising TSH/falling fT4 | Rising TSH/rising fT4 | Falling TSH/falling fT4 | Falling TSH/rising fT4 | |
|---|---|---|---|---|
| Initial age (years) | ||||
| 60–69 | 3.1 [0.6–15.5] | 1.6 [0.5–5.8] | 0.4 [0.1–1.6] | 2.9 [0.8–11] |
| 70–79 | 7.7 [1.6–35]# | 1.2 [0.3–5.2] | 1.1 [0.3–3.7] | 4.0 [1.0–15.2]* |
| 80+ | 8.5 [1.6–43]* | 4.9 [1.2–19.7]* | 1.9 [0.6–6.4] | 6.6 [1.6–26.6]# |
| Male | 1.2 [0.5–2.6] | 1.0 [0.4–2.4] | 0.8 [0.3–1.8] | 0.6 [0.3–1.3] |
| White | 1.1 [0.4–2.8] | 0.4 [0.2–1.1] | 0.7 [0.3–1.9] | 0.8 [0.3–1.9] |
| Smoker | 1.0 [1.3–8.3] | 2.5 [0.5–11.9] | 7.2 [1.9–27.4]# | 2.1 [0.4–9.7] |
| Baseline TSH (mIU/L) | 1.1 [0.8–1.5] | 1.1 [0.8–1.6] | 2.0 [1.5–2.6]** | 1.4 [1.1–1.7]* |
| Baseline fT4 (0.1 ng/dL) | 1.1 [0.9–1.5] | 0.7 [0.5–1.0] | 1.9 [1.4–2.5]** | 0.7 [0.5–0.9]* |
Hazard ratio and confidence intervals for each risk factor by phenotype. Statistically significant values are shown in bold.
p < 0.05; #p < 0.01; **p < 0.001.
Baseline levels predicted increased odds of changes in patterns consistent with regression to the mean. Higher baseline TSH predicted falling TSH with an OR of 1.4 per 1 mIU/L increase in baseline TSH [CI 1.1–1.7] among those with falling TSH/rising fT4, and an OR of 2.0 [CI 1.5–2.6] for those with falling TSH/falling fT4. Similarly, higher initial fT4 was positively associated with falling TSH/falling fT4 (OR = 1.9 per 0.1 ng/dL [CI 1.4–2.5]), while initial fT4 was negatively associated with patterns of rising fT4 for both falling TSH/rising fT4 and rising TSH/rising fT4 (OR = 0.7 per 0.1 ng/dL [CI 0.5–0.9 and 0.5–1.0, respectively]). The only group with no significant relationship to baseline thyroid function was the pattern of rising TSH/falling fT4.
Among the demographic variables analyzed, smoking within the past 10 years increased the risk of a central falling TSH/falling fT4 pattern, with an OR of 7.2 [CI 1.9–27.4]. Race/ethnicity and sex were not associated with thyroid aging pattern.
Survival by thyroid aging phenotype
The study examined whether changes in thyroid function over time were associated with differential survival. In multivariate Cox models, participants with changing thyroid function had an increased risk of death relative to those with stable thyroid function controlling for age, sex, race/ethnicity, and smoking status. The change in TSH or fT4 as a continuous variable, and the baseline values for these hormones, did not predict survival. However, using the phenotype to evaluate risk, the overall hazard ratio for changing versus stable thyroid function was 5.4 [CI 3.1–9.5], p < 0.001). Analyzed by individual phenotype, the point estimates of the hazard ratio were of a similar magnitude (Table 3). There was a loss of significance for the hazard estimate for those with central pattern of rising TSH/rising fT4, whereas the hazard estimate increased for the two primary patterns. Age and smoking were also independent risk factors for death. Initial TSH and fT4 levels were not independently associated with survival.
Table 3.
