Abstract
Background:
Ventilator-induced lung injury (VILI) is a side effect of mechanical ventilation. Lung inflammation and pulmonary activation of coagulation are induced by mechanical stress. Clinical and preclinical studies show that heparin possesses anti-inflammatory properties. Therefore, we assessed the effects of nebulized heparin in VILI.
Methods:
Sixty critically ill adult patients who require mechanical ventilation for more than 48 h were included in this prospective, nonrandomized controlled study. Patients received nebulized heparin (10,000 U every 6 h) for 5 days. The matched control group received nebulized budesonide as routine practice in our center. This study assessed changes in partial pressure of oxygen to inspired fraction of oxygen ratio (PaO2/FiO2) and rapid shallow breathing index (RSBI) during the study as primary endpoints.
Results:
The average daily PaO2/FiO2 ratio was not statistically significant between both groups (187 ± 11.6 vs. 171 ± 11.6, P = 0.35). The RSBI also did not differ between groups (P = 0.58). Heparin administration was associated with a higher number of ventilator-free days among survivors but not significantly (7.7 ± 10.6 vs. 5.1 ± 8, 95% confidence interval − 2.2–7.5, P = 0.28). Successful weaning from mechanical ventilation was higher in the heparin group (P = 0.42). We did not observe any serious or increased adverse effects from nebulized heparin.
Conclusion:
The results of this study show that the overall effectiveness of nebulized heparin is at least as comparable with a potent corticosteroid (budesonide). Heparin could be a safe and effective modality for patients who at risk of VILI.
Keywords: Heparin, mechanical ventilation, nebulizer
INTRODUCTION
Utilization of invasive mechanical ventilation (IMV) increased in recent years. The epidemiological data showed that IMV increased from 178.9/100,000 in 1993 to 310.9/100,000 US adults in 2009.[1] Regardless of the underlying etiology, patients underwent mechanical ventilation are at high risk of developing lung damage.[2] Overdistention of alveoli and cyclic atelectasis could lead to alveolar injury which is called ventilator-induced lung injury (VILI).[3] Besides of lung distention, inflammatory responses and mediators play an important role in the development of VILI. Endothelial cell disruptions, inflammatory cell recruitment, secretion of mediators (such as tumor necrosis factor α [TNF-α], interleukin 6 [IL-6]), and fibrin deposition in the pulmonary microcirculation are some of the based inflammatory mechanisms in VILI.[4,5,6]
On the other hand, microvascular thrombi and fibrin deposition are hallmarks of acute lung injury (ALI). Activated coagulation factors may impair alveolar perfusion, promote fibrosis, and exaggerate lung injury.[7,8] Clinical and experimental models have demonstrated that heparin and its derivatives have anti-inflammatory effects alongside their anticoagulant mechanisms.[9,10,11,12] These effects suggest that heparin may improve clinical outcome in VILI and ALI. Reduction of pulmonary edema, reduced leukocyte activation, inhibition of bacteria and viruses to respiratory surface, binding of heparin to some of the cytokines and neutralizing them and induction of apoptosis are some of the anti-inflammatory effects of heparin that may be beneficial and improve clinical outcomes.[13,14,15,16] A systematic review by Tuinman et al.,[12] showed that local anticoagulant therapy through nebulization attenuates pulmonary coagulopathy and also inflammation. They suggest that nebulized heparin could be a safe and beneficial option in an ALI, but data are very limited. It is logical to hypothesize that local anticoagulants reduce the potential for systemic bleeding associated with intravenous administration.[17]
Most of the strategies to prevent VILI are focused to minimize alveolar overdistension and cyclic atelectasis; such as low tidal volume ventilation and a low plateau airway pressure.[18] Therefore, considering both role of coagulopathy and inflammation in the induction of VILI, we assessed whether nebulized heparin improved lung function in patients expected to require prolonged mechanical ventilation.
METHODS
A prospective, nonrandomized controlled study was undertaken in a single center from September 2015 to December 2016. The study population consisted of consecutive patients admitted to the Intensive Care Unit (ICU) of Alzahra hospital (Isfahan, Iran), a tertiary-level university-affiliated hospital. The ethics committee of Isfahan University of Medical Sciences approved the study. Informed consent was obtained from patients or next of kin, or appropriate surrogate before participation in the study. The study was registered with the Iranian registry of clinical trials (IRCT201701011497N6).
