Abstract
Rheumatoid arthritis (RA) is a chronic, definitely disabling, and potentially severe autoimmune disease. Although an increasing number of patients are affected, a key treatment for all patients has not been discovered. High-mobility group box-1 (HMGB1) is a nuclear protein passively and actively released by almost all cell types after several stimuli. HMGB1 is involved in RA pathogenesis, but a convincing explanation about its role and possible modulation in RA is still lacking. Microbiome and its homeostasis are altered in patients with RA, and the microbiota restoration has been proposed to patients with RA. The purpose of the present review is to analyze the available evidences regarding HMGB1 and microbiome roles in RA and the possible implications of the crosstalk between the nuclear protein and microbiome in understanding and possibly treating patients affected by this harmful condition.
1. Introduction
Among the autoimmune diseases, rheumatoid arthritis (RA) represents one of the most relevant [1, 2]. In fact, patients affected by RA have a poor quality of life, due to articular pain and functional impairment [3–7]. In addition, RA causes an increased risk of other pathological conditions, including cardiovascular diseases [8–16]. Furthermore, immunosuppressant for RA can often determine dangerous and potentially lethal side effects, among which are infections, organ failure, and even death [7, 17–24]. Although RA has been studied over the last decades and several researchers have been focused on identifying new potential drugs, a definite treatment is not available and the disease can progress to severe disability [4, 7, 17, 19, 25–30]. One of the reasons of the delayed defeat of the disease is the lack of a full understanding of the causes responsible for the RA onset. Indeed, while several pathways and mechanisms have been clarified, such as lymphocyte, interleukin, and tumor necrosis factor (TNF) roles, the very initial trigger has not been discovered [1, 23, 27, 28, 31–41]. As in other autoimmune conditions, an infectious event has been proposed to explain the altered immune response and the RA initiation [42]. In this scenario, microbiome obviously represents an attractive candidate. In fact, the altered crosstalk between microbiome and the immune system could underlie the disease onset [43–54]. Among the well-known pathways, the high-mobility group box-1 (HMGB1) plays a role in RA. In fact, this nuclear protein is involved in synovial inflammation observed in RA and could represent a new therapeutic target [55–68]. Recent data demonstrated that the HMGB1 pathway is important in a model of bowel inflammation [69]. The aims of the present review are to evaluate the available data about the role of HMGB1 in the crosstalk between gut microbiome and RA-altered immune response, to try to better understand the mechanisms underlying this disease, and to see whether it could represent a therapeutic target and, eventually, whether it would be more cost-effective to inhibit or stimulate the activity of HMGB1 in these conditions.
2. Rheumatoid Arthritis
RA is an autoimmune disease, characterized by chronic inflammation of joints and several other tissues, including those of the lungs, vessels, blood, eye, skin, and heart [70, 71]. RA is not a rare disease; in fact, out of every 100,000 people, about 40 are diagnosed with RA every year [72]. In general, at the onset, RA affects the small joints of hands. However, also hips, shoulders, and knees may be involved, and RA can potentially hit every joint [25, 33, 73]. Quality of life of patients affected by RA is worsened by pain, swelling, stiffness, and loss of function in the joints [4, 5, 74]. Furthermore, patients with RA have a reduced life expectancy due to an increased mortality for cardiovascular events, infections, and drug side effects [9–11]. In fact, it has been definitely demonstrated that patients with RA have an increased risk of myocardial infarction and of stroke [75]. The principal reason is that the typical chronic inflammation observed in the RA scenario plays a pivotal role in atherosclerotic plaque formation and destabilization [10, 11, 76, 77]. In addition, the immune dysregulation of T and B cell network can affect other cardiovascular risk factors, such as hypertension and lipid metabolism [9, 78, 79]. Furthermore, sedentary lifestyle and weight gain due to joint impairment could be additional factors. Other morbidity causes are certainly infections. In fact, immunosuppressant therapy and RA itself increase the risk of infectious complication, and about a quarter of deaths are caused by infections [42, 80–86]. Finally, several of the most effective treatments commonly used in patients with RA can have many side effects, including organ failure, cancer and, sometimes, death [18–20, 22].
Although the relevance and the impact of RA are clearly important, an effective treatment has not been yet discovered. The reason of this delay may reside in the relatively unknown initial pathological event. Indeed, several mechanisms have been clarified to explain the fundamental injury: the synovitis and the joint destruction [3]. First, a genetic susceptibility is known. In fact, an association between RA onset and major histocompatibility complex (MHC) class II antigens, specifically the shared epitope found on HLA-DRB1, has been demonstrated [3, 70, 71, 87–89]. However, RA does not seem to be a genetically transmitted disease, and DNA in the strict sense plays a minor role. Regarding the genetic heritage and regulation, novel mechanisms have been elucidated in the last decade, in particular the epigenetic regulatory systems, including the microRNA (miRNA) pathways [41, 90, 91]. Moreover, miRNAs can regulate gene expression and protein function of several cytokines, growth factors, and receptors involved in RA [41]. Alongside the genetic susceptibility, a trigger is required to initiate RA; in fact, studies performed on twins have demonstrated that identical genetics are not sufficient to develop similar disease [92]. Several potential environmental triggers have been implicated, among which are cigarette smoking and infections [93–95]. Taking into account infectious event, the relationship between RA and infective disease is dependent on the immune and inflammatory activation caused by pathogens [42, 96–98]. The T and B cell activation and the beginning of the autoimmune response are the mechanisms involved in the RA onset [32, 79, 99–103]. Another important event is represented by the protein citrullination, a normal posttranslational modification required in several physiological processes [104–107]. In RA, there is an autoimmune activity against citrullinated peptides detected as anti-citrullinated peptide antibodies (ACPA), a prototypical biomarker of the disease. After T and B cell activation and autoantibody production, additional cell types come into play to propagate and amplify inflammation, among which are macrophages that produce interleukin- (IL-) 1, IL-6, IL-8, and tumor necrosis factor- (TNF-) α [108–113]. All these phenomena translate into the main event of the disease: joint damage.
3. High-Mobility Group Box-1
The high-mobility group box-1 (HMGB1) is a highly conserved DNA-binding protein, present in the nucleus, that acts as a damage-associated molecular pattern (DAMP) molecule [114]. HMGB1 belongs to the family of the high-mobility group (HMG) chromosomal proteins, distinguished on the basis of their rapid mobility on electrophoresis gels [115]. These nuclear proteins were discovered more than 40 years ago and are subdivided into three superfamilies: the HMGB, HMGN, and HMGA superfamilies [116]. Of the HMGB family that includes HMGB1, HMGB2, HMGB3, and SP100HMG, HMGB1 is the most abundant nonhistone DNA-binding protein [114]. HMGB1 is the typical DAMP molecule, and it is involved in the setting of both sepsis and sterile inflammation [114]. This nuclear protein belongs to the “alarmin” family, a group of signaling effectors that acts as an injury-induced response in mammals [117]. DAMPs interact with several ancestral receptors and pathways and share a significant number of signaling systems with the pathogen-associated molecular patterns (PAMPs) [118]. DAMPs and PAMPs can activate the immune system by using the same ways, starting from completely different pathological triggers. In this scenario, HMGB1 represents the prototypical molecule that can stimulate a lot of immune responses against external injury. In this sense, HMGB1 could be considered exclusively a defensive protein. However, this protein plays also a dangerous and harmful role in numerous conditions by activating detrimental pathways so that many authors suggest the blockade of its function [119–121]. The role of HMGB1 in normal and in disease conditions was originally attributed to the passive release in the extracellular space after the cell damage [122]. Subsequently, a more complex mechanism of action was identified for HMGB1: it is also actively secreted by almost all types of cells, in response to several stimuli, and it can activate different pathways, depending on the tissue where the signaling is triggered and on the kind of receptor involved [118, 123]. The most recent findings have highlighted that the effect of HMGB1 is also closely dependent on the redox status of the milieu where the protein is released [124].
