SUMMARY
Adult stem cells provide a renewable source of differentiated cells for a wide variety of tissues and generally give rise to multiple cell types. Basic principles of stem cell organization and regulation underlying this behavior are emerging. Local niche signals maintain stem cells, while different sets of signals act outside the niche to diversify initially equivalent stem cell progeny. Here we show that Drosophila ovarian Follicle Stem Cells (FSCs) produced two distinct cell types directly. This cell fate choice was determined by the A/P position of an FSC and by the magnitude of spatially graded Wnt pathway activity. These findings reveal a paradigm of immediate diversification of stem cell derivatives according to stem cell position within a larger population, guided by a graded niche signal. We also found that FSCs strongly resemble mammalian intestinal stem cells in many aspects of their organization, including population asymmetry and dynamic heterogeneity.
An adult stem cell usually produces more than one type of differentiated cell, but the mechanisms underlying this key organizational principle are not well understood. One demonstrated strategy involves the initial production of a generic non-stem cell derivative, which later adopts different fates, sometimes with intervening transit-amplifying divisions, in response to spatial differences in external signals distant from the stem cell niche1, 2. An alternative hypothetical possibility is that a stem cell may be able to produce two or more distinct cell types directly.
Drosophila ovarian Follicle Stem Cells (FSCs) are model epithelial stem cells that offer major benefits of relative simplicity, direct visualization and genetic manipulation. FSCs produce Follicle Cells (FCs), which proliferate, diversify and collaborate with Germline Stem Cell (GSC) derivatives to support the life-long formation of egg chambers and their maturation into eggs3. Lineage analyses reported to date have concluded there are two or three relatively stable FSCs located midway through the germarium4–7, which lies at the anterior end of each ovariole (Fig. 1a,b). FSCs do not express the surface protein Fasciclin 3 (Fas3), which is expressed by all or most FCs in the posterior half of the germarium (Fig. 1a)4–7. The anterior half of the germarium harbors somatic Escort Cells (ECs), which envelop developing germline cells and ensure normal germline differentiation8, 9. ECs regenerate during adult life and were most recently reported to do so through occasional division of mature ECs8, 9.
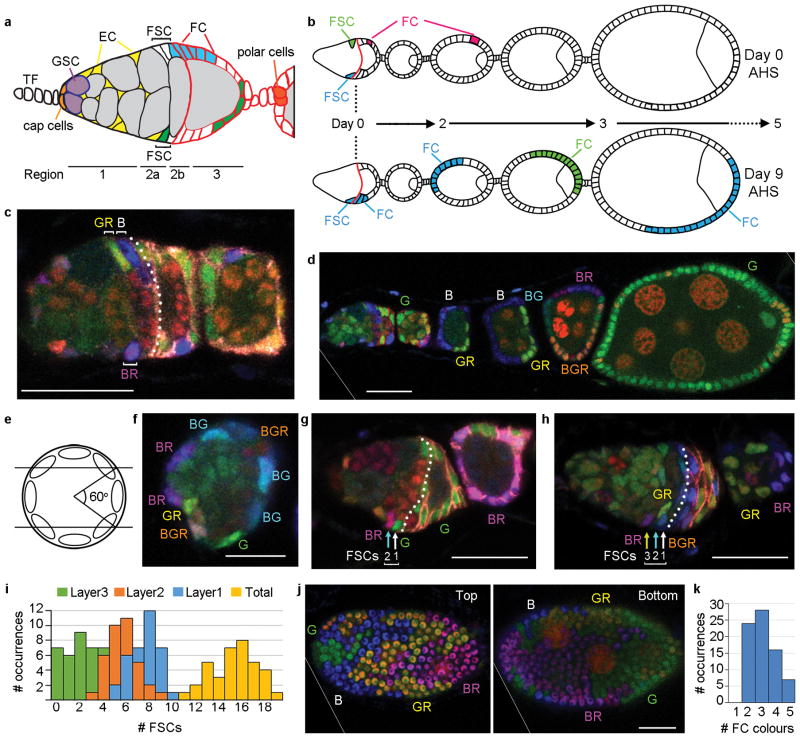
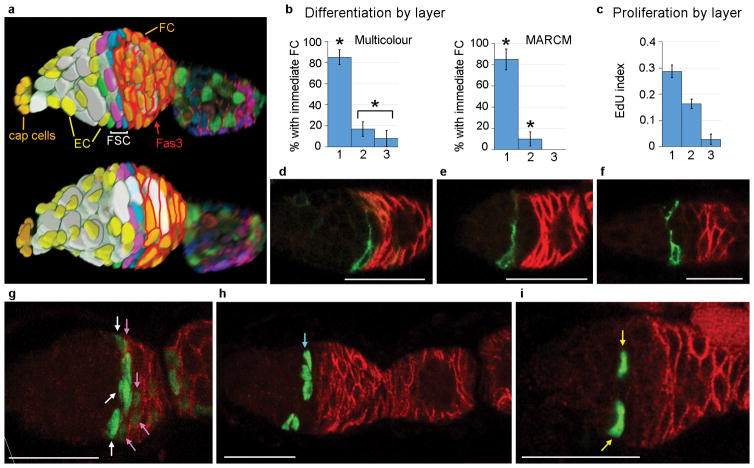
Figure 1. Location of FSCs in a germarium.
(a) Germarium cartoon showing Terminal Filament (TF) cells, Germline Stem Cells (GSCs), GSC daughters developing into 16-cell germline cysts (grey), Escort Cells (EC, yellow), Follicle Stem Cells (FSC) and Follicle Cells (FC) from anterior (left) to the newest egg chamber. Two potential FSC lineages are shown (blue, green) together with the Fas3 staining pattern (red). (b) Dividing FCs marked by heat-shock induced genetic recombination (red) will exit the ovariole (late egg chambers not shown) within 5 days after heat-shock (AHS). Any marked FCs present 5 or more days AHS derive from an FSC, whether the FSC still remains (blue lineage) or not (green lineage). (c,d) Ovariole from multicolour lineage analysis 9d AHS with (c) three FSC phenotypes (B, GR, BR) anterior to Fas3 staining (pink overlaid by dotted white line) in this z-section and (d) patches of all six possible FC colours. Letters indicate the presence of a given transgene (B- Blue lacZ, G-Green GFP, R- Red, RFP; see Supplementary Fig. 1a–c). (e) Radial location of single candidate FSC nuclei was scored according to z-section location as falling within central (5 cases), upper (5 cases) or lower thirds (6 cases) of the germarial circumference. (f) Cross-section of radially distributed FSC nuclei of the indicated colours surrounding central germline cells. (g,h) Germaria 9d AHS. (g) The only BR FSC candidate is in layer 2 (“BR”, cyan arrow), anterior to another FSC (“G”, white arrow). (h) The only BR FSC candidate is in layer 3 (“BR”, yellow arrow) with two FSCs (cyan and white arrows) that are more posterior (right) but still anterior to Fas3 staining (enhanced as dotted white line). (i) Frequency of germaria with the indicated numbers of labeled FSCs (summing all lineages) in layers 1, 2, 3 and in total. (j) Two surfaces (combined z-sections 1–3 and 11–14) of an egg chamber populated by four different FC colours. (k) Frequency of egg chambers with the indicated number of distinct FC colours among all egg chambers from ovarioles with at least four distinct FSC lineages. Scale bar in (f) 10μm; all others 20μm. (d,j) Diagonal white lines indicate an edge of the original image (shown in Supplementary Fig. 9).