Patients of both gender and more than 18 years old were included if they were expected to require IMV for more than 48 h. They were excluded if they receive mechanical ventilation for more than 24 h before enrollment, required mechanical ventilation for more than 48 h in a previous admission to the ICU during current hospital admission and received therapeutic doses of heparin or low-molecular-weight heparin, warfarin or dabigatran at the time of screening. Furthermore, they were excluded if have an allergy to heparin (including any history of heparin-induced thrombocytopenia), a pulmonary hemorrhage in the previous 3 months, uncontrolled bleeding or a significant bleeding disorder, an intracranial hemorrhage in the past 12 months (a clipped or subarachnoid aneurysm was acceptable), or an epidural catheter in place or likely to be placed in the next 48 h.
Eligible patients were enrolled, received heparin sodium 5000 IU/2 ml (Caspian Tamin, Tehran, Iran). Patients were administered heparin 10000 IU every 6 h for 5 days. The dose was selected based on data from other studies that used heparin as nebulizer (inhaler).[19,20,21] No dose adjustment was made for heparin administration for deep vein thrombosis (DVT) prophylaxis or if therapeutic anticoagulation was initiated. Nebulization of budesonide/twice daily is a routine practice in our ICU for ventilated patients, we hold this medication during 5 days of our study, but the control group received it as routine. The study medication was withheld at the physician's decision if any of the following occurred; excessive blood staining of the sputum or bronchial secretion, other significant bleeding, an excessively elevated activated partial thromboplastin time (APTT), or thrombocytopenia (50% fall from the baseline).
Heparin was nebulized through Hamilton C2 ventilator (Hamilton Medical AG, Bonaduz, Switzerland) which contain integrated pneumatic nebulizer that is fully synchronized with the inspiration and exhalation timing. The nebulizer generates droplets with a mass median aerodynamic diameter between 1.5 and 4.5 μm in size. The heparin diluted with 5 ml of normal saline. The cup was placed in the aspiratory limb just before the Y-piece. This pneumatic nebulizer allow as to nebulizer drug without interruption of ventilation.
A suitable mode of ventilation was used according to patients. The target tidal volume was set at not more than 8 ml/kg of predicted body weight; this was a routine practice at the time of the study. Weaning was undertaken with a spontaneous pressure support mode. The level of pressure support was adjusted to maintain the target tidal volume. Patients were considered suitable for extubation if they were cooperative and hemodynamically stable with an oxygen saturation of at least 95%, while ventilated on pressure support of not more than 10 cm H2O, positive end-expiratory pressure of not more than 5 cm H2O, and FiO2 of not more than 50%. Patients who were not suitable for extubation after 7 days of mechanical ventilation and who had not demonstrated clinical improvement were considered for tracheostomy.
The primary outcomes were the average daily ratio of partial pressure of oxygen to FiO2 (PaO2/FiO2) during the 5 days of study and also the rapid shallow breathing index (RSBI) which is measured as the ratio of respiratory rate to tidal volume. Secondary outcomes included: ventilator-free days among surviving patients, tracheostomy rate, length of stay in ICU and hospital and mortality rate.
Data collection
The PaO2/FiO2 ratio and RSBI were measured and recorded daily. Daily APTT levels were recorded to assess the systemic effects of nebulized heparin. Demographic data were collected on study entry, and ventilation parameters, clinical data, medication usage, and adverse events including blood in bronchial secretion or thrombocytopenia were recorded daily while the patient remained mechanically ventilated. Ventilator-free days were defined as the number of days patients were breathing without mechanical ventilation during admission and hospital stay.
Statistical analysis
Based on the study of Dixon et al.,[22] the study was performed to demonstrate an improvement in the average daily PaO2/FiO2 ratio from 250 to 300 mmHg over the period of mechanical ventilation, assuming a standard deviation (SD) of 50, alpha = 0.05, and power of 0.8. Data were analyzed on an intention-to-treat analysis. Data were expressed as mean ± SD and were compared using independent t-test and repeated measure analysis of variance. Categorical variables compared using Chi-square tests. The rate of freedom from ventilator was analyzed according to the Kaplan-Meier method, and the results were compared with the log-rank test. All reported P value were two-sided. P ≤ 0.05 was considered as statistical significance. The analysis was performed with SPSS software version 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
Thirty patients were allocated to the nebulized heparin group. We matched another 30 patients as a control group. The baseline characteristics of the two groups were similar [Table 1]. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was better at baseline in the control group (17.2 ± 1.6) compared to the treatment group (18.3 ± 2.3) (P = 0.73). The difference was not significant.
Table 1.
