Abstract
Aim of the Study:
Pleiotropic effect of statins can modulate inflammation in septic shock. We tested the hypothesis whether statins can reduce mortality in septic shock.
Patients and Methods:
We conducted a randomized double-blinded trial with treatment (40 mg dose of atorvastatin for 7 days) and control (placebo) arm in adult septic shock patients admitted to the Intensive Care Unit. Primary (28-day mortality) and secondary (vasopressor-, ventilation-, and renal replacement therapy-free days) outcomes, with lipid profile and adverse effects, were documented. Inflammatory biomarkers (interleukin [IL]-1, IL-6, tumor-necrosis-factor [TNF]-α, interferon [IFN], and C-reactive protein [CRP]), were also measured before (day 1 [D1]) and after start of trial drug (D4 and D7).
Results:
Seventy-three septic shock patients with 36 and 37 included in the atorvastatin and placebo group, respectively. Both groups were equally matched. Twenty-eight-day mortality, event-free days, lipid profile, and adverse effects were also not significantly different between groups. Reduced levels of IL-1, IL-6, TNF-α, IFN, and CRP were observed in the atorvastatin group. Also observed were significant day-wise changes in inflammatory biomarkers.
Conclusions:
Atorvastatin-induced changes in inflammatory biomarkers did not confer mortality benefit in septic shock (ClinicalTrials.govNCT02681653).
Keywords: Inflammatory biomarkers, mortality, septic shock, statins
INTRODUCTION
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection.[1] Septic shock is a subset of sepsis, in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality.[1] In spite of advancement in management of septic shock in the last two decades, mortality remains unacceptably high. Furthermore, escalating cost of intensive care with limited health insurance in developing countries is only aggravating the problem. These conditions continue to fuel search for new adjunctive therapies in septic shock to reduce mortality, cost of therapy, and length of stay.
Statins (hydroxymethyl glutaryl coenzyme A reductase inhibitors) have been shown to have anti-inflammatory and immunomodulatory properties capable of attenuating inflammatory response in sepsis.[2,3,4] Their lipid-lowering capabilities have been well acknowledged specifically in patients at risk of atherosclerotic cardiovascular events. Pleiotropic properties of statins in the prevention and mitigation of sepsis have also been intensely investigated. In a meta-analysis of 26 observational studies on infection and sepsis, statins were associated with a significant decrease in mortality.[5] However, in stark contrast to these observational studies, the meta-analysis of randomized controlled trials (RCTs) on this issue did not report any survival benefit.[5,6]
Despite superiority over observational studies, existing RCTs are also limited by profound variability. The variability exists in dose, duration, and type of statin, primary and secondary outcomes, severity of sepsis, prior statin use, and nonuniform quantification of adverse effects of statins. Furthermore, impact of statins in an exclusive cohort of septic shock has never been previously studied. To test the hypothesis that statins have survival benefit in septic shock, we conducted a prospective randomized double-blinded phase II trial with treatment (atorvastatin) and control (placebo) arm. The present study has used the older definitions of sepsis, severe sepsis, and septic shock as were prevalent at the time of conception, design, implementation, and completion of the study.[7,8,9]
PATIENTS AND METHODS
The study was conducted in a tertiary care referral hospital and academic institute in north of India. A combined adult pediatric medical and surgical Intensive Care Unit (ICU) of 12 beds with an annual admission rate of 250 was used for this purpose.
Ethics, consent, and permissions
The study was approved and funded by the ethics (A-02:PGI/IMP/IEC/56/19.8.11) and research committee of the institute, respectively. Written informed consent was obtained from patients or their surrogates as appropriate.
Study design
The study was a prospective, randomized, double-blinded placebo-controlled trial to assess the impact of atorvastatin in septic shock patients. The trial was registered with the ClinicalTrials.gov with registration number NCT02681653.
Study population
Screening, enrollment, and randomization of the study population were as illustrated in Figure 1.
Figure 1.