Here we revise and extend current understanding of FSC behaviour. We find that a germarium contains about 14–16 FSCs maintained by population asymmetry, with high rates of loss or amplification of individual FSCs. FSCs are arranged in three layers along the anterior-posterior (A/P) axis, but individual cells can move between layers. Posterior FSCs directly produce proliferative FCs, while anterior FSCs directly produce quiescent ECs. These alternative outcomes and the A/P position of individual cells within the FSC community are governed by Wnt pathway activity. Thus, FSCs present a potentially general paradigm where alternative direct stem cell products are specified according to the location of a stem cell within a larger population. This behaviour is guided by the strength of a spatially graded niche signal.
RESULTS
Multiple FSCs reside in a narrow A/P domain of radially symmetric rings
To define the number and location of FSCs in a germarium we used multicolour lineage analysis. Here, mitotic recombination eliminates GFP (G-green), β-galactosidase (B-blue) or RFP (R-red) transgenes, resulting in any one of six distinguishable colours (G, B, BG, GR, BR or BGR) (Supplementary Fig. 1a–c). Recombination was initiated by a heat-shock induced recombinase and the number of distinct FSC lineages was counted 9 days after the last of a series of heat-shocks. Transient clones arising in FCs pass through the ovariole within 5 days4 (Fig. 1b) and control animals with no heat-shock included only three recombinant clones among 161 ovarioles. We can therefore be certain that almost all experimental clones (over 150 in 50 ovarioles) were induced by heat-shock 9 days earlier and derived from recombination in a stem cell.
An FSC clone that survived for 9 days was defined as including at least one coherent group (“patch”) of FCs and at least one cell in region 2a/b of the germarium (an FSC) of the same colour (B in Fig. 1b; B, G, GR, and BR in Fig. 1c,d and Fig. 2a–d). Many ovarioles included four or more FSC clone colours and two had all six (Fig. 2i). The distribution of FSC clone colour numbers under-estimates the number of surviving FSCs because two or more FSCs may have the same colour (Supplementary Fig. 1c); it best matches expectations for an average of five surviving FSC lineages per germarium 9d after marking (see Supplementary Note and Supplementary Fig. 2a).
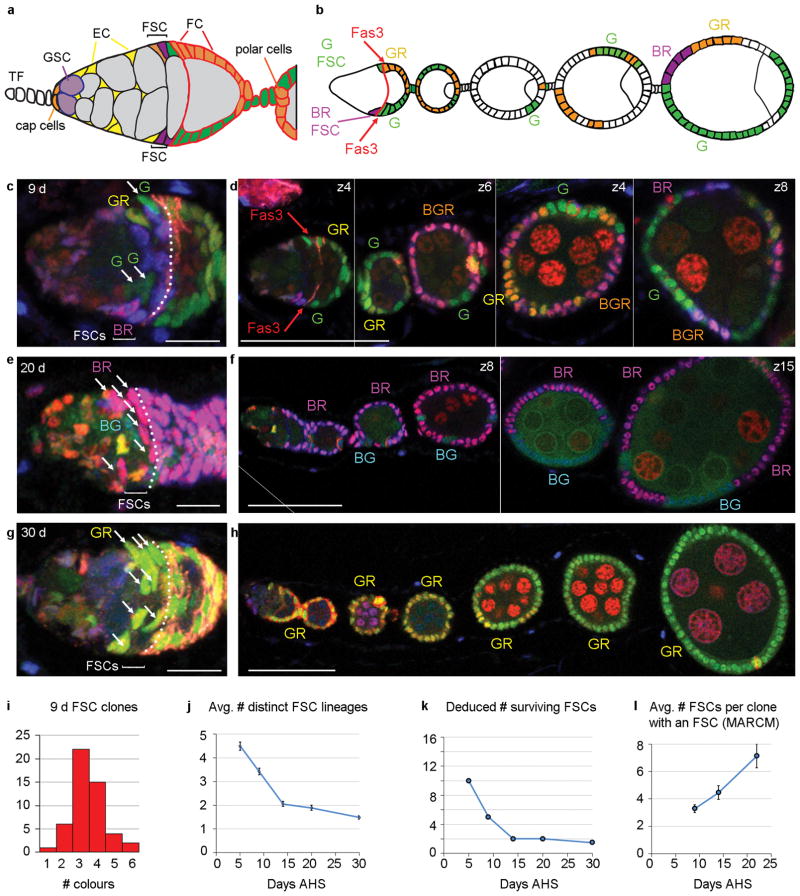
Figure 2. FSC population asymmetry.
(a,b) Diagram of (a) a germarium and (b) anterior portion of an ovariole illustrating the clones observed in (c,d). There are G (green), BR (purple), GR (orange) and BGR (white in (b)) FSC clones containing at least one candidate FSC in region 2a/b (BGR FSC is in a different z-section) and one FC patch. (c–h) Examples of (d,f,h) ovarioles (single z-sections) and (c,e,g) their germaria (3–5 z-sections combined) at higher magnification from multicolour lineage experiments 9, 20 and 30 days AHS. The number of distinct FSC lineages declines from (c,d) four at 9d to (e,f) two (BG, BR) at 20d and (g,h) just one (GR) at 30d (single-channel images show all cells have GFP and RFP). (c,e,g) The number of FSCs of one colour (white arrows in (c) G, (e) BR and (g) GR) is higher when fewer FSC lineages are present (Fas3 staining enhanced by white dotted line). Scale bars (c,e,g) 10μm, (d,f,h) 50μm. (d,f) Vertical white lines separate different z-sections (indicated top right) for different regions of the ovariole (full original images for each z-section in Supplementary Fig. 9). (f) Diagonal white line indicates an edge of the original image (shown in Supplementary Fig. 9). (i) Number of ovarioles (among 50) with the indicated number of distinct FSC clone colours 9d AHS. (j) Mean number of distinct multicolour FSC lineages observed and (k) deduced number of FSC lineages surviving for indicated number of days after heat-shock (AHS). (j) Error bars show SEM for n=50 (5d), 50 (9d), 53 (14d), 47 (20d) and 60 (30d) biologically independent ovarioles. Mean number of distinct FSC lineages differed significantly by Pearson’s chi-squared test between d5 and d9 (p<0.0005), d9 and d14 (p=1×10−6), d20 and d30 (p<0.01). The observation of several distinct FSC lineages was not contingent on using multiple heat-shocks for clone induction. An analogous experiment 12d after a single heat-shock produced ovarioles with one (5/45), two (18/45), three (15/45), four (5/45) and five (2/45) FSC clone colours with FSCs in the same locations as described earlier (Supplementary Fig. 3). (l) Mean number of FSCs labeled per MARCM clone at different times AHS. Error bars show SEM for n=151 (9d), 77 (14d) and 112 (22d) biologically independent clones. Mean number of FSC per clone differed significantly by Pearson’s chi-squared test between d9 and d14 (p<0.05), d14 and d22 (p<0.05).