Baseline and patient characteristics
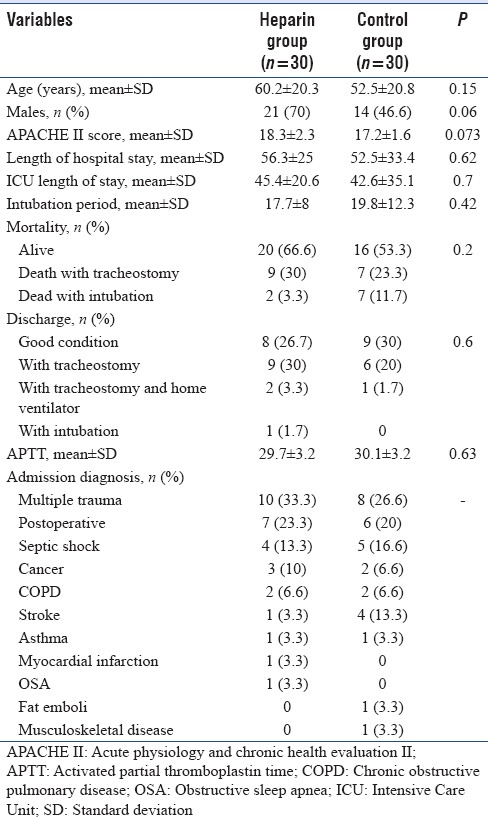
The primary endpoint, the average daily PaO2/FiO2 ratio during the study period was not statistically different between both groups (187 ± 11.6 vs. 171 ± 11.6, P = 0.35). The PaO2/FiO2 ratio levels over the first 5 days are presented in Figure 1. Based on the ANOVA test, the PaO2/FiO2 ratio at day 5 did not differ significantly between groups (P = 0.3). As illustrated, although not statistically significant, the PaO2/FiO2 ratio trends were better in the heparin group. The levels were increased in both groups [Table 2].
Figure 1.
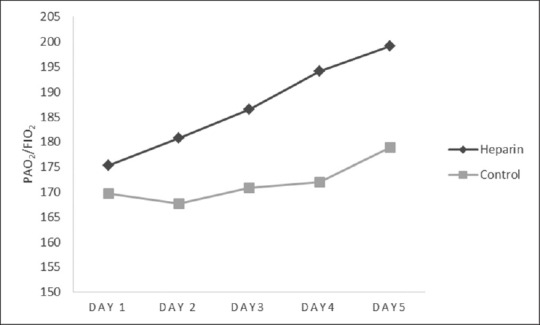
Changes in PaO2/FiO2 ratio over the first 5 days of the study
Table 2.
Values of partial pressure of oxygen to inspired fraction of oxygen ratio and during the 5 days of the study
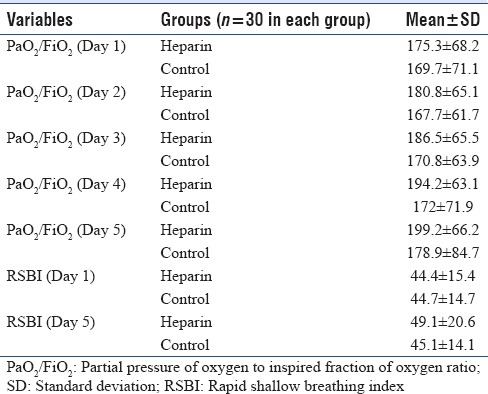
We had successful weaning in 16 patients in both groups (8 patients in each group [16%]). The intubation period was 17.7 ± 8.1 in the heparin group versus 19.8 ± 12.3 in the control group, which is lower in the treatment group but the difference was not significant (P = 0.42). Heparin administration was associated with a higher number of ventilator-free days among survivors at discharge from hospital (7.7 ± 10.6 vs. 5.1 ± 8, 95% confidence interval (CI) −2.2–7.5, P = 0.28). The difference between groups was not statistically significant regarding the ventilator-free days. Figure 2 shows the rate of freedom from ventilator among survivors. Overall, it was higher in heparin group, but not significantly (P = 0.26, log-rank test). The RSBI did not differ statistically between groups (44.4 ± 15 and 49.1 ± 20.6 in day 1 and 5 vs. 44.7 ± 14 and 45.1 ± 14.8, respectively, P = 0.58).
Figure 2.