The first information about HMGB1 activity has been collected in models of sepsis and systemic infections [125]; the idea that this alarmin is involved in the sterile inflammation and fibrosis rapidly increased [55, 114, 117, 126] and fibrosis [127]. During the last decade, additional data were collected regarding more variegated effects of this nuclear protein in terms of tissue remodeling and angiogenesis, not necessarily related to septic conditions [115, 128–131].
4. High-Mobility Group Box-1 and Rheumatoid Arthritis
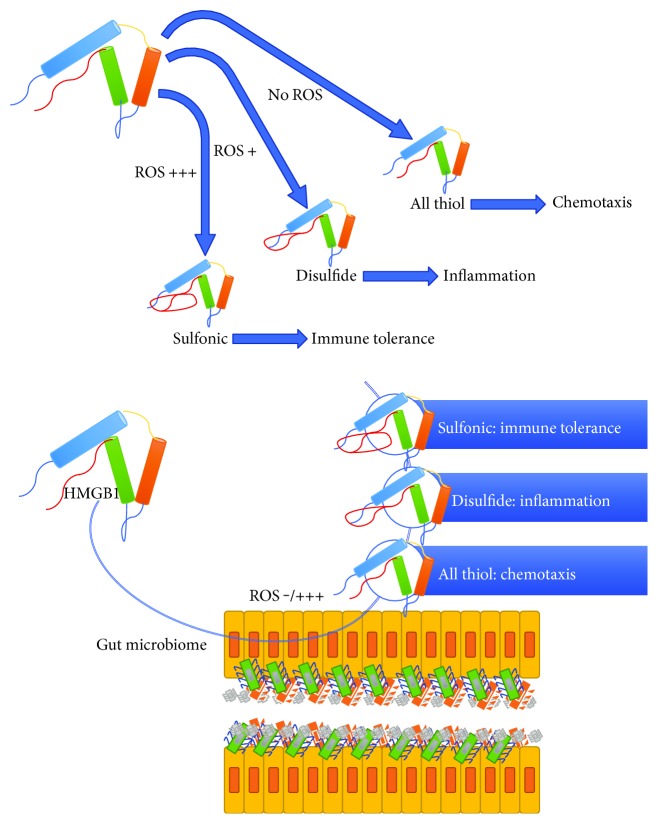
There are several data supporting the role of HMGB1 in RA, particularly suggesting that it plays a role in initiating the synovium inflammation and in maintaining the joint damage mediated by proinflammatory cytokines. Since the first studies by Andersson and coworkers, it has been clarified that HMGB1 can stimulate the release of IL-1, IL-6, and TNF-α [122] and it determines the beginning and the development of inflammation in different experimental models of arthritis. Furthermore, HMGB1 is increased in synovium and synovial fluid of patients with RA, compared with patients with osteoarthritis [132, 133]. Moreover, HMGB1 blockade reduces arthritis induction in experimental models [55, 56, 59, 63, 67, 134, 135]. Finally, HMGB1 administration induces synovial angiogenesis through a vascular endothelial growth factor- (VEGF-) dependent mechanism [55]. Although multiple mechanisms involved in RA pathogenesis have been discovered, there is no fully comprehensive explanation about the HMGB1 pathway in this scenario. In particular, HMGB1 function depends on two principal factors: the oxidation/reduction status and the extracellular milieu where different receptor systems can be found. While the second point is enough studied and we know now that the TLRs, the receptor for advanced glycation end-products (RAGE) and the IL-1 receptor, represent the most important extracellular pathways [61], we less know about the factors that modify the oxidation/reduction status of HMGB1. In fact, depending on oxidation/reduction status, HMGB1 can be in three different conformations: sulfonic, disulfide, or all-thiol form [58, 136, 137]. According to the redox status and following different structures, HMGB1 explicates various functions. For instance, the sulfonic form acts as an immune tolerance inducer, while the disulfide one is a major player in inflammation. In this sense, the HMGB1 pathway is notably plastic and dynamic and depends on the redox status of the extracellular setting, not only on the receptor quality and content [61]. However, it is not yet clear how the environment can modify the redox state and what cell types are involved in this process.
5. Microbiome
The term microbiome refers to the genetic characterization of the entire microbiota in a specific tissue [138]. We know several microbiomes, depending on localization, such as skin, lung, and oral microbiomes [139]. Certainly, the gut microbiome is one of the most important because, together with activities shared with other microbiomes, it plays a fundamental role in digestion and transformation of food [43, 45, 51, 140, 141]. However, the principal function of microbiome is the crosstalk with the immune system to modulate and regulate the immune response against the host. Gut is colonized by billions of bacteria immediately after birth, and the mucosal interface of the intestinal tract is characterized by several types of immune cells and systems, organized in aggregates and organs [140]. The location of these systems is strategically at the border with the outside world, and they require a multipotent and versatile network of signals and receptors. In fact, there we have the pattern recognition receptors (PRRs), an ancestral part of the immune system that can recognize several pathogens with the same pathway [142]. Among PPRs, toll-like receptors (TLRs) are the prototypical receptors that bind elemental fragments of bacteria, such as lipopolysaccharides (LPSs), and also of microbiota [142, 143]. However, given the number and the different types of species of gut microbiome, it seems unlikely that these bacteria activate the immune response normally. Most likely, the interaction between microbiome and intestinal immune system determines a continuous modulation of the two players [43, 144].
6. Microbiome and Rheumatoid Arthritis
The connection between gut and joints was hypothesized several decades ago, when researchers studied different models of inflammatory arthritides, in particular spondyloarthropathies related to inflammatory bowel diseases and secondary to intestinal resections [49]. The interaction between genetic profile and environmental triggers is important in the pathogenesis and development of RA. Oral chronic colonization or infection sustained by Porphyromonas gingivalis was linked to RA development [145, 146], and traces of bacteria were found in synovial fluid of patients with RA. Furthermore, prolonged antibiotic therapy against certain bacterial infections is effective in RA disease control [147]. Breaking tolerance in RA could occur in reaction to these pathogens. However, Porphyromonas gingivalis is not the only implicated in RA. In fact, data regarding other bacteria are available, and a single infection seems to be not likely as the sole cause. Moreover, the analysis of microbiome from mice prone to arthritis development revealed that microbiome can influence the arthritis susceptibility [148]. Several reports demonstrated that a subpopulation of patients with early RA harbored intestinal microbiota dominated by Prevotella copri and that SKG mice harboring the same microbiota had an increased number of intestinal Th17 cells and developed severe arthritis due to autoreactive T cells [149]. Interestingly, a taxon-level analysis-based study revealed an expansion of rare taxa with a decrease in abundant taxa in microbiome of patients with RA, compared with controls; this finding was related to the production of proinflammatory cytokines, such as IL17 [150]. Microbiome alterations do not only affect the expression level of TLRs of cells that exhibit antigens but also contribute to the Treg/Th17 deregulation. Epigenetic modifications triggered by external factors are important pathways leading to an altered gene expression. Crosstalk between microbiome and the mucosal immune system has been demonstrated being a crucial activator of epigenetic pathways in mammalian, including humans [43]. The most compelling evidence that gut infection-inflammation is a key moment in the occurrence of arthritis comes from the K/BxN and IL1RA−/− mice that do not develop arthritis in a germ-free setting [151]. On the other hand, the evidence that a normal gut microbiota is fundamental in maintaining the homeostasis is shown in the streptococcal cell wall arthritis, in which the normal flora protects against the occurrence of arthritis [152].