The majority of FSC clones included more than one candidate FSC. To determine where FSCs reside, we considered clones with only a single candidate FSC in region 2a/b. The single candidate FSC was found at similar frequency in each of the 7–10 confocal z-sections spanning the germarium, not just in the middle sections as previously proposed5 (Fig. 1e). We infer that FSCs can occupy any position around the germarial circumference (Fig. 1f).
Along the A/P axis, 24 FSC lineages had a single candidate FSC immediately anterior to Fas3-positive FCs; we call this “layer 1” (B in Fig. 1c and Supplementary Fig. 1i–n). In 19 lineages the only candidate FSC was in the penultimate Fas3-negative ring (“layer 2”) (BR in Fig. 1g) and in five lineages it was one cell further anterior (“layer 3”) (BR in Fig. 1h).
We counted the total number of candidate FSCs in layers 1–3 for all lineages in 36 germaria (Supplementary Fig. 1i–n). We found an average of 7.6 cells in layer 1, 5.6 cells in layer 2 and 2.0 cells in layer 3, in a distribution centered around a total of 15–16 FSCs (Fig. 1i). The ratio of cells present in layers 1–3 (50%:37%:13%) was very similar to the percentage of ovarioles with the only candidate FSC in layer 1, 2 or 3 (50%:40%:10%), suggesting that all cells in these three layers are FSCs. We also used the MARCM method10 to label FSC clones with GFP and found a very similar distribution of all candidate FSCs among layers 1–3 (46%:38%:16%, n= 273).
FSC population asymmetry: rapid loss and amplification of FSCs
Although occasional FSC replacement is acknowledged, each FSC is thought to be relatively long-lived, mostly undergoing repeated divisions with asymmetric outcomes4, 5, 7, 11, 12. We measured the rate of FSC loss by counting surviving FSC clone colours 14d, 20d and 30d after clone induction. Because it takes 4 days for an FC to transit from birth to the latest egg chambers scored (Fig. 1b) we could also infer the number of FSC colours present 5d after clone induction by counting the number of FC colours in 9d ovarioles. The number of FSC colours observed (Fig. 2j) and the inferred number of surviving FSC lineages (Fig. 2c–k, see Supplementary Note) declined rapidly over time and, by extrapolation, indicates the initial presence of about 16 FSCs (Fig. 2k), very similar to the number of candidate FSCs in layers 1–3 (Fig. 1i).
If some FSC lineages are lost, others must amplify for the total number of FSCs to remain constant. When the average number of surviving FSC lineages was five at day 9 the average number of FSCs per lineage was 3.2 among 50 B, G and BG lineages. A similar number of FSCs per lineage (3.3 at day 9) was measured in a MARCM analysis. The number of FSCs per lineage increased over time (Fig. 2c–h,l).
The rapid loss of some FSCs and amplification of others contradicts earlier models of predominantly asymmetric division of FSCs4–6, 11 and instead conforms to a model of population asymmetry, where the fates of two daughters of a stem cell are not necessarily different. Stem cell populations maintained by population asymmetry undergo neutral drift, in which the number of stem cell lineages declines stochastically over time, eventually leaving only one randomly selected lineage per developmental unit13, 14. We observed exactly this behaviour from 5 to 30d following clone induction (Fig. 2c–l), resulting in just one or two lineages in most ovarioles by 20–30d after clone induction. Correspondingly, the number of FSCs per lineage exhibited stochastic variations at any one time, centered on larger clone sizes as time progressed (Supplementary Fig. 2k–m).
We also counted the number of distinct colours present on each egg chamber (Fig. 1j,k) and the average proportion of each egg chamber contributed by a single colour to deduce that each egg chamber is seeded on average by about four founding FCs (see Supplementary Note). Consistent with the considerable excess of FSCs over founding FCs, individual FSC lineages contributed only sporadically to individual egg chambers (83 of 185 egg chambers for B, G and BG clones at 9d; 179 of 337 egg chambers for MARCM clones at 9d) and some FSCs had no matching FCs in the entire ovariole (14 of 63 (22%) B, G and BG clones; 20 of 83 (24%) 9d MARCM clones; Supplementary Fig. 2d–g).
FSCs are radially mobile
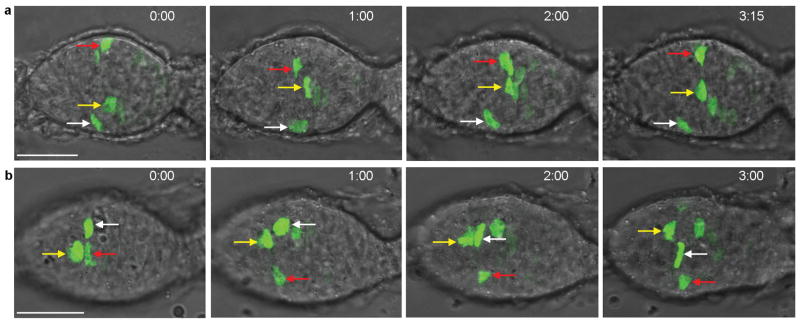
We used live imaging to follow FSCs marked by GFP after embedding dissected ovarioles in Matrigel. Throughout the 3–11h imaging period, labeled cell bodies in FSC locations moved radially along the germarial circumference (Fig. 3a,b; Supplementary videos 1–6). We quantified relative movement between pairs of marked cells (Supplementary Fig. 4). Radial movement was observed between all 34 pairs tracked. Cell pairs switched between periods of moving towards each other or further apart at an average rate of 13% of the circumference (about 45 degrees) per hour and FSCs sometimes crossed paths (Supplementary Fig. 4 and videos 2,3,5). These movements support the idea that FSCs can occupy any radial position and the fluid exchange of radial positions suggests functional equivalency in this dimension.
Figure 3. Radial movement of FSCs visualized by live imaging.
(a,b) Time-stamped frames up to (a) 3h 15min and (b) 3h 00min from live imaging of MARCM-labeled FSCs showing frequent radial and independent movement of FSCs (each marked by a coloured arrow). Each image has several z-sections combined, so circumferential movement appears like movement across the interior of the germarium. Panel (a) frames are taken from Supplementary video 1, panel (b) frames from Supplementary video 2. Supplementary Fig. 4 shows FSC movements tracked in two dimensions around the germarial circumference (from Supplementary video 6). Scale bars are 20μm.
FSCs produce Escort Cells
It was first suggested that ECs replenished during adulthood derive from an escort stem cell adjacent to the GSCs8. Because there is virtually no somatic cell division anterior to region 2a/b, a more recent study concluded that new ECs derive from occasional division of mature ECs, principally in region 2a/b9. We have found that the entire region 2a/b proliferative domain appears to be occupied by FSCs and therefore hypothesized that FSCs produce ECs as well as FCs.