Survival Analysis for Thyroid Aging Phenotype
| Unadjusted hazard ratio [CI] | Hazard ratio [CI] | p-Value | |
|---|---|---|---|
| Initial age (years) | <0.001 | ||
| <60 | 1 | 1 | |
| 60–69 | 2.5 [0.6–10.0] | 2.1 [0.5–9.0] | |
| 70–79 | 9.2 [2.7–31.7] | 8.0 [2.2–29.4] | |
| 80+ | 48 [14.8–156.5] | 44.0 [12.4–155.9] | |
| Male | 1.9 [1.1–3.3] | 1.5 [0.8–2.7] | 0.15 |
| White | 4.03 [2.1–8.7] | 2.0 [0.9–4.5] | 0.11 |
| Smoker | 1.5 [0.5–5.1] | 8.1 [2.2–30.3] | <0.01 |
| Baseline TSH (mIU/L) | 1.2 [1.1–1.4] | 1.0 [0.9–1.3] | 0.70 |
| Baseline fT4 (01 ng/dL) | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 0.74 |
| Stable thyroid function | 1 | 1 | <0.001 |
| Rising TSH/falling fT4 | 14.5 [6.9–30.2] | 9.6 [4.4–21.0] | |
| Rising TSH/rising fT4 | 1.6 [0.4–6.6] | 3.3 [0.7–15.3] | |
| Falling TSH/falling fT4 | 5.8 [2.8–12.0] | 3.8 [1.7–8.2] | |
| Falling TSH/rising fT4 | 2.5 [0.8–8.2] | 6.4 [1.8–22.6] |
Discussion
Analyses of serum thyroid hormone and TSH levels in the BLSA confirm the findings of multiple previous cross-sectional studies that mean TSH values are higher in older populations. Furthermore, this unique longitudinally studied cohort demonstrates that (i) the majority of individuals of any age have relatively stable thyroid function parameters, (ii) a survival bias may explain the observed higher TSH in older populations, and (iii) significant and persistently changing thyroid hormone and TSH levels arise from diverse physiologic mechanisms that have divergent impacts on survival. This suggests the need for a nuanced and individualized approach to the interpretation of TSH in older adults.
The observation that most individuals are stable with respect to TSH and fT4 but that population averages change across age groups suggests that survival bias is present, although the population is too small and diverse to measure it directly. If those with a lower TSH are less likely to survive than those with a higher TSH, the sampled population mean in older age groups will be higher than in younger groups, even if every individual remains stable. In addition, it was found that changes are more likely in older individuals, which would widen the distribution of TSH levels in older populations. Although there are conflicting data on thyroid measures and survival, the observation is consistent with the Leiden 85+ study (10) and others (11), which demonstrated a protective effect of higher TSH. In contrast, a recent study on National Health and Nutrition Examination Survey data reported increased risk of death with higher TSH within the normal range (12). Other studies have reported increased risk associated with higher initial fT4 but no TSH association (13–15). Meta-analyses have suggested decreased survival for more extreme values of TSH <0.1 mIU/L (16) and >10 mIU/L (17). The present study suggests that the divergence of results may originate in the diversity of the underlying physiology.
It was found that a subset of individuals have significantly and persistently changing thyroid function tests. By examining the simultaneous movement of TSH and fT4, evidence is seen of both thyroid and pituitary causes for these aging-related changes in different individuals. Possible mechanisms include altered hormone metabolism (18), decreased TSH bio-activity (19), changes in functional set point (20,21), and increased prevalence of thyroid autoimmunity (22) and autonomy (23). In addition, some of the changes seen likely represent regression to the mean, since initial values are negatively correlated with later changes, both for lower initial TSH and across the spectrum of fT4. Regression to the mean has also been reported by Bremner et al. for those with lower TSH (24) and by Pearce et al. for those with higher TSH levels (25). High rates of reversion to normal TSH after a single finding of elevated TSH have been reported in several studies (26–28). This suggests a high prevalence of transient perturbations among older populations. The widening distribution of TSH in older cohorts may be driven by complex interactions of all these factors.
Our suggestion that the mechanisms of hormonal changes are diverse helps explain the discrepancies between three longitudinal studies that looked at average population changes of TSH and fT4. Of the two studies to observe increasing mean TSH over time, one found fT4 increased and fT3 fell (13), while the other found no change (25). The third, reporting stable TSH, reported increasing fT4 levels (29). By reporting mean changes, these studies combine diverse aging phenotypes at unknown ratios.