RA is a chronic multifactorial autoimmune disease where the immune event represented by the ACPA production can start even 15 years before symptoms, thus suggesting that the initial pathogen phenomenon is not necessarily present in the joints. In this scenario, microbiome represents the ideal theater [44]. Starting from animal models, about forty years ago, several researchers found that the administration of specific bacteria fragments, such as LPS, can induce arthritis and that the presence of gut microbiota is protective against the injury. Furthermore, additional evidence suggested that the balance of the intestinal germ population is fundamental in maintaining homeostasis and protection against environmental pathogens [153]. Recent data demonstrated that alteration of the gut microbiome can influence the balance of pro- and anti-inflammatory immune cells, such as T reservoir, and promote the development of RA [154]. Moreover, it has been found that TLRs play a crucial role in influencing the Th17 differentiation and the Treg inhibition caused by gut microbiome in animal and human models [142]. However, although a lot of possible mechanisms have been elucidated to demonstrate the role of microbiome in RA, a definitive, omnicomprehensive, and convincing explanation has not been yet found.
7. High-Mobility Group Box-1 and Microbiome
Since LPS is one of the most important experimental activators of the HMGB1 pathway, it seems fair to assume that intestinal bacterial flora is involved in HMGB1 modulation. However, there is a lack of evidence about the crosstalk between HMGB1 and microbiome due, at least in part, to the difficulty of measuring tissue and fluid protein concentrations in its extracellular form. In fact, once released after cellular injury or activation, HMGB1 can be found in at least three conformations, depending on the oxidation/reduction status, and the commonly used experimental kits are not capable to detect all the conformations [58, 136, 137]. Furthermore, the complexity of the gut and the difficulty of obtaining reproducible data about the redox state of microbiota make it even more difficult task. However, HMGB1 surely plays a role in oral and intestinal homeostasis [155], and recent data demonstrated that this nuclear protein is involved in the inflammatory response of the gut and that the HMGB1 blockade is able to inhibit the LPS-induced injury by a TLR4-dependent mechanism [69]. In this model, TLR4 is considered a pivotal receptor for inflammation and the interaction between HMGB1 and TLR4 of mucosal tissue is important in inducing the intestinal inflammation. However, the inflammatory milieu is rich in oxidizing agents, and the HMGB1 translocation in this scenario could promote the structural modification of the protein. Furthermore, microbiome represents an important source of redox-based signals that modulate critical microbial and host cell functions [156–158]. Moreover, the microbiome modulates the redox status of the host by modifying the glutathione metabolism [159]. In addition, recent data obtained in both in vivo and in vitro models demonstrated a novel HMGB1-RAGE-mediated redox signaling pathway involved in intestinal inflammation induced by a liver dysfunction model [160]. As shown in Figure 1, HMGB1 conformational modulation depending on microbiome homeostasis could lead to different redox states and consequent activities. In this respect, the maintenance of a proper homeostasis of the microbiome may be important to prevent damage caused by HMGB1 overexpression.
Figure 1.
A schematic representation of the interaction and the crosstalk between HMGB1 and gut microbiome in RA pathogenesis. Depending on the oxidation/reduction status after the passage through the gut microbiome, HMGB1 can play several and different roles in RA initiation and maintenance.
8. Therapeutic Implications
A definitive treatment for all RA patients has not been discovered [23, 161–164]. A multitarget approach is required to better control the disease, and several pathways must be considered to completely treat RA. However, immunosuppressive drugs are not always sufficient [165–167]. For this reason, new therapeutical strategies are desirable and a better knowledge of HMGB1 interaction with microbiome in RA could provide new elements to achieve it. In this regard, a possible attempt could be the HMGB1 pathway blockade. In fact, several data demonstrated that, together with the commonly used monoclonal antibody-based therapies, monoclonal antibodies directed versus HMGB1 can protect against arthritis in experimental models [168, 169]. In particular, in two notably different models of arthritis, collagen-induced arthritis (CIA) and a genetic model of arthritis, Schierbeck and colleagues demonstrated that anti-HMGB1 monoclonal antibody administration significantly ameliorated the clinical courses in these experimental conditions. However, there is no evidence about the redox status and the possible role of microbiome in these models, and further data are needed to better understand the possible implications of an altered homeostasis of microbiome in HMGB1-dependent arthritis and in anti-HMGB1 therapy efficacy. Moreover, in a model where germ-free piglets were orally colonized with enteric bacterial pathogens, HMGB1 result significantly increased, suggesting that the upset balance of the microbiome can affect the HMGB1 pathway equilibrium [170]. Since the protein redox state can significantly modify the HMGB1 activity, a therapy capable of controlling the microbiome-oxidizing capacity could represent a new interesting approach. In this respect, probiotics need to be cited. Probiotic administration restores homeostasis of the gut microbiome and can have several beneficial effects [52]. Among the autoimmune disorders, RA seems to benefit from the probiotic therapy [54, 171]. Results obtained from animal models demonstrated that oral therapy with Lactobacillus casei ameliorated CIA by downregulating T helper 1 effector functions [172] and by reducing proinflammatory cytokines [173]. Also, data from humans have been achieved. In particular, in 46 patients with RA, Lactobacillus casei was orally administrated for 8 weeks and the disease activity score and serum proinflammatory cytokines were significantly decreased by the intervention [174]. In this setting, it is possible to speculate that homeostasis of microbiome could regulate HMGB1 activities in these patients. However, additional data are required to confirm this hypothesis.
9. Conclusions
RA is a chronic, harmful, and potentially severe disease for which there is no yet a decisive treatment. HMGB1 and microbiome alterations are involved in pathogenesis of RA, and the crosstalk between the protein and the microbiome deserves to be studied more carefully in order to offer a new therapeutic tool for patients with this serious disease.
Conflicts of Interest
The authors declare that they have no conflicts to disclose.