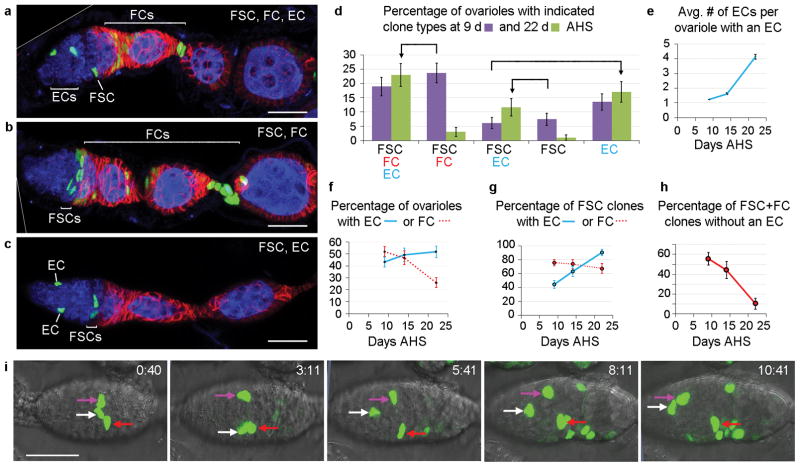
MARCM labeling revealed many ovarioles with marked ECs, FSCs and FCs, consistent with a common FSC origin of both FCs and ECs (Fig. 4a). However, some ovarioles contained only marked FSCs and FCs (Fig. 4b), or only marked ECs, with or without FSCs (Fig. 4c). It has been argued from similar data that ECs and FCs have separate origins; co-incident marking was invoked to explain the presence of marked FSCs, FCs and ECs in the same ovariole8, 9. We clarified the origin of ECs by examining a time course of MARCM clones.
Figure 4. FSCs also produce Escort Cells.
(a–c) Ovarioles with GFP-labeled MARCM clones 9d after heat-shock (AHS) showing marked (a) FSC, FCs and ECs, (b) FSCs and FCs only or (c) FSCs and ECs only. Fas3 (red) and Vasa (blue) stain FCs and germline cells, respectively. Multiple z-sections are combined in (a–c) to capture all labeled cells. (a,b) Diagonal white lines indicate an edge of the original image (shown in Supplementary Fig. 9). (d) Percentage of ovarioles with different clone types 9d (purple) and 22d (green) AHS. Arrows highlight major trends. FSC/FC/EC and FSC/EC categories increase at the expense of FSC/FC and FSC categories, respectively, as more FSCs produce ECs. The percentage of ovarioles with marked FSCs declines from 57% at 9d to 42% at 22d, limiting the observed FSC/FC/EC and FSC/EC increases, adding to FSC/FC and FSC decreases, and increasing the EC category as some FSCs are lost after producing ECs. Error bars show SEM for n=148 (9d) and n=112 (22d) biologically independent ovarioles. (e–h) Data from the same experiment in (d), showing changes in (e) the mean number of ECs in an ovariole that contains at least one EC, (f) the percentage of ovarioles with at least one marked EC (blue) or FC (red), (g) the percentage of ovarioles with a marked FSC that also include one or more marked EC (blue) or FC (red), and (h) the percentage of all ovarioles with marked FSC and FCs (FSC/FC + FSC/FC/EC) that have no marked ECs (35/63 at d9, 15/34 at d14, 3/29 at d22). Error bars show SEM for (d,f) n=148 (9d), n=77 (14d) and n=112 (22d), (e) n=66 (9d), n=50 (14d) and n=52 (22d) biologically independent ovarioles, (g) n=86 (9d), n=46 (14d) and n=42 (22d) and (h) n=65 (9d), n=34 (14d) and n=29 (22d) biologically independent clones. (e) The mean number of ECs per ovariole with an EC differed significantly by Pearson’s chi-squared test between d9 and d14 (p<0.05), d14 and d22 (p<0.005). Raw data are in Supplementary Table 1. (i) A time-stamped series of live imaging frames up to 10h 41min where one marked cell (white arrow) and then another (magenta arrow) moves anteriorly from the FSC region to EC territory (taken from Supplementary video 3). All scale bars 20μm.
The average number of labeled ECs in EC-containing ovarioles increased over time (Fig. 4e), indicating repeated divisions of a cell labeled at time zero and hence a stem cell origin. The percentage of ovarioles with at least one labeled EC also increased (Fig. 4f), especially when considering only ovarioles that included an FSC (Fig. 4g). Labeled ECs in germaria previously lacking any labeled ECs must derive from a cell type other than an EC. The tight correlation of new ECs with ovarioles containing labeled FSCs strongly suggests that FSCs are the source of new ECs.
The diversity of MARCM clone types can also be readily explained. First, some FSC lineages lack FCs or ECs because an individual FSC only produces these cells sporadically. Accordingly, the proportion of FSC/FC clones lacking ECs declines over time to almost zero, as each surviving FSC clone eventually produces a relatively stable EC (Fig. 4h). Second, individual FSC lineages are also lost frequently (Fig. 2j,k), leaving a residue of any marked ECs produced and hence an increasing proportion of “EC-only” clones over time (Fig. 4d).
We also saw evidence of EC production from FSCs in live imaging studies. In at least four cases we observed extensive migration of a GFP-labeled cell from FSC territory to an anterior position characteristic of ECs (Fig. 4i, Supplementary Fig. 5 and videos 3–6).
Heterogeneity among FSCs
To investigate possible heterogeneity in the FSC population (Fig. 5a) we examined proliferation rates, morphology, gene expression markers and cell fate. We measured proliferation rates by labeling MARCM FSC clones with EdU immediately after ovary removal. EdU incorporated into a greater proportion of layer 1 FSCs (29%) than layer 2 FSCs (16%), while layer 3 FSC labeling (3%) was barely higher than for quiescent ECs (<1%) (Fig. 5c).
Figure 5. FSCs are heterogeneous along the A/P axis.
(a) Two surfaces of a 3D reconstruction traced from a germarium with multicolour FSC clones, showing germline cells (grey/white), cap cells (pale orange), ECs (yellow) FCs (orange with red Fas3 outlines) and FSCs in layer 1 (blue), layer 2 (pink) and layer 3 (green). (b) Percentage of all clones with FSC(s) in only the indicated layer that are represented in the first layer of Fas3-positive FCs for multicolour clones and MARCM clones. The proportion of layer 1 FSC clones and layer 2/3 FSC clones (combined) with recent FCs were both significantly different to the proportion of all single layer FSC clones with recent FCs (multicolour, n=48 [24 layer 1, 19 layer 2, 5 layer 3] biologically independent clones; MARCM, n=30 [13 layer 1, 13 layer 2, 4 layer 3] biologically independent clones) by Fisher’s exact two-tailed test (* p<0.05). SEMs indicated by error bars. (c) Fraction of wild-type MARCM-labeled FSCs in each layer labeled by EdU (error bars show SEM from five experiments with n=375, 191 and 70 total FSCs scored for layers 1, 2 and 3, respectively). EdU indices for layer 1 vs 2 and layer 2 vs 3 are significantly different (p<0.005 by Fisher’s exact two-tailed test). (d–f) Single mid-section layer 1, 2 or 3 FSCs labeled with surface CD8-GFP in Fas3 (red)-stained germaria. (d) Layer 1 FSC processes contact the posterior surface of a stage 2b cyst. (e) Layer 2 and (f) layer 3 FSCs contact the anterior surface of stage 2b cysts. All FSC processes span the germarium. (g–i) Examples of MARCM clones where the FSCs labeled (green) are in (g) layer 1 (white arrows), (h) layer 2 (cyan arrow) or (i) layer 3 (yellow arrows). Only layer 1 FSCs (g) are associated with (green) labeled FCs (pink arrows) in the first layer of cells expressing Fas3 (red). (g) and (h) have six z-sections combined. (g) Diagonal white line indicates an edge of the original image (shown in Supplementary Fig. 9). Scale bars 20μm.