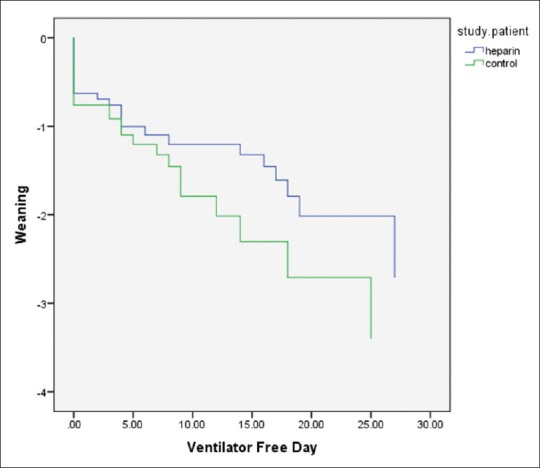
Rate of weaning and freedom from mechanical ventilation
The number of tracheostomies was higher in the heparin group (20/30 [66.7%] vs. 14/30 [46.7%], relative risk: 0.65, 95% CI 0.37–1.1, P = 0.21). The difference between groups was not significant. A tracheostomy, if required was undertaken an average of 12.5 ± 13.3 days after enrollment. This was 13.3 ± 11 days in heparin group and 11.8 ± 15.6 in the control group with the range of 0–53 days.
The ICU and hospital length of stay was 45.4 ± 20.5 versus 42.6 ± 35.1 (range, 16–128 days, P = 0.7) and 56.3 ± 25 versus 52.5 ± 33.8 (range, 9–128 days, P = 0.6) which is similar in both groups and did not differ significantly. Table 1 shows the average of APTT in both groups during the 5 days of the study, which is not statistically significant (P = 0.64). The heparin was well tolerated. We suspected two cases of thrombocytopenia in heparin group, with further evaluation, those were not related to heparin administration. We did not observe cases with pulmonary hemorrhage or blood-stained sputum during the study. No patients had blood loss or transfusion requirements attributable to the study medication.
Table 3 shows the results of respiratory cultures which is positive in all patients, but similar in both groups (P = 0.06).
Table 3.
Results of tracheal cultures
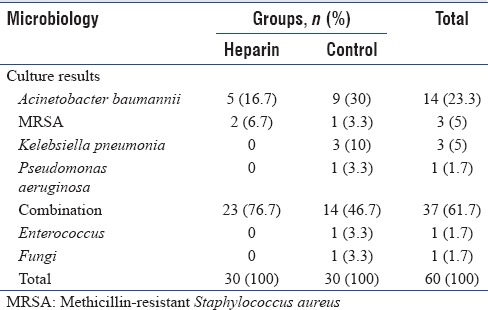
Heparin for DVT prophylaxis administered in 73.3% of patients in the heparin group and 66.6% of patients in the control group after enrollment.
DISCUSSION
In this nonrandomized controlled trial, we found that nebulized heparin had no effect on weaning rate from mechanical ventilation, tracheostomy rate, PaO2/FiO2 ratio, and RSBI index. Although compared to the control group, nebulized heparin was associated with fewer days of mechanical ventilation and intubation period. As we mentioned earlier, nebulized budesonide is a routine practice in our ICU for critically ill patients under mechanical ventilation. The nonstatistically difference between both groups show that heparin at least as effective as budesonide (an inhaled corticosteroids).
Inflammatory responses and mediators are responsible for some part of the injury from mechanical ventilation.[23] Corticosteroids as potent anti-inflammatory drugs have been used in many respiratory diseases such as ALI.[24,25] Ju et al.,[26] in the experimental study, assessed the effects of budesonide on VILI. Experimental VILI was induced in Wistar rats by means of mechanical ventilation at a high tidal volume. Twenty-two rats were randomized into three groups: a ventilation group, ventilation/budesonide, and sham group. Compared with that in the ventilation group, the PaO2/FiO2 ratio was significantly increased by treatment with budesonide. The levels of inflammatory mediators such as TNF-α and IL-6 were decreased in the budesonide group. The authors concluded that budesonide ameliorated lung injury likely by a reduction in inflammation and reducing apoptosis in VILI. In our center, patients candidate for tracheostomy on average of 2 weeks after ventilation (back to our results after 12.5 days); chance of VILI is very high during this period. Nebulized budesonide is available in our setting and because of some reports of effectiveness; our physicians prescribed this drug for patients who require prolonged mechanical ventilation; however, the total cost of nebulized budesonide is high in our country, and this drug is not available all the time. Heparin has low cost and easily available; the equal effectiveness of these two drugs and good safety profile of nebulized heparin, indicate that we could replace usage of budesonide with heparin or at least add these two drugs for the reduction of VILI.