References
- 1.Catrina A. I., Svensson C. I., Malmström V., Schett G., Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nature Reviews Rheumatology. 2017;13(2):79–86. doi: 10.1038/nrrheum.2016.200. [DOI] [PubMed] [Google Scholar]
- 2.Grøn K. L., Ornbjerg L. M., Hetland M. L., et al. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis. Results from 34 countries participating in the Quest-RA program. Clinical and Experimental Rheumatology. 2014;32(6):869–877. [PubMed] [Google Scholar]
- 3.Firestein G. S., McInnes I. B. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones G., Nash P., Hall S. Advances in rheumatoid arthritis. The Medical Journal of Australia. 2017;206(5):221–224. doi: 10.5694/mja16.01287. [DOI] [PubMed] [Google Scholar]
- 5.Katz P. Fatigue in rheumatoid arthritis. Current Rheumatology Reports. 2017;19(5):p. 25. doi: 10.1007/s11926-017-0649-5. [DOI] [PubMed] [Google Scholar]
- 6.Martelli L., Olivera P., Roblin X., Attar A., Peyrin-Biroulet L. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. Journal of Gastroenterology. 2017;52(1):19–25. doi: 10.1007/s00535-016-1266-1. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Q., Huang J., Lin Y., et al. Polymorphisms and pharmacogenomics for the toxicity of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96(11, article e6337) doi: 10.1097/MD.0000000000006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadley I., Pera A., Morrow G., Davies K. A., Kern F. Expansions of cytotoxic CD4(+)CD28(−) T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Frontiers in Immunology. 2017;8:p. 195. doi: 10.3389/fimmu.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chodara A. M., Wattiaux A., Bartels C. M. Managing cardiovascular disease risk in rheumatoid arthritis: clinical updates and three strategic approaches. Current Rheumatology Reports. 2017;19(4):p. 16. doi: 10.1007/s11926-017-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao K. P. Cardiovascular disease in patients with rheumatoid arthritis. Trends in Cardiovascular Medicine. 2017;27(2):136–140. doi: 10.1016/j.tcm.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoudi M., Aslani S., Fadaei R., Jamshidi A. R. New insights to the mechanisms underlying atherosclerosis in rheumatoid arthritis. International Journal of Rheumatic Diseases. 2017;20 doi: 10.1111/1756-185X.12999. [DOI] [PubMed] [Google Scholar]
- 12.Bellucci E., Terenzi R., La Paglia G. M., et al. One year in review 2016: pathogenesis of rheumatoid arthritis. Clinical and Experimental Rheumatology. 2016;34(5):793–801. [PubMed] [Google Scholar]
- 13.Bonek K., Głuszko P. Cardiovascular risk assessment in rheumatoid arthritis - controversies and the new approach. Reumatologia. 2016;54(3):128–135. doi: 10.5114/reum.2016.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougados M. Comorbidities in rheumatoid arthritis. Current Opinion in Rheumatology. 2016;28(3):282–288. doi: 10.1097/BOR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 15.Faccini A., Kaski J. C., Camici P. G. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. European Heart Journal. 2016;37(23):1799–1806. doi: 10.1093/eurheartj/ehw018. [DOI] [PubMed] [Google Scholar]
- 16.Fent G. J., Greenwood J. P., Plein S., Buch M. H. The role of non-invasive cardiovascular imaging in the assessment of cardiovascular risk in rheumatoid arthritis: where we are and where we need to be. Annals of the Rheumatic Diseases. 2017;76(7):1169–1175. doi: 10.1136/annrheumdis-2016-209744. [DOI] [PubMed] [Google Scholar]
- 17.Cantini F., Niccoli L., Nannini C., et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Seminars in Arthritis and Rheumatism. 2017 doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Lenert A., Lenert P. Tapering biologics in rheumatoid arthritis: a pragmatic approach for clinical practice. Clinical Rheumatology. 2017;36(1):1–8. doi: 10.1007/s10067-016-3490-8. [DOI] [PubMed] [Google Scholar]
- 19.Verhoef L. M., Tweehuysen L., Hulscher M. E., Fautrel B., den Broeder A. A. bDMARD dose reduction in rheumatoid arthritis: a narrative review with systematic literature search. Rheumatology and Therapy. 2017;4(1):1–24. doi: 10.1007/s40744-017-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi G., Caporali R., Todoerti M., Mattana P. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Advances in Therapy. 2016;33(3):369–378. doi: 10.1007/s12325-016-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Llanio Comella N., Fernández Matilla M., Castellano Cuesta J. A. Have complementary therapies demonstrated effectiveness in rheumatoid arthritis? Reumatologia Clinica. 2016;12(3):151–157. doi: 10.1016/j.reuma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira J. F., Ahmed Mohamed A. A., Emery P. Glucocorticoids and rheumatoid arthritis. Rheumatic Diseases Clinics of North America. 2016;42(1):33–46. doi: 10.1016/j.rdc.2015.08.006. vii. [DOI] [PubMed] [Google Scholar]
- 23.Gavrilă B. I., Ciofu C., Stoica V. Biomarkers in rheumatoid arthritis, what is new? Journal of Medicine and Life. 2016;9(2):144–148. [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar L. D., Karthik R., Gayathri N., Sivasudha T. Advancement in contemporary diagnostic and therapeutic approaches for rheumatoid arthritis. Biomedicine & Pharmacotherapy. 2016;79:52–61. doi: 10.1016/j.biopha.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Alivernini S., Tolusso B., Petricca L., et al. Synovial features of patients with rheumatoid arthritis and psoriatic arthritis in clinical and ultrasound remission differ under anti-TNF therapy: a clue to interpret different chances of relapse after clinical remission? Annals of the Rheumatic Diseases. 2017;76(7):1228–1236. doi: 10.1136/annrheumdis-2016-210424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergrath E., Gerber R. A., Gruben D., Lukic T., Makin C., Wallenstein G. Tofacitinib versus biologic treatments in moderate-to-severe rheumatoid arthritis patients who have had an inadequate response to nonbiologic DMARDs: systematic literature review and network meta-analysis. International Journal of Rheumatology. 2017;2017:15. doi: 10.1155/2017/8417249.8417249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venuturupalli S. Immune mechanisms and novel targets in rheumatoid arthritis. Immunology and Allergy Clinics of North America. 2017;37(2):301–313. doi: 10.1016/j.iac.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Kolarz B., Majdan M. Epigenetic aspects of rheumatoid arthritis: contribution of non-coding RNAs. Seminars in Arthritis and Rheumatism. 2017;46(6):724–731. doi: 10.1016/j.semarthrit.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Singh J. A., Hossain A., Tanjong Ghogomu E., et al. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews. 2017;3, article CD012591 doi: 10.1002/14651858.CD012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M., Feng X., Ding J., Chang F., Chen X. Nanotherapeutics relieve rheumatoid arthritis. Journal of Controlled Release. 2017;252:108–124. doi: 10.1016/j.jconrel.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Leblond A., Allanore Y., Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmunity Reviews. 2017;16(6):594–601. doi: 10.1016/j.autrev.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Schinnerling K., Aguillón J. C., Catalán D., Soto L. The role of IL-6 signaling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clinical and Experimental Immunology. 2017;189(1):12–20. doi: 10.1111/cei.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tracy A., Buckley C. D., Raza K. Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Seminars in Immunopathology. 2017;39(4):423–435. doi: 10.1007/s00281-017-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson S. M., Nansen A. Pharmacological value of murine delayed-type hypersensitivity arthritis: a robust mouse model of rheumatoid arthritis in C57BL/6 mice. Basic & Clinical Pharmacology & Toxicology. 2017;120(2):108–114. doi: 10.1111/bcpt.12657. [DOI] [PubMed] [Google Scholar]