Surface labeling of single cells showed that layer 1 FSCs extended processes over the posterior surface of stage 2b cysts, while layer 2 and 3 FSC processes generally contacted the anterior surface of 2b cysts (Fig. 5d–f and Supplementary Fig. 6a–c). By contrast, region 1 ECs had short processes and region 2a EC processes wrapped around stage 2a cysts (Supplementary Fig. 6d–f), as noted previously9.
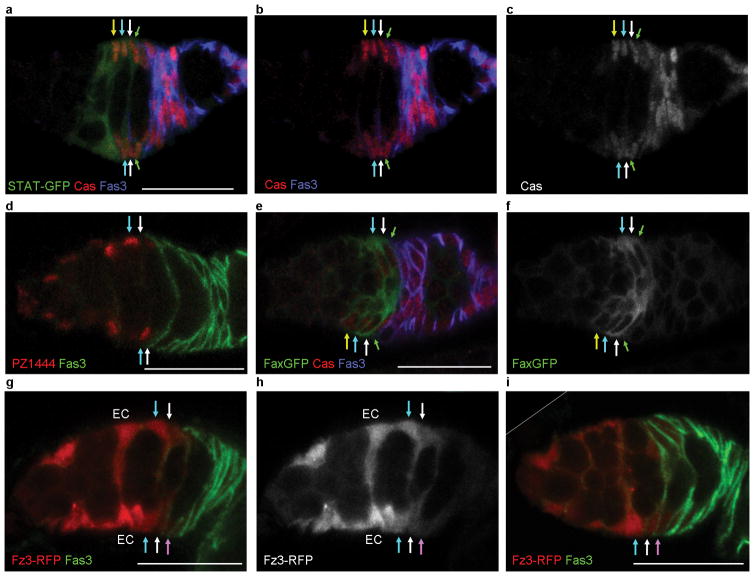
Some markers showed graded expression among FSCs but all were additionally expressed in FCs, ECs or both. Castor15 and 109-30-GAL412, 16 were expressed in all FSC layers and FCs but were largely absent from ECs (Fig. 6a–c and Supplementary Fig. 6g–i). Conversely, PZ14444 and C587-GAL417 were expressed in ECs, declined over FSC layers, and were largely absent from FCs (Fig. 6d and Supplementary Fig. 6j–l). Fax-GFP18 was strongly expressed in all FSCs and a limited number of nearby cells (Fig. 6e,f).
Figure 6. Heterogeneous gene expression among FSCs.
(a–i) Expression of markers along the A/P axis; arrows indicate the border of Fas3 expression (green), layer 1 (white), 2 (cyan) and 3 (yellow) FSCs. (a–c) Castor (Cas, red in a, b, gray in c) stains FCs and all FSCs, while the stat-GFP (green) JAK-STAT pathway reporter also extends into region 2a ECs. (d) PZ1444- lacZ (red) and (g–i) the Fz3-RFP Wnt pathway reporter (red in g, i) show strong expression in ECs and weaker expression in FSCs, with reduced or undetectable expression in layer 1 FSCs (white arrows) and FCs (pink arrows); Fas3 (green). (h) shows Fz3-RFP (gray) from the same germarium as (g). (e,f) Fax-GFP (green in e, gray in f) is expressed more strongly in FSCs than in FCs or ECs. (i) Diagonal white line indicates an edge of the original image (shown in Supplementary Fig. 9). Scale bars 20μm.
To examine functional diversity among FSCs we scored how often germaria with labeled FSCs in only one layer were associated with labeled FCs in the most anterior Fas3-positive cells, indicating very recent FC production. Recently produced FCs were very frequently associated with layer 1 FSCs (85%), but much less frequently with FSCs in layer 2 (17%) or 3 (8%) in 9d multicolour clones and in MARCM clones (Fig. 5b,g–i). We conclude that layer 1 FSCs produce FCs much more frequently than FSCs in other layers. We propose that FCs only derive directly from layer 1 FSCs.
Conversely, we suggest that ECs derive directly from their immediate neighbors in layer 3 or 2, as might be expected simply on a mechanical basis. Consistent with this proposal, marked FSCs that moved into EC positions during live imaging were always anterior to other FSCs in the same germarium (Fig. 4i and Supplementary Fig. 5).
Do FSCs move between layers? Approximately two-thirds of RFP-negative multicolour clones (23/34) and MARCM clones (39/57) with two or more marked FSCs included FSCs in more than one layer, even though the overall frequency of those clones suggests that the majority originated from a single marked cell. There must therefore be movement of FSCs between layers. We also saw several examples of FSCs exchanging A/P positions during live imaging (Supplementary videos 1,2). On the other hand, a relatively high proportion of 9d MARCM FSC clones contained FCs but no ECs or vice versa (Fig. 4b–d), suggesting that FSCs can spend several days in a single layer, producing only one type of derivative. Thus, the FSC population exists in a relatively slow equilibrium along the A/P axis, with individual FSCs occasionally moving from one layer to another and consequently switching between EC and FC production.
Short-term twin-spot studies confirm FSC origin of ECs and FSC exchange between layers
We generated multicolor clones using a minimal heat-shock and stained ovarioles 72h later to capture all cells derived from recombination in a single FSC. After recombination the two daughters have known “twin-spot” colour pairings (G-BR, B-GR, GR-BR; BG-GBR; Supplementary Fig. 7). Both daughters of an FC necessarily populate the same egg chamber. Unpaired FC patches must therefore derive from recombination in an FSC and were found up to the fourth egg chamber from the germarium. We concluded that marked FSC derivatives populated the germarium (generally two FC-associated cysts), the first three egg chambers and sometimes the fourth, consistent with budding of new egg chambers every 12h 4.
The average contribution of each FSC lineage colour to the entire FC population spanning egg chambers 1 to 3 or 4 (as appropriate for each ovariole) was 9.2% (n=90), approaching expectations for just one of the 14–16 FSCs present. Moreover, we could identify and exclude several exceptional lineages founded by two FSCs based on the presence of overlapping twin-spot pairs (e.g. GR lineages in ovarioles with B-GR and BR-GR pairs) to ensure that almost all lineages studied derived from a single cell.
Among the sixteen lineages with at least one EC, nine had matching FCs; in six cases the twin-spot lineage also included FCs (Fig. 7a–d). Of the remaining seven lineages, the twin-spot sister clone included FCs in five cases, showing that the parent FSC produced both EC and FC cell types. Altogether, we found twenty examples where both ECs and FCs derived from a single FSC, confirming the FSC origin of ECs.