The study by Dixon et al.,[21] on nebulized heparin in critically ill patients who require prolonged mechanical ventilation showed that nebulized heparin was associated with fewer days of mechanical ventilation (P = 0.02). The levels of inflammatory biomarkers (such as IL-6, IL-8, and TNF-α) in pulmonary lavage fluid were similar between nebulized heparin and the placebo group. Therefore, it seems that despite a theoretical anti-inflammatory effect of heparin, other underlying mechanisms are significant for heparin action. Hence, the addition of heparin to present preventive modality in VILI could be more effective as nebulization of heparin as a sole therapy. Further clinical trials are necessary to test this hypothesis. Mohammad et al.,[17] (2016) performed a similar study on 50 ICU patients who were in need of mechanical ventilation for more than 48 h. Treatment group received nebulized heparin with the dose of 25000 U every 4 h until weaning or for a maximum of 14 days. The changes in plateau pressure and compliance rate were statistically different between groups (P = 0.003 and P = 0.015, respectively). The PaO2/FiO2, ICU-free days on day 28, ventilator-free days at day 28, acute renal failure-free days at day 28, vasopressor-free days on day 28, and mortality and sputum culture results on day 4 did not differ significantly within groups.
Recently, Glas et al.[27] conducted an individual patient data meta-analysis of nebulized heparin for patients under mechanical ventilation. Data from five studies (one randomized controlled trial, one open-label study, and three studies using historical controls) were included in the meta-analysis, compassing 286 patients. The number of ventilator-free days and patients alive at day 28 was higher in patients treated with nebulized heparin compared to control group (14 [IQR 0–23] vs. 6 [IQR 0–22]), though the difference did not reach statistical significance (P = 0.459). Whereas, we found no statistically difference, the ventilator-free days were higher in the heparin group and total days of intubation were lower. Glas et al.[27] also reported that patients treated with nebulized heparin had significantly higher ICU-free days and alive at day 28 and the lung injury score were significantly lower in them, but in the propensity score-matched analysis, there was no difference in any of the endpoints. The final conclusion of this meta-analysis is that nebulized heparin was not beneficial in intubated and ventilated ICU patients.
Based on the meta-analysis by Glas et al.,[27] the dosage of heparin varied from 30,000 to 400,000 U/day. Several studies suggested a dose-dependent effect of heparin nebulization in which dosages of 30,000 U/day improved outcomes in pediatric patients but failed to improve outcomes in adults. This meta-analysis could not confirm this. Types of nebulizers and its position in the circuit may affect the delivery of nebulized drugs in ventilated patients. Nebulized heparin is cleared slowly from the lungs, and 40% was still present in the lungs after nebulization of a single dose.[19] In the 2008 study by Dixon et al.,[28] the authors were investigating dose-dependent effects of nebulized heparin. The first group was administered a total of 50,000 U/day, the second group 100,000 U/day, the third group 200,000 U/day, and the fourth group 400,000 U/day. The changes over time for the PaO2/FiO2 ratio, lung compliance, and the alveolar dead space fraction levels were similar for all doses. Therefore, it seems that even higher doses of heparin compared to our study, did not differ the results significantly
The small patient numbers and methodological shortcomings of our study (such as non-randomized), administration of heparin for 5 days and conduction of study in a single center are the major limitations of our study, but, as we mentioned before, heparin could replace or add to budesonide as a safe and effective practice in critically ill patients under mechanical ventilation. It is also probable that the dosage, timing, and administration of heparin were not optimized to this population and that larger doses, more frequent administration or longer duration of treatment may have shown benefit. Ideally, a multicenter randomized controlled trial would be conducted to provide a definitive answer to the question of clinical benefit of heparin in VILI.