- 35.Bessis N., Decker P., Assier E., Semerano L., Boissier M. C. Arthritis models: usefulness and interpretation. Seminars in Immunopathology. 2017;39(4):469–486. doi: 10.1007/s00281-017-0622-4. [DOI] [PubMed] [Google Scholar]
- 36.Lin L., Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunology. 2017;18(1):p. 2. doi: 10.1186/s12865-016-0187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araki Y., Mimura T. The mechanisms underlying chronic inflammation in rheumatoid arthritis from the perspective of the epigenetic landscape. Journal of Immunology Research. 2016;2016:10. doi: 10.1155/2016/6290682.6290682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldring S. R. Differential mechanisms of de-regulated bone formation in rheumatoid arthritis and spondyloarthritis. Rheumatology (Oxford) 2016;55(Supplement 2):ii56–ii60. doi: 10.1093/rheumatology/kew345. [DOI] [PubMed] [Google Scholar]
- 39.Quiñonez-Flores C. M., González-Chávez S. A., Pacheco-Tena C. Hypoxia and its implications in rheumatoid arthritis. Journal of Biomedical Science. 2016;23(1):p. 62. doi: 10.1186/s12929-016-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurowska-Stolarska M., Alivernini S., Melchor E. G., et al. MicroRNA-34a dependent regulation of AXL controls the activation of dendritic cells in inflammatory arthritis. Nature Communications. 2017;8, article 15877 doi: 10.1038/ncomms15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alivernini S., Kurowska-Stolarska M., Tolusso B., et al. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nature Communications. 2016;7, article 12970 doi: 10.1038/ncomms12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitchon C. A., El-Gabalawy H. S. Infection and rheumatoid arthritis: still an open question. Current Opinion in Rheumatology. 2011;23(4):352–357. doi: 10.1097/BOR.0b013e3283477b7b. [DOI] [PubMed] [Google Scholar]
- 43.Chen B., Sun L., Zhang X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. Journal of Autoimmunity. 2017 doi: 10.1016/j.jaut.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Catrina A. I., Deane K. D., Scher J. U. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford) 2016;55(3):391–402. doi: 10.1093/rheumatology/keu469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forbes J. D., Van Domselaar G., Bernstein C. N. The gut microbiota in immune-mediated inflammatory diseases. Frontiers in Microbiology. 2016;7:p. 1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leszczyszyn J. J., Radomski M., Leszczyszyn A. M. Intestinal microbiota transplant - current state of knowledge. Reumatologia. 2016;54(1):24–28. doi: 10.5114/reum.2016.58758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda Y., Kumanogoh A., Takeda K. Altered composition of gut microbiota in rheumatoid arthritis patients. Nihon Rinshō Men'eki Gakkai Kaishi. 2016;39(1):59–63. doi: 10.2177/jsci.39.59. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbaum J. T., Asquith M. J. The microbiome: a revolution in treatment for rheumatic diseases? Current Rheumatology Reports. 2016;18(10):p. 62. doi: 10.1007/s11926-016-0614-8. [DOI] [PubMed] [Google Scholar]
- 49.Scher J. U., Littman D. R., Abramson S. B. Microbiome in inflammatory arthritis and human rheumatic diseases. Arthritis & Rhematology. 2016;68(1):35–45. doi: 10.1002/art.39259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steves C. J., Bird S., Williams F. M., Spector T. D. The microbiome and musculoskeletal conditions of aging: a review of evidence for impact and potential therapeutics. Journal of Bone and Mineral Research. 2016;31(2):261–269. doi: 10.1002/jbmr.2765. [DOI] [PubMed] [Google Scholar]
- 51.Gomez A., Luckey D., Taneja V. The gut microbiome in autoimmunity: sex matters. Clinical Immunology. 2015;159(2):154–162. doi: 10.1016/j.clim.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang H. J., Im S. H. Probiotics as an immune modulator. Journal of Nutritional Science and Vitaminology (Tokyo) 2015;61(Supplement):S103–S105. doi: 10.3177/jnsv.61.S103. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Zhang D., Jia H., et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature Medicine. 2015;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 54.Bedaiwi M. K., Inman R. D. Microbiome and probiotics: link to arthritis. Current Opinion in Rheumatology. 2014;26(4):410–415. doi: 10.1097/BOR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 55.Biscetti F., Flex A., Pecorini G., et al. The role of high-mobility group box protein 1 in collagen antibody-induced arthritis is dependent on vascular endothelial growth factor. Clinical and Experimental Immunology. 2016;184(1):62–72. doi: 10.1111/cei.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nefla M., Holzinger D., Berenbaum F., Jacques C. The danger from within: alarmins in arthritis. Nature Reviews Rheumatology. 2016;12(11):669–683. doi: 10.1038/nrrheum.2016.162. [DOI] [PubMed] [Google Scholar]
- 57.Park S. Y., Lee S. W., Kim H. Y., Lee W. S., Hong K. W., Kim C. D. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. European Journal of Immunology. 2015;45(4):1216–1227. doi: 10.1002/eji.201444908. [DOI] [PubMed] [Google Scholar]
- 58.Yang H., Wang H., Chavan S. S., Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Molecular Medicine. 2015;21(Supplement 1):S6–S12. doi: 10.2119/molmed.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y., Sun W., Gao R., et al. The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis. Rheumatology (Oxford) 2013;52(10):1739–1747. doi: 10.1093/rheumatology/ket134. [DOI] [PubMed] [Google Scholar]
- 60.Schaper F., Heeringa P., Bijl M., Westra J. Inhibition of high-mobility group box 1 as therapeutic option in autoimmune disease: lessons from animal models. Current Opinion in Rheumatology. 2013;25(2):254–259. doi: 10.1097/BOR.0b013e32835cee2d. [DOI] [PubMed] [Google Scholar]
- 61.Harris H. E., Andersson U., Pisetsky D. S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nature Reviews Rheumatology. 2012;8(4):195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 62.He Z., Shotorbani S. S., Jiao Z., et al. HMGB1 promotes the differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14+ monocytes from patients with rheumatoid arthritis. Scandinavian Journal of Immunology. 2012;76(5):483–490. doi: 10.1111/j.1365-3083.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 63.Guo H. F., Liu S. X., Zhang Y. J., Liu Q. J., Hao J., Gao L. X. High mobility group box 1 induces synoviocyte proliferation in rheumatoid arthritis by activating the signal transducer and activator transcription signal pathway. Clinical and Experimental Medicine. 2011;11(2):65–74. doi: 10.1007/s10238-010-0116-3. [DOI] [PubMed] [Google Scholar]
- 64.Andersson U., Harris H. E. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochimica et Biophysica Acta. 2010;1799(1-2):141–148. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Voll R. E., Urbonaviciute V., Herrmann M., Kalden J. R. High mobility group box 1 in the pathogenesis of inflammatory and autoimmune diseases. The Israel Medical Association Journal. 2008;10(1):26–28. [PubMed] [Google Scholar]
- 66.Mantell L. L., Parrish W. R., Ulloa L. Hmgb-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25(1):4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 67.Andersson U., Erlandsson-Harris H. HMGB1 is a potent trigger of arthritis. Journal of Internal Medicine. 2004;255(3):344–350. doi: 10.1111/j.1365-2796.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- 68.Andersson U., Tracey K. J. HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheumatic Diseases Clinics of North America. 2004;30(3):627–637. doi: 10.1016/j.rdc.2004.04.007. xi. [DOI] [PubMed] [Google Scholar]