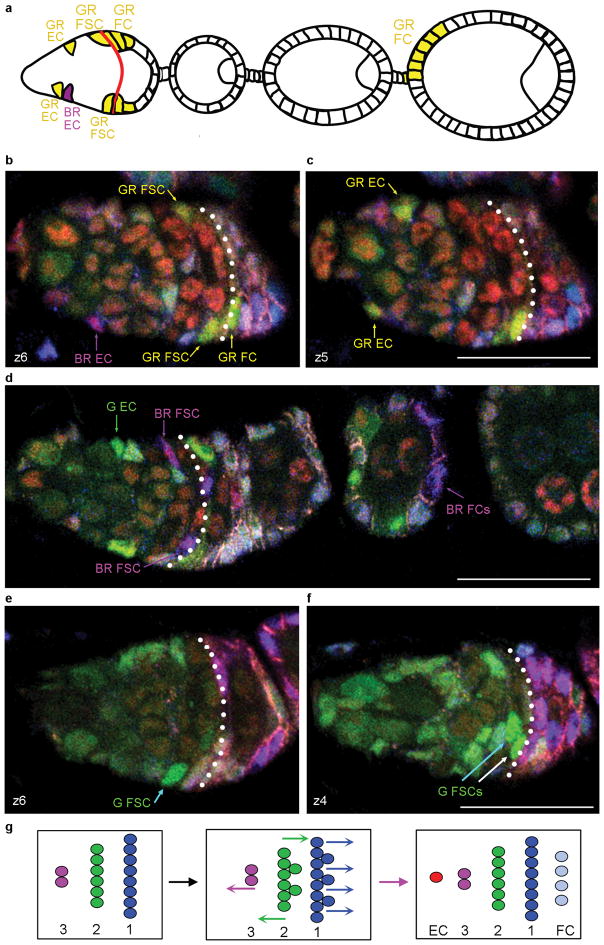
Figure 7. Twin-spot clone evidence for a common FSC origin of FCs and ECs.
(a–f) Analysis of multicolour FSC twin-spot clones 72h after induction. Known twin-spot pairings (Supplementary Fig. 7) allow identification of the two sister lineages derived from recombination in an FSC. (a) Illustration of the germarium and egg chambers populated by derivatives of GR and BR daughters of an FSC. (b,c) Two z-sections of the germarium illustrated in (a). The BR EC has no corresponding FSC or FC patch, while the GR lineage includes ECs, FSCs and FCs. The twin-spot parent FSC (and the GR daughter) therefore produced both ECs and FCs. (d) A G EC has no corresponding FSC or FC (the only G cell is indicated by a green arrow; all other cells with a similar colour in this image are GR cells) but the twin-spot BR lineage includes FCs and FSCs (as well as an EC in a different z-section). The twin-spot parent FSC therefore produced both ECs and FCs. (e,f) Two z-sections of the same germarium show one G FSC in layer 1 (white arrow) and two G FSCs in layer 2 (cyan arrows), showing that FSCs can move between layers. In (b,c) the two GR FSCs are both in layer 1 and in (d) there are BR FSCs in both layer 1 and 2. (g) Summary of FSC dynamics through one cycle (left to right), inferred from the rates of FC production, EC production and FSC division, as well as graded rates of FSC proliferation in layers 1–3. On average, 3–4 of 7–8 FSCs in layer 1 (blue) and 1–2 of 5–6 FSCs in layer 2 (green) divide, while at least four layer 1 FSCs become FCs and one layer 2 or 3 FSC becomes an EC. Consequently, in some cycles there must also be net movement of a FSC in layer 2 to replenish layer 1 or 3. Scale bars 20μm.
In these twenty lineages, the progeny of a single FSC must have moved between layers in order to produce an FC from layer 1 and an EC from layer 2 or 3. On the other hand, over 40% of EC-containing lineages had no matching FCs (7/16) and almost 80% of FC-containing lineages included no ECs (33/42), suggesting that FSCs also often dwell in an EC-producing or FC-producing layer. When there were two or more surviving FSCs of a single colour, they were confined to the same layer in eight cases and occupied more than one layer in sixteen cases, again indicating a moderate rate of movement between layers (Fig. 7b–f).
Altogether, 43 ECs and 166 FC patches were produced from a total of 152 marked FSCs, suggesting that ECs are produced at a frequency about four-fold lower than FCs, or roughly one per cycle of egg chamber budding (Fig. 7g). Based on the number of FSCs in different layers and their relative EdU indices (Fig. 5c), we estimate that FSC division in layer 1 may not quite suffice to supply FCs without net FSC loss, and hence that there is likely a slow net flow from layer 2 to layer 1 (Fig. 7g).
Wnt signaling controls A/P position and conversion of FSCs to ECs
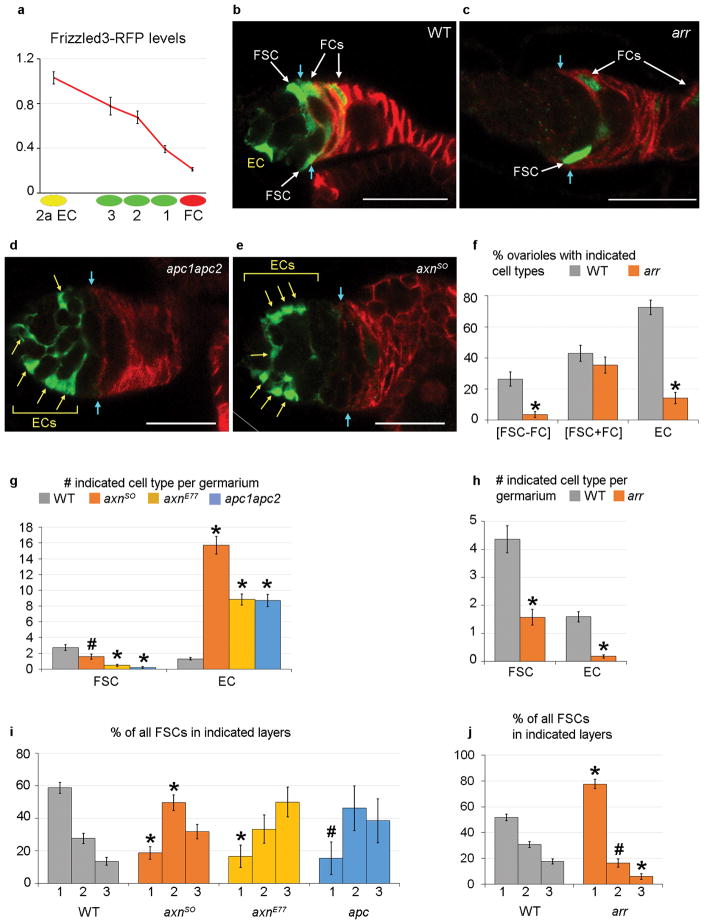
To study how FSC fate is regulated we examined extracellular signals that act on FSCs. Both Hedgehog (Hh) and JAK-STAT signaling promote FSC maintenance7, 19. Hh signaling was strong in ECs, FSCs and early FCs, with lower levels in more posterior FCs12, 19–21 (Supplementary Fig. 6m–o). A JAK-STAT pathway reporter19, 22 was expressed in FCs, FSCs, and region 2a ECs but barely at all in region 1 ECs (Fig. 6a–c). Fz3-RFP reports Wnt pathway activity in the germarium based on a robust signal that is greatly altered by genetic changes in Wnt pathway activity23. Fz3-RFP was expressed most strongly in ECs and anterior FSCs, with clearly lower levels in layer 1 FSCs and low or undetectable levels in FCs (Fig. 6g–i). Quantitation showed a consistent decline across the FSC domain (Fig. 8a). FSC clones are poorly maintained in adults if Wnt pathway activity is eliminated or increased19, 24 but it is not known how Wnt signaling affects FSC maintenance.