Importantly, the present study found that those with changing thyroid function have decreased survival compared to those with stable function, after correcting for age. Given the small numbers in each phenotypic subgroup, caution in interpretation is needed. Nonetheless, it may be that changes driven by altered primary gland function, such as developing hypo- or hyperthyroidism, confer higher risk compared to pituitary changes.
It is critical to recognize that the association observed with changes in TSH and survival is not a causal demonstration that the change in thyroid function itself is underlying increased risk. It is plausible and perhaps even likely that in some participants, TSH changes reflect the development of underlying comorbidities, in parallel with non-thyroidal illness, which could drive the association with reduced survival. The observation of a lower average T3 in the oldest age group would be consistent with this interpretation, but there is not a sufficiently large sample to add T3 into the phenotyping at this time. The smoking association with change phenotype is also intriguing in this connection, but it is important to be cautious with interpretation because the numbers of smokers with changing phenotypes are composed of fewer than five individuals in the subgroups.
In summary, the results suggest that thyroid function tests change for many reasons: reversion to the mean, aging and disease-related changes in pituitary and thyroid function, and response to other comorbidities. This diversity of pathophysiology complicates the interpretation of thyroid tests in older individuals. Not all rising TSH levels will be associated with the same risk or carry the same treatment implications. Even a persistent elevation in the TSH does not guarantee that it is elevated due to subclinical hypothyroidism, and this complicates the interpretation of treatment studies in older adults and could explain negative results (30). Looking at the full thyroid function panel may improve interpretation. However, no clinical tool is yet available to distinguish these phenotypic variants based on a single time point. Therefore, testing over time may be critical in older patients, for example to distinguish subclinical hypothyroidism from central thyroid hormone resistance, or to avoid treating transient abnormalities, which the present data suggest are more common in older adults. Phenotyping will also be critical to studies evaluating the appropriate use of therapy in this population, since the efficacy of thyroid hormone replacement may vary depending on the underlying physiologic mechanisms.
Supplementary Material
Acknowledgments
Funding was provided by K23DK095954 National Institute of Diabetes, Digestive, and Kidney Diseases (J.S.M.) and the Intramural Research Program National Institute on Aging, National Institutes of Health (E.M.S.). J.S.M. is also grateful for support from the Turock Family Foundation.
Author Disclosure Statement
No competing financial interests exist for any author.
References
- 1.Surks MI, Hollowell JG. 2007. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- 2.Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. 2013. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab 98:1147–1153 [DOI] [PubMed] [Google Scholar]
- 3.Boucai L, Surks MI. 2009. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 70:788–793 [DOI] [PubMed] [Google Scholar]
- 4.Taylor PN. Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas SL, Dayan C. 2014. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 174:32–39 [DOI] [PubMed] [Google Scholar]
- 5.Mammen JS, McGready J, Oxman R, Chia CW, Ladenson PW, Simonsick EM. 2015. Thyroid hormone therapy and risk of thyrotoxicosis in community-resident older adults: findings from the Baltimore Longitudinal Study of Aging. Thyroid 25:979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodondi N, Aujesky D, Vittinghoff E, Cornuz J, Bauer DC. 2006. Subclinical hypothyroidism and the risk of coronary heart disease: a meta-analysis. Am J Med 119:541–551 [DOI] [PubMed] [Google Scholar]
- 7.Jonklaas J. 2016. Update on the treatment of hypothyroidism. Curr Opin Oncol 28:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaptein EM, Beale E, Chan LS. 2009. Thyroid hormone therapy for obesity and nonthyroidal illnesses: a systematic review. J Clin Endocrinol Metab 94:3663–3675 [DOI] [PubMed] [Google Scholar]