- 69.Wang F. C., Pei J. X., Zhu J., et al. Overexpression of HMGB1 A-box reduced lipopolysaccharide-induced intestinal inflammation via HMGB1/TLR4 signaling in vitro. World Journal of Gastroenterology. 2015;21(25):7764–7776. doi: 10.3748/wjg.v21.i25.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jalil S. F., Arshad M., Bhatti A., et al. Rheumatoid arthritis: what have we learned about the causing factors? Pakistan Journal of Pharmaceutical Sciences. 2016;29(2):629–645. [PubMed] [Google Scholar]
- 71.Suzuki A., Yamamoto K. From genetics to functional insights into rheumatoid arthritis. Clinical and Experimental Rheumatology. 2015;33(4) Supplement 92:S40–S43. [PubMed] [Google Scholar]
- 72.Benucci M., Rogai V., Atzeni F., Hammen V., Sarzti-Puttini P., Migliore A. Costs associated with rheumatoid arthritis in Italy: past, present, and future. ClinicoEconomics and Outcomes Research. 2016;8:33–41. doi: 10.2147/CEOR.S91006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hennessy K., Woodburn J., Steultjens M. Clinical practice guidelines for the foot and ankle in rheumatoid arthritis: a critical appraisal. Journal of Foot and Ankle Research. 2016;9:p. 31. doi: 10.1186/s13047-016-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKenna S., Donnelly A., Fraser A., Comber L., Kennedy N. Does exercise impact on sleep for people who have rheumatoid arthritis? A systematic review. Rheumatology International. 2017;37(6):963–974. doi: 10.1007/s00296-017-3681-x. [DOI] [PubMed] [Google Scholar]
- 75.Zha A. M., Di Napoli M., Behrouz R. Prevention of stroke in rheumatoid arthritis. Current Neurology and Neuroscience Reports. 2015;15(12):p. 77. doi: 10.1007/s11910-015-0600-y. [DOI] [PubMed] [Google Scholar]
- 76.Tocci G., Goletti D., Marino V., et al. Cardiovascular outcomes and tumour necrosis factor antagonists in chronic inflammatory rheumatic disease: a focus on rheumatoid arthritis. Expert Opinion on Drug Safety. 2016;15(Supplement 1):55–61. doi: 10.1080/14740338.2016.1218469. [DOI] [PubMed] [Google Scholar]
- 77.Yang X., Chang Y., Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators of Inflammation. 2016;2016:9. doi: 10.1155/2016/6813016.6813016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.George M. D., Baker J. F. The obesity epidemic and consequences for rheumatoid arthritis care. Current Rheumatology Reports. 2016;18(1):p. 6. doi: 10.1007/s11926-015-0550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferraccioli G., Tolusso B., Bobbio-Pallavicini F., et al. Biomarkers of good EULAR response to the B cell depletion therapy in all seropositive rheumatoid arthritis patients: clues for the pathogenesis. PLoS One. 2012;7(7, article e40362) doi: 10.1371/journal.pone.0040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minozzi S., Bonovas S., Lytras T., et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opinion on Drug Safety. 2016;15(Supplement 1):11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 81.Bijlsma J. W. J., Buttgereit F. Adverse events of glucocorticoids during treatment of rheumatoid arthritis: lessons from cohort and registry studies. Rheumatology (Oxford) 2016;55(Supplement 2):ii3–ii5. doi: 10.1093/rheumatology/kew344. [DOI] [PubMed] [Google Scholar]
- 82.Singh J. A. Infections with biologics in rheumatoid arthritis and related conditions: a scoping review of serious or hospitalized infections in observational studies. Current Rheumatology Reports. 2016;18(10):p. 61. doi: 10.1007/s11926-016-0609-5. [DOI] [PubMed] [Google Scholar]
- 83.Turesson C. Comorbidity in rheumatoid arthritis. Swiss Medical Weekly. 2016;146, article w14290 doi: 10.4414/smw.2016.14290. [DOI] [PubMed] [Google Scholar]
- 84.Strand V., Ahadieh S., French J., et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Research & Therapy. 2015;17:p. 362. doi: 10.1186/s13075-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bijlsma J. W., Jacobs J. W., Buttgereit F. Glucocorticoids in the treatment of rheumatoid arthritis. Clinical and Experimental Rheumatology. 2015;33(4) Supplement 92:S34–S36. [PubMed] [Google Scholar]
- 86.Cacciapaglia F., Zuccaro C., Iannone F. Varicella-zoster virus infection in rheumatoid arthritis patients in the anti-tumour necrosis factor era. Clinical and Experimental Rheumatology. 2015;33(6):917–923. [PubMed] [Google Scholar]
- 87.Kim K., Bang S. Y., Lee H. S., Bae S. C. Update on the genetic architecture of rheumatoid arthritis. Nature Reviews Rheumatology. 2017;13(1):13–24. doi: 10.1038/nrrheum.2016.176. [DOI] [PubMed] [Google Scholar]
- 88.Rodríguez-Elías A. K., Maldonado-Murillo K., López-Mendoza L. F., Ramírez-Bello J. Genetics and genomics in rheumatoid arthritis (RA): an update. Gaceta Médica de México. 2016;152(2):218–227. [PubMed] [Google Scholar]
- 89.Sparks J. A., Costenbader K. H. Genetics, environment, and gene-environment interactions in the development of systemic rheumatic diseases. Rheumatic Diseases Clinics of North America. 2014;40(4):637–657. doi: 10.1016/j.rdc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma A. R., Sharma G., Lee S. S., Chakraborty C. miRNA-regulated key components of cytokine signaling pathways and inflammation in rheumatoid arthritis. Medicinal Research Reviews. 2016;36(3):425–439. doi: 10.1002/med.21384. [DOI] [PubMed] [Google Scholar]
- 91.Ferraccioli G., Alivernini S., Gremese E. Biomarkers of joint damage in rheumatoid arthritis: where are we in 2013? The Journal of Rheumatology. 2013;40(8):1244–1246. doi: 10.3899/jrheum.130566. [DOI] [PubMed] [Google Scholar]
- 92.Frisell T., Saevarsdottir S., Askling J. Family history of rheumatoid arthritis: an old concept with new developments. Nature Reviews Rheumatology. 2016;12(6):335–343. doi: 10.1038/nrrheum.2016.52. [DOI] [PubMed] [Google Scholar]
- 93.Sparks J. A., Karlson E. W. The roles of cigarette smoking and the lung in the transitions between phases of preclinical rheumatoid arthritis. Current Rheumatology Reports. 2016;18(3):p. 15. doi: 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chatzidionisyou A., Catrina A. I. The lung in rheumatoid arthritis, cause or consequence? Current Opinion in Rheumatology. 2016;28(1):76–82. doi: 10.1097/BOR.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 95.Malaise O., von Frenckell C., Malaise M. G. Genetic and environmental interactions on the development of rheumatoid arthritis. Revue Médicale de Liège. 2012;67(5-6):305–313. [PubMed] [Google Scholar]
- 96.Leech M. T., Bartold P. M. The association between rheumatoid arthritis and periodontitis. Best Practice & Research: Clinical Rheumatology. 2015;29(2):189–201. doi: 10.1016/j.berh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Benham H., Robinson P. C., Baillet A. C., Rehaume L. M., Thomas R. Role of genetics in infection-associated arthritis. Best Practice & Research: Clinical Rheumatology. 2015;29(2):213–225. doi: 10.1016/j.berh.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Kumar P. S. Oral microbiota and systemic disease. Anaerobe. 2013;24:90–93. doi: 10.1016/j.anaerobe.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Mellado M., Martínez-Muñoz L., Cascio G., Lucas P., Pablos J. L., Rodríguez-Frade J. M. T cell migration in rheumatoid arthritis. Frontiers in Immunology. 2015;6:p. 384. doi: 10.3389/fimmu.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paulissen S. M., van Hamburg J. P., Dankers W., Lubberts E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine. 2015;74(1):43–53. doi: 10.1016/j.cyto.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Canestri S., Totaro M. C., Serone E., et al. Association between the response to B cell depletion therapy and the allele∗2 of the HS1,2A enhancer in seropositive rheumatoid arthritis patients. Reumatismo. 2012;64(6):368–373. doi: 10.4081/reumatismo.2012.368. [DOI] [PubMed] [Google Scholar]