Figure 8. Wnt pathway activity regulates FSC position and FC/EC production.
(a) Fz3-RFP levels (mean and SEM) for region 2a ECs, layer 1–3 FSCs and immediate FCs relative to region 1 ECs (set at 1.0) from 18 biologically independent germaria (n=31 region 2a ECs, n=13 layer 3 FSCs, n=27 layer 2 FSCs, n=32 layer 1 FSCs, n=31 FCs), showing a Wnt signaling gradient across the FSC region. The Fz3-RFP signal differed significantly by Student’s t-test between region 2a ECs and layer 3 FSCs (p<0.01), layer 2 and layer 1 FSCs (p<0.0001), layer 1 FSCs and immediate FCs (p<0.0001). (b–j) MARCM clones analyzed 12d after clone induction with (b–e) Fas3 (red) borders indicated by cyan arrows. (b) WT clone with labeled EC, FSCs and FCs. (c) arr mutant clone with labeled layer 1 FSC and FCs but no labeled ECs. (d) apc1apc2 and (e) axn mutant clones with many labeled ECs (yellow arrows) but no labeled FSC or FCs. (e) Diagonal white line indicates an edge of the original image (shown in Supplementary Fig. 9). (g,i) Increased Wnt pathway activity due to axn or apc mutations (g) decreased the average number of FSCs and drastically increased the average number of labeled ECs per germarium, and (i) decreased the proportion of FSCs in layer 1 in favor of more anterior positions. (f,h,j) Conversely, loss of Wnt pathway activity (f) increased the proportion of FSC clones associated with FCs and decreased the percentage of ovarioles with labeled ECs, (h) decreased the average number of labeled ECs and FSCs per germarium, and (j) increased the proportion of FSCs in layer 1 versus layers 2 and 3. Error bars show SEM for (g) n=75 (WT), n=67 (axnSO), n=64 (axnE77) and n=63 (apc1apc2) biologically independent germaria, (i) n=206 (WT), n=107 (axnSO), n=30 (axnE77) and n=13 (apc1apc2) FSCs, (f, h) n=91 (WT) and n=85 (arr) biologically independent germaria, and (j) n=397 (WT) and n=134 (arr) FSCs. (g, h) Significant differences from controls (WT) were assessed by Pearson’s chi-squared test (* p<1×10−4). (f,i,j) Significant differences from controls (WT) were assessed by Fisher’s exact two-tailed test (* p<0.001, # p<0.05). Scale bars 20μm. See Supplementary Table 1 for supporting data.
Increased Wnt pathway activity induced by loss of Axin (Axn) or Adenomatous Polyposis Coli (APC) activity led to a decline in the frequency of FC-producing FSC clones over time (Fig. 8d,e and Supplementary Fig. 8a,d), as noted previously19, 24. There were also significantly fewer FSCs per ovariole than in controls by 12 days (Fig. 8g and Supplementary Fig. 8b). Strikingly, all ovarioles contained ECs by 12d (Supplementary Fig. 8d) with a huge increase in the average number of ECs per ovariole (Fig. 8d,e,g), suggesting FSCs were lost principally because they became ECs at an unusually high rate. The large number of axn or apc mutant cells in the location of ECs did not incorporate EdU (0/499 at 6d; 0/1093 at 12d). Hence, these cells did not amplify after becoming ECs; they exhibit a key characteristic of normal EC cells- quiescence.
The loss of FSCs was slower for axnS0 than for axnE77 and apc1 apc2, but axnSO clones eventually produced the most ECs per ovariole (Fig. 8g and Supplementary Fig. 8b). This inverse correlation is consistent with greater initial survival and amplification of axnSO FSCs (relative to axnE77 and apc1 apc2 FSCs) prior to conversion of the majority of FSCs to ECs, providing further evidence that ECs normally derive from FSCs.
axn and apc mutant FSCs accumulated significantly in layer 3 compared to controls by day 6 (Supplementary Fig. 8c) and by day 12 only 15–20% of surviving FSCs were in layer 1 compared to almost 60% for controls (Fig. 8i). Thus, FSCs with elevated Wnt pathway activity moved to increasingly anterior positions.
EdU incorporation was significantly lower in axn and apc mutant FSCs than in controls, even when considering FSCs only in layer 1 (Supplementary Fig. 8h). We have examined many other mutations that reduce FSC proliferation by similar or greater amounts25–27 but they did not significantly alter FSC location or increase the production of ECs.
When Wnt pathway activity was eliminated by a null arrow mutation FSC/FC clones were lost at a modestly enhanced rate (Fig. 8c,f and Supplementary Fig. 8e), as noted previously19, 24. Strikingly, we found that almost no arr mutant ECs were produced (Fig. 8c,f,h and Supplementary Fig. 8e,f). Reduced EC production cannot be attributed simply to loss of FSCs because the ratio of ECs to FSCs was much lower for arr than for controls (0.06 vs 0.19 at 6 days; 0.11 vs 0.37 at 12 days). FSC proliferation was not significantly altered by loss of arr (Supplementary Fig. 8i).
The disposition of arr FSCs was skewed substantially towards layer 1, especially by 12d (Fig. 8j and Supplementary Fig. 8g). Most clones containing only layer 1 FSCs were associated with marked FCs in the first Fas3-positive cells for arr (86%), as in controls (82%), indicating recent FC production. The concentration of arr mutant FSC in layer 1 can therefore explain why almost all ovarioles with marked arr mutant FSCs also included marked FCs (Fig. 8f and Supplementary Fig. 8e) and further substantiates the hypothesis that FCs derive directly only from layer 1 FSCs.
In summary, eliminating Wnt signaling in FSCs led to a shift to layer 1, increased FC production by surviving FSCs and an almost complete failure to produce ECs, while elevation of Wnt pathway activity led to FSC movement out of layer 1, reduced FC production by surviving FSCs and a very large increase in EC production. These correlations provide further evidence that ECs derive directly from anterior FSCs while FCs derive directly from posterior FSCs. The results also show that FSC location and FSC fates are guided by the magnitude of graded Wnt signaling.
DISCUSSION
We have uncovered several interesting facets of somatic stem cell organization in the Drosophila ovary. First, a relatively large population of FSCs is maintained by population asymmetry. Second, FSCs produce not only proliferating FCs but also quiescent ECs. Third, FSCs have heterogeneous properties correlated with their position along the A/P axis; most importantly, FCs derive from posterior FSCs while ECs derive from anterior FSCs. Fourth, FSCs are radially mobile and can exchange A/P positions. Fifth, the level of Wnt signaling is graded over the FSC domain, dictating the A/P position of FSCs and whether FSCs produce FCs or ECs.
In this study we identified and counted clones originating in FSCs by the presence of marked FCs at least 9 days after marking, several days beyond the lifetime of an FC, which inevitably passes through the ovariole without delay in association with a germline cyst. We also identified single marked cells of these lineages that persisted in the germarium to define the precise location of FSCs. These criteria meet the fundamental defining characteristics of stem cells.