- 9.Shock NW, Gruelich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. 1984. Normal human aging: the Baltimore Longitudinal Study of Aging. National Institutes of Health, Washington, DC, pp 47–55 [Google Scholar]
- 10.Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 11.Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pedersen C, Gislason GH. 2014. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 99:2372–2382 [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Tsujimoto T, Saito J, Sugiyama T. 2016. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid 26:1457–1465 [DOI] [PubMed] [Google Scholar]
- 13.Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR. 2012. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab 97:3944–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, Chubb SA. 2013. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the Health In Men Study. Eur J Endocrinol 169:401–408 [DOI] [PubMed] [Google Scholar]
- 15.van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SW. 2005. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab 90:6403–6409 [DOI] [PubMed] [Google Scholar]
- 16.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Asvold BO, Sgarbi JA, Volzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N; Thyroid Studies Collaboration 2012. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 172:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J; Thyroid Studies Collaboration 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaffney GW, Gregerman RI, Shock NW. 1962. Relationship of age to the thyroidal accumulation, renal excretion and distribution of radioiodide in euthyroid man. J Clin Endocrinol Metab 22:784–794 [DOI] [PubMed] [Google Scholar]
- 19.Klug TL, Adelman RC. 1978. Age-dependent accumulation of an immunoreactive species of thyrotropin (TSH) which inhibits production of thyroid hormones [proceedings]. Adv Exp Med Biol 97:259–264 [PubMed] [Google Scholar]
- 20.Brown SJ, Bremner AP, Hadlow NC, Feddema P, Leedman PJ, O'Leary PC, Walsh JP. 2016. The log TSH-free T4 relationship in a community-based cohort is nonlinear and is influenced by age, smoking and thyroid peroxidase antibody status. Clin Endocrinol (Oxf) 85:789–796 [DOI] [PubMed] [Google Scholar]
- 21.Over R, Mannan S, Nsouli-Maktabi H, Burman KD, Jonklaas J. 2010. Age and the thyrotropin response to hypothyroxinemia. J Clin Endocrinol Metab 95:3675–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 23.Laurberg P, Andersen S, Bulow Pedersen I, Carle A. 2005. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging 22:23–38 [DOI] [PubMed] [Google Scholar]
- 24.Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, Wilson SG, O'Leary PC, Walsh JP. 2012. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 97:1554–1562 [DOI] [PubMed] [Google Scholar]
- 25.Pearce SH, Razvi S, Yadegarfar ME, Martin-Ruiz C, Kingston A, Collerton J, Visser TJ, Kirkwood TB, Jagger C. 2016. Serum thyroid function, mortality and disability in advanced old age: the Newcastle 85+ study. J Clin Endocrinol Metab 101:4385–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somwaru LL, Rariy CM, Arnold AM, Cappola AR. 2012. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab 97:1962–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F. 1995. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- 28.Imaizumi M, Sera N, Ueki I, Horie I, Ando T, Usa T, Ichimaru S, Nakashima E, Hida A, Soda M, Tominaga T, Ashizawa K, Maeda R, Nagataki S, Akahoshi M. 2011. Risk for progression to overt hypothyroidism in an elderly Japanese population with subclinical hypothyroidism. Thyroid 21:1177–1182 [DOI] [PubMed] [Google Scholar]
- 29.Waring AC, Harrison S, Samuels MH, Ensrud KE, LeBLanc ES, Hoffman AR, Orwoll E, Fink HA, Barrett-Connor E, Bauer DC; Osteoporotic Fractures in Men (MrOS) Study 2012. Thyroid function and mortality in older men: a prospective study. J Clin Endocrinol Metab 97:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RG, Mooijaart SP, Sattar N, Aubert CE, Aujesky D, Bauer DC, Baumgartner C, Blum MR, Browne JP, Byrne S, Collet TH, Dekkers OM, den Elzen WP, Du Puy RS, Ellis G, Feller M, Floriani C, Hendry K, Hurley C, Jukema JW, Kean S, Kelly M, Krebs D, Langhorne P, McCarthy G, McCarthy V, McConnachie A, McDade M, Messow M, O'Flynn A, O'Riordan D, Poortvliet RK, Quinn TJ, Russell A, Sinnott C, Smit JW, Van Dorland HA, Walsh KA, Walsh EK, Watt T, Wilson R, Gussekloo J; TRUST Study Group 2017. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 376:2534–2544 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.