- 102.Gremese E., Tolusso B., Fedele A. L., Canestri S., Alivernini S., Ferraccioli G. ZAP-70+ B cell subset influences response to B cell depletion therapy and early repopulation in rheumatoid arthritis. The Journal of Rheumatology. 2012;39(12):2276–2285. doi: 10.3899/jrheum.120153. [DOI] [PubMed] [Google Scholar]
- 103.Michelutti A., Gremese E., Morassi F., et al. B-cell subsets in the joint compartments of seropositive and seronegative rheumatoid arthritis (RA) and no-RA arthritides express memory markers and ZAP70 and characterize the aggregate pattern irrespectively of the autoantibody status. Molecular Medicine. 2011;17(9-10):901–909. doi: 10.2119/molmed.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trouw L. A., Rispens T., Toes R. E. M. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nature Reviews Rheumatology. 2017;13(6):331–339. doi: 10.1038/nrrheum.2017.15. [DOI] [PubMed] [Google Scholar]
- 105.Gazitt T., Lood C., Elkon K. B. Citrullination in rheumatoid arthritis-a process promoted by neutrophil lysis? Rambam Maimonides Medical Journal. 2016;7(4) doi: 10.5041/RMMJ.10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shoda H. Citrullination and rheumatoid arthritis. Nihon Rinsho. 2016;74(6):902–6. [PubMed] [Google Scholar]
- 107.Bingham C. O., Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Current Opinion in Rheumatology. 2013;25(3):345–353. doi: 10.1097/BOR.0b013e32835fb8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Avci A. B., Feist E., Burmester G. R. Targeting GM-CSF in rheumatoid arthritis. Clinical and Experimental Rheumatology. 2016;34(4) Supplement 98:39–44. [PubMed] [Google Scholar]
- 109.Cutolo M., Sulli A., Paolino S., Pizzorni C. CTLA-4 blockade in the treatment of rheumatoid arthritis: an update. Expert Review of Clinical Immunology. 2016;12(4):417–425. doi: 10.1586/1744666X.2016.1133295. [DOI] [PubMed] [Google Scholar]
- 110.Kim K. W., Kim H. R. Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis. The Korean Journal of Internal Medicine. 2016;31(4):634–642. doi: 10.3904/kjim.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsumoto I. Monocyte/macrophage and TNFα-induced adipose related protein (TIARP) in rheumatoid arthritis. Nihon Rinshō Men'eki Gakkai Kaishi. 2016;39(5):455–459. doi: 10.2177/jsci.39.455. [DOI] [PubMed] [Google Scholar]
- 112.Udalova I. A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nature Reviews Rheumatology. 2016;12(8):472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 113.Tolusso B., Sacco S., Gremese E., La Torre G., Tomietto P., Ferraccioli G. F. Relationship between the tumor necrosis factor receptor II (TNF-RII) gene polymorphism and sTNF-RII plasma levels in healthy controls and in rheumatoid arthritis. Human Immunology. 2004;65(12):1420–1426. doi: 10.1016/j.humimm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 114.Tsung A., Tohme S., Billiar T. R. High-mobility group box-1 in sterile inflammation. Journal of Internal Medicine. 2014;276(5):425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 115.Biscetti F., Ghirlanda G., Flex A. Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Current Vascular Pharmacology. 2011;9(6):677–681. doi: 10.2174/157016111797484125. [DOI] [PubMed] [Google Scholar]
- 116.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Molecular and Cellular Biology. 1999;19(8):5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andersson U., Tracey K. J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual Review of Immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bianchi M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 119.Hirata Y., Kurobe H., Higashida M., et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013;231(2):227–233. doi: 10.1016/j.atherosclerosis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 120.Kokkola R., Li J., Sundberg E., et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis and Rheumatism. 2003;48(7):2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 121.Rong L. L., Trojaborg W., Qu W., et al. Antagonism of RAGE suppresses peripheral nerve regeneration. The FASEB Journal. 2004;18(15):1812–1817. doi: 10.1096/fj.04-1899com. [DOI] [PubMed] [Google Scholar]
- 122.Andersson U., Wang H., Palmblad K., et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. The Journal of Experimental Medicine. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hori O., Brett J., Slattery T., et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. The Journal of Biological Chemistry. 1995;270(43):25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 124.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biological Chemistry. 2014;395(2):203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 125.Wang H., Yang H., Czura C. J., Sama A. E., Tracey K. J. HMGB1 as a late mediator of lethal systemic inflammation. American Journal of Respiratory and Critical Care Medicine. 2001;164, 10, Part 1:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 126.Sims G. P., Rowe D. C., Rietdijk S. T., Herbst R., Coyle A. J. HMGB1 and RAGE in inflammation and cancer. Annual Review of Immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 127.Li L. C., Gao J., Li J. Emerging role of HMGB1 in fibrotic diseases. Journal of Cellular and Molecular Medicine. 2014;18(12):2331–2339. doi: 10.1111/jcmm.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitola S., Belleri M., Urbinati C., et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. Journal of Immunology. 2006;176(1):12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 129.Straino S., Di Carlo A., Mangoni A., et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. The Journal of Investigative Dermatology. 2008;128(6):1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 130.Germani A., Limana F., Capogrossi M. C. Pivotal advances: high-mobility group box 1 protein—a cytokine with a role in cardiac repair. Journal of Leukocyte Biology. 2007;81(1):41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- 131.Biscetti F., Straface G., De Cristofaro R., et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 2010;59(6):1496–1505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palmblad K., Sundberg E., Diez M., et al. Morphological characterization of intra-articular HMGB1 expression during the course of collagen-induced arthritis. Arthritis Research & Therapy. 2007;9(2, article R35) doi: 10.1186/ar2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pullerits R., Jonsson I. M., Verdrengh M., et al. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis and Rheumatism. 2003;48(6):1693–1700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- 134.Yang H., Tracey K. J. Targeting HMGB1 in inflammation. Biochimica et Biophysica Acta. 2010;1799(1-2):149–156. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kokkola R., Sundberg E., Ulfgren A. K., et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis and Rheumatism. 2002;46(10):2598–2603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 136.Cai J., Wen J., Bauer E., Zhong H., Yuan H., Chen A. F. The role of HMGB1 in cardiovascular biology: danger signals. Antioxidants & Redox Signaling. 2015;23(17):1351–1369. doi: 10.1089/ars.2015.6408. [DOI] [PubMed] [Google Scholar]