CONCLUSION
Despite the mentioned limitation and nonsignificant results, the overall effectiveness of nebulized heparin is at least comparable with a potent inhaled corticosteroid (budesonide). Therefore, we suggest further well-designed trials to confirm these findings, especially in our country. Because our sources are limited and replacement of drug with better efficacy and low cost are very crucial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–53. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Saddy F, Sutherasan Y, Rocco PR, Pelosi P. Ventilator-associated lung injury during assisted mechanical ventilation. Semin Respir Crit Care Med. 2014;35:409–17. doi: 10.1055/s-0034-1382153. [DOI] [PubMed] [Google Scholar]
- 3.Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37:633–46. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2012;46:566–72. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung Injury: Clinical implications. Chest. 2016;150:1109–17. doi: 10.1016/j.chest.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Benítez NE, Valladares F, García-Hernández S, Ramos-Nuez Á, Martín-Barrasa JL, Martínez-Saavedra MT, et al. Altered profile of circulating endothelial-derived microparticles in ventilator-induced lung injury. Crit Care Med. 2015;43:e551–9. doi: 10.1097/CCM.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 7.Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: More than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141:916–22. doi: 10.5858/arpa.2016-0342-RA. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen BS, Maltesen RG, Pedersen S, Kristensen SR. Early coagulation activation precedes the development of acute lung injury after cardiac surgery. Thromb Res. 2016;139:82–4. doi: 10.1016/j.thromres.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Bowler SD, Smith SM, Lavercombe PS. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am Rev Respir Dis. 1993;147:160–3. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- 10.Bissonnette EY, Tremblay GM, Turmel V, Pirotte B, Reboud-Ravaux M. Coumarinic derivatives show anti-inflammatory effects on alveolar macrophages, but their anti-elastase activity is essential to reduce lung inflammation in vivo. Int Immunopharmacol. 2009;9:49–54. doi: 10.1016/j.intimp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Hofstra JJ, Vlaar AP, Cornet AD, Dixon B, Roelofs JJ, Choi G, et al. Nebulized anticoagulants limit pulmonary coagulopathy, but not inflammation, in a model of experimental lung injury. J Aerosol Med Pulm Drug Deliv. 2010;23:105–11. doi: 10.1089/jamp.2009.0779. [DOI] [PubMed] [Google Scholar]
- 12.Tuinman PR, Dixon B, Levi M, Juffermans NP, Schultz MJ. Nebulized anticoagulants for acute lung injury - A systematic review of preclinical and clinical investigations. Crit Care. 2012;16:R70. doi: 10.1186/cc11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–52. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig RJ. Therapeutic use of heparin beyond anticoagulation. Curr Drug Discov Technol. 2009;6:281–9. doi: 10.2174/157016309789869001. [DOI] [PubMed] [Google Scholar]
- 15.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: A Systematic review. Adv Pharmacol Sci. 2015;2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017;117:437–44. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad RS, El-Maraghi SK, El-Sorougi WM, Sabri SM, Mohammad MF. Role of nebulized heparin inhalation on mechanically ventilated critically ill patients. Egypt J Bronchology. 2016;10:179. [Google Scholar]
- 18.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Med. 2013;11:85. doi: 10.1186/1741-7015-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köhler D. Aerosolized heparin. J Aerosol Med. 1994;7:307–14. doi: 10.1089/jam.1994.7.307. [DOI] [PubMed] [Google Scholar]
- 20.Bendstrup KE, Newhouse MT, Pedersen OF, Jensen JI. Characterization of heparin aerosols generated in jet and ultrasonic nebulizers. J Aerosol Med. 1999;12:17–25. doi: 10.1089/jam.1999.12.17. [DOI] [PubMed] [Google Scholar]
- 21.Dixon B, Schultz MJ, Hofstra JJ, Campbell DJ, Santamaria JD. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit Care. 2010;14:445. doi: 10.1186/cc9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ, et al. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: A randomized controlled trial. Crit Care. 2010;14:R180. doi: 10.1186/cc9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson MR, Takata M. Inflammatory mechanisms of ventilator-induced lung injury: A time to stop and think? Anaesthesia. 2013;68:175–8. doi: 10.1111/anae.12085. [DOI] [PubMed] [Google Scholar]
- 24.Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: A systematic review and meta-analysis. Crit Care Med. 2009;37:1594–603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 25.Festic E, Carr GE, Cartin-Ceba R, Hinds RF, Banner-Goodspeed V, Bansal V, et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju YN, Yu KJ, Wang GN. Budesonide ameliorates lung injury induced by large volume ventilation. BMC Pulm Med. 2016;16:90. doi: 10.1186/s12890-016-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glas GJ, Serpa Neto A, Horn J, Cochran A, Dixon B, Elamin EM, et al. Nebulized heparin for patients under mechanical ventilation: An individual patient data meta-analysis. Ann Intensive Care. 2016;6:33. doi: 10.1186/s13613-016-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dixon B, Santamaria JD, Campbell DJ. A phase 1 trial of nebulised heparin in acute lung injury. Crit Care. 2008;12:R64. doi: 10.1186/cc6894. [DOI] [PMC free article] [PubMed] [Google Scholar]