Our measurement of 14–16 FSCs may not be precise because we could only count six distinct surviving lineages directly and we had to estimate how many lineages were lost prior to our earliest sampling time (Fig. 2k). Nevertheless, both the total number of FSCs and their distribution among layers deduced from lineage analyses matched the total somatic cell content of layers 1–3 almost perfectly. Several factors contributed to finding more FSCs than previously appreciated. More lineage colours, a high frequency of clone induction and comprehensive cell by cell analyses increased our opportunity to count surviving FSCs, while analyses at multiple time-points allowed us to deduce that FSCs are maintained by population asymmetry, with many lineages lost very quickly. Earlier estimates of far fewer FSCs were based, in essence, on counting only those stem cells that survived for relatively long periods of time4, 5, 7.
The FSCs we identify have heterogeneous properties at any one instant, with only layer 1 FSCs poised to produce FCs directly and an A/P gradient of proliferation rates. However, FSCs also exchange A/P positions over time and therefore constitute a single stem cell population. Retrospective analysis shows that some FSCs were short-lived and some produced only FCs, while others produced ECs, but these behaviors cannot be forecast for individual FSCs. At any one instant, all FSCs in the population have the potential to produce FCs and ECs, and have an unpredictable lifespan.
Many aspects of FSC organization reported here are remarkably similar to the organization of mammalian intestinal stem cells 1, 31. FSCs and Lgr5-positive murine intestinal stem cells exist in communities of similar size. Both are maintained by population asymmetry, engendering neutral competition among individual stem cells, but there is also spatial heterogeneity coupled to dynamic exchange (“dynamic stem cell heterogeneity”31) along the major developmental axis32. A key difference is that dynamic heterogeneity guides alternative differentiation outcomes only for FSCs. In the gut, diverse secretory cells and transit-amplifying cells are defined, shortly after their derivation from stem cells, by different levels of Wnt and Notch pathway activities. However, it is currently thought that each cell type is initially produced at the same location immediately above the stem cell population, with stochastic expression of Notch ligands determining which cells adopt secretory fates through lateral inhibition33–36.
The choice of an FSC to move anteriorly and become one cell type, to move posteriorly and become a different cell type, or to remain within the stem cell niche presents a potentially general stem cell paradigm. Here, these choices are guided by the magnitude of Wnt signaling at multiple locations within the stem cell domain. Increased Wnt signaling caused layer 1 FSCs to move anteriorly and accelerated the conversion of layer 2/3 FSCs to ECs. Conversely, loss of Wnt signaling caused posterior movement and reduced EC production in layer 2/3 FSCs. Dose-dependent responses at different positions and the observed gradient of Wnt signaling declining from anterior to posterior over the FSC domain suggest that normal FSC behaviour is guided by graded Wnt signaling.
Supplementary Material
Acknowledgments
We thank Yokarla Veras, Raphaël Toueg and Natania Field for technical assistance, Daniel Rabinowitz and Harmen Bussenmaker for help with statistical analyses, Ward Odenwald, Ramanuj DasGupta, Erika Bach, the Developmental Studies and the Bloomington Stock Center for antibodies and fly stocks, Tulle Hazelrigg, Michael Shen, Meehan Crist and Jamie Little for comments on the manuscript. This work was supported by the National Institutes of Health (RO1 GM079351 to D.K.); D.M. was supported in part by an NIH training grant.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, A.R. and D.K.; Methodology, A.R., E.M., N.T., G. V., and D.K.; Formal Analysis, A.R., D.M. and D.K.; Investigation, A.R., D.M., K.S.P., A.B., and S.F.; Writing-Original Draft, D.K.; Writing-Review & Editing, A.R., D.M, and D.K.; Visualization, A.R. and D.K.; Funding Acquisition, D.K.. The authors have no competing financial interests.
References
- 1.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliazer S, Buszczak M. Finding a niche: studies from the Drosophila ovary. Stem cell research & therapy. 2011;2:45. doi: 10.1186/scrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 5.Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Nystul T, Spradling A. Regulation of epithelial stem cell replacement and follicle formation in the Drosophila ovary. Genetics. 2010;184:503–515. doi: 10.1534/genetics.109.109538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001;410:599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- 8.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Developmental Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138:5087–5097. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends in Neurosciences. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [erratum appears in Trends Neurosci 2001 Jul;24(7):385] [DOI] [PubMed] [Google Scholar]
- 11.Kronen MR, Schoenfelder KP, Klein AM, Nystul TG. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS One. 2014;9:e101085. doi: 10.1371/journal.pone.0101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahai-Hernandez P, Nystul TG. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development. 2013;140:4490–4498. doi: 10.1242/dev.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nature reviews. Molecular cell biology. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- 14.Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Jang AC, Lin CH, Montell DJ. Castor is required for Hedgehog-dependent cell-fate specification and follicle stem cell maintenance in Drosophila oogenesis. Proc Natl Acad Sci U S A. 2013;110:E1734–1742. doi: 10.1073/pnas.1300725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman TR, et al. Novel tools for genetic manipulation of follicle stem cells in the Drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation. Genetics. 2015;199:935–957. doi: 10.1534/genetics.114.173617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 18.Buszczak M, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vied C, Reilein A, Field NS, Kalderon D. Regulation of stem cells by intersecting gradients of long-range niche signals. Dev Cell. 2012;23:836–848. doi: 10.1016/j.devcel.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996;122:3283–3294. doi: 10.1242/dev.122.10.3283. [DOI] [PubMed] [Google Scholar]
- 21.Vied C, Kalderon D. Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development. 2009;136:2177–2186. doi: 10.1242/dev.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bach EA, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Page-McCaw A. A matrix metalloproteinase mediates long-distance attenuation of stem cell proliferation. J Cell Biol. 2014;206:923–936. doi: 10.1083/jcb.201403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Kalderon D. Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J Cell Biol. 2014;205:325–338. doi: 10.1083/jcb.201309141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ZA, Huang J, Kalderon D. Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nature communications. 2012;3:769. doi: 10.1038/ncomms1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZA, Kalderon D. Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc Natl Acad Sci U S A. 2009;106:21701–21706. doi: 10.1073/pnas.0909272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matunis EL, Stine RR, de Cuevas M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis. 2012;2:137–144. doi: 10.4161/spmg.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat Cell Biol. 2016;18:349–355. doi: 10.1038/ncb3332. [DOI] [PubMed] [Google Scholar]
- 30.Voog J, et al. Escargot restricts niche cell to stem cell conversion in the Drosophila testis. Cell Rep. 2014;7:722–734. doi: 10.1016/j.celrep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger T, Simons BD. Dynamic stem cell heterogeneity. Development. 2015;142:1396–1406. doi: 10.1242/dev.101063. [DOI] [PubMed] [Google Scholar]
- 32.Ritsma L, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basak O, et al. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. EMBO J. 2014;33:2057–2068. doi: 10.15252/embj.201488017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buczacki SJ, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 35.Schuijers J, et al. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell. 2015;16:158–170. doi: 10.1016/j.stem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Stamataki D, et al. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One. 2011;6:e24484. doi: 10.1371/journal.pone.0024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.