- 137.Yang H., Antoine D. J., Andersson U., Tracey K. J. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. Journal of Leukocyte Biology. 2013;93(6):865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thaiss C. A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 139.Brusca S. B., Abramson S. B., Scher J. U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Current Opinion in Rheumatology. 2014;26(1):101–107. doi: 10.1097/BOR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim D., Yoo S. A., Kim W. U. Gut microbiota in autoimmunity: potential for clinical applications. Archives of Pharmacal Research. 2016;39(11):1565–1576. doi: 10.1007/s12272-016-0796-7. [DOI] [PubMed] [Google Scholar]
- 141.Actis G. C. The gut microbiome. Inflammation & Allergy Drug Targets. 2014;13(4):217–223. doi: 10.2174/1871528113666140623113221. [DOI] [PubMed] [Google Scholar]
- 142.Rogier R., Koenders M. I., Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. Journal of Immunology Research. 2015;2015:8. doi: 10.1155/2015/527696.527696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joosten L. A., Abdollahi-Roodsaz S., Dinarello C. A., O'Neill L., Netea M. G. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nature Reviews Rheumatology. 2016;12(6):344–357. doi: 10.1038/nrrheum.2016.61. [DOI] [PubMed] [Google Scholar]
- 144.Börnigen D., Morgan X. C., Franzosa E. A., et al. Functional profiling of the gut microbiome in disease-associated inflammation. Genome Medicine. 2013;5(7):p. 65. doi: 10.1186/gm469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bender P., Bürgin W. B., Sculean A., Eick S. Serum antibody levels against Porphyromonas gingivalis in patients with and without rheumatoid arthritis - a systematic review and meta-analysis. Clinical Oral Investigations. 2017;21(1):33–42. doi: 10.1007/s00784-016-1938-5. [DOI] [PubMed] [Google Scholar]
- 146.Totaro M. C., Cattani P., Ria F., et al. Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: analysis of various compartments including the synovial tissue. Arthritis Research & Therapy. 2013;15(3, article R66) doi: 10.1186/ar4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scher J. U., Bretz W. A., Abramson S. B. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Current Opinion in Rheumatology. 2014;26(4):424–429. doi: 10.1097/BOR.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu X., Zeng B., Zhang J., et al. Role of the gut microbiome in modulating arthritis progression in mice. Scientific Reports. 2016;6, article 30594 doi: 10.1038/srep30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maeda Y., Kurakawa T., Umemoto E., et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis & Rhematology. 2016;68(11):2646–2661. doi: 10.1002/art.39783. [DOI] [PubMed] [Google Scholar]
- 150.Chen J., Wright K., Davis J. M., et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Medicine. 2016;8(1):p. 43. doi: 10.1186/s13073-016-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van den Berg W. B. Lessons from animal models of arthritis over the past decade. Arthritis Research & Therapy. 2009;11(5):p. 250. doi: 10.1186/ar2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu H. J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scher J. U., Abramson S. B. The microbiome and rheumatoid arthritis. Nature Reviews Rheumatology. 2011;7(10):569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mankia K., Emery P. Is localized autoimmunity the trigger for rheumatoid arthritis? Unravelling new targets for prevention. Discovery Medicine. 2015;20(109):129–135. [PubMed] [Google Scholar]
- 155.Vasconcelos R. M., Sanfilippo N., Paster B. J., et al. Host-microbiome cross-talk in oral mucositis. Journal of Dental Research. 2016;95(7):725–733. doi: 10.1177/0022034516641890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koboziev I., Reinoso Webb C., Furr K. L., Grisham M. B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radical Biology & Medicine. 2014;68:122–133. doi: 10.1016/j.freeradbiomed.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Espey M. G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radical Biology & Medicine. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 158.Daschner P. J., Grisham M. B., Espey M. G. Redox relationships in gut-microbiome interactions. Free Radical Biology & Medicine. 2017;105:1–2. doi: 10.1016/j.freeradbiomed.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 159.Mardinoglu A., Shoaie S., Bergentall M., et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Molecular Systems Biology. 2015;11(10):p. 834. doi: 10.15252/msb.20156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chandrashekaran V., Seth R. K., Dattaroy D., et al. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biology. 2017;13:8–19. doi: 10.1016/j.redox.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bastida C., Ruíz V., Pascal M., Yagüe J., Sanmartí R., Soy D. Is there potential for therapeutic drug monitoring of biologic agents in rheumatoid arthritis? British Journal of Clinical Pharmacology. 2016;85(5):962–975. doi: 10.1111/bcp.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown P. M., Pratt A. G., Isaacs J. D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nature Reviews Rheumatology. 2016;12(12):731–742. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 163.D'Agostino M. A., Terslev L., Wakefield R., et al. Novel algorithms for the pragmatic use of ultrasound in the management of patients with rheumatoid arthritis: from diagnosis to remission. Annals of the Rheumatic Diseases. 2016;75(11):1902–1908. doi: 10.1136/annrheumdis-2016-209646. [DOI] [PubMed] [Google Scholar]
- 164.Kawahito Y. Guidelines for the management of rheumatoid arthritis. Nihon Rinsho. 2016;74(6):939–943. [PubMed] [Google Scholar]
- 165.Nogueira E., Gomes A., Preto A., Cavaco-Paulo A. Update on therapeutic approaches for rheumatoid arthritis. Current Medicinal Chemistry. 2016;23(21):2190–2203. doi: 10.2174/0929867323666160506125218. [DOI] [PubMed] [Google Scholar]
- 166.Nam J. L. Rheumatoid arthritis management of early disease. Current Opinion in Rheumatology. 2016;28(3):267–274. doi: 10.1097/BOR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 167.Mahmood S., Lesuis N., van Tuyl L. H., van Riel P., Landewé R. Quality in rheumatoid arthritis care. Best Practice & Research: Clinical Rheumatology. 2015;29(4-5):664–679. doi: 10.1016/j.berh.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 168.Bossaller L., Rothe A. Monoclonal antibody treatments for rheumatoid arthritis. Expert Opinion on Biological Therapy. 2013;13(9):1257–1272. doi: 10.1517/14712598.2013.811230. [DOI] [PubMed] [Google Scholar]
- 169.Schierbeck H., Lundbäck P., Palmblad K., et al. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Molecular Medicine. 2011;17(9-10):1039–1044. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Splichalova A., Splichal I., Chmelarova P., Trebichavsky I. Alarmin HMGB1 is released in the small intestine of gnotobiotic piglets infected with enteric pathogens and its level in plasma reflects severity of sepsis. Journal of Clinical Immunology. 2011;31(3):488–497. doi: 10.1007/s10875-010-9505-3. [DOI] [PubMed] [Google Scholar]
- 171.Wang P., Tao J. H., Pan H. F. Probiotic bacteria: a viable adjuvant therapy for relieving symptoms of rheumatoid arthritis. Inflammopharmacology. 2016;24(5):189–196. doi: 10.1007/s10787-016-0277-0. [DOI] [PubMed] [Google Scholar]
- 172.So J. S., Kwon H. K., Lee C. G., et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Molecular Immunology. 2008;45(9):2690–2699. doi: 10.1016/j.molimm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 173.Amdekar S., Singh V., Singh R., Sharma P., Keshav P., Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. Journal of Clinical Immunology. 2011;31(2):147–154. doi: 10.1007/s10875-010-9457-7. [DOI] [PubMed] [Google Scholar]
- 174.Vaghef-Mehrabany E., Alipour B., Homayouni-Rad A., Sharif S. K., Asghari-Jafarabadi M., Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30(4):430–435. doi: 10.1016/j.nut.2013.09.007. [DOI] [PubMed] [Google Scholar]