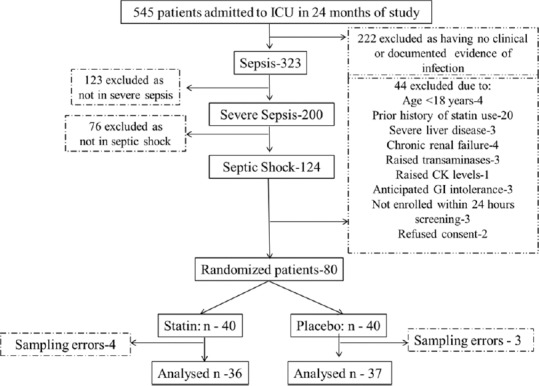
Flow diagram of randomization of study population. CK: Creatine kinase; GI: Gastrointestinal
Inclusion criteria
Adult patients of 18 years and above in septic shock and admitted to the ICU were included in the study. Septic shock was defined according to the Surviving Sepsis Campaign Guidelines of 2012.[7]
Exclusion criteria
Patients were excluded from the study on grounds of prior statin use in previous 3 months of inclusion in the study, myopathy or rhabdomyolysis, severe liver disease (acute liver failure, chronic liver disease [Child-Pugh Classification C]), serum alanine aminotransferase (ALT) level >2.5 times the upper limit of normal (ULN) or a raised serum creatine kinase (CK) level higher than the ULN, chronic renal failure, anticipated gut intolerance (e.g., postoperative abdominal surgery), pregnant or breastfeeding females, concurrent drug administration (cimetidine, warfarin, ketoconazole, macrolides, immunosuppressants, except steroids, HIV protease inhibitors, and other lipid-lowering drugs), or refusal for consent.
Randomization
Patients were randomized to receive either atorvastatin or placebo (methylcellulose). Randomization was performed in 1:1 ratio using a computer-generated random number list (conducted by a person not involved in the study). Trial packs of drug were prepared by an independent pharmacy and contained atorvastatin or an equally matched placebo in size, shape, color, and weight. Allocation concealment was by sealed opaque envelopes. Participants, caregivers, investigators, and outcome assessors were all blinded to the study group assignment.
Study protocol and intervention
The patients were included in the study within 24 h of having met the inclusion criteria. Trial drug was administered through nasogastric tube after randomization and was continued daily for 7 days of ICU admission, discharge, or death whichever occurred earlier. Patients were treated with either 40 mg of atorvastatin or placebo. Patients received the first trial drug dose at the earliest opportunity (but not exceeding 24 h) of randomization. The trial drug was dissolved in 20 ml of distilled water before being given enterally.
Data collection
All patients' data were codified and stored using a study identification number on a single computer in a protected database. Basic demographic data included age, gender, coexisting medical conditions, concurrent medications, and severity scores (acute physiology and chronic health evaluation II [APACHE-II] score and sequential organ failure assessment [SOFA] score). Life support measures and other ICU interventions, vasopressors (type, dose, and duration), invasive ventilation (ventilation support, PaO2/FiO2 ratio, and PaCO2), and renal replacement therapy (RRT) (modality ± diuretics [dose]) were recorded. Physiological parameters of hemodynamics (heart rate and mean arterial pressure [mmHg], central venous oxygen saturation [%]) and lactate [mg/dL]), acid–base status, urinary output, and fluid balance were also recorded. Baseline biochemical measurements including lipid profile, CK, liver function tests, complete blood count, renal function tests (serum creatinine [Scr], blood urea nitrogen [BUN]), arterial blood gas (pH, PaO2, PaCO2, Na+, K+, Cl−, and HCO3−), procalcitonin, and cultures were recorded. Blood samples were collected before (day 1 [D1]) and after (D4 and D7) starting of trial drug for the measurement of inflammatory biomarkers interleukin (IL)-1, 6, tumor-necrosis-factor alpha (TNF-α), interferon (IFN) and C-reactive protein (CRP). Lipid profiles (nonfasting, total cholesterol [TC], triglycerides, high-density lipoprotein, low-density lipoprotein [LDL] and very LDL) were measured on D1, D4, and D7 by enzymatic colorimetric method. BD Biosciences manufactured kits with catalog numbers for IL-1 (557953), IL-6 (555220), TNF (555212), IFN (555142), and CRP by nephelometry technique on the Siemens BN ProSpec machine were used for estimation.
Drug discontinuation
Each patient was assessed on the day of randomization, before (D1) and after starting of trial drug (D4 and D7) and then subsequently on D14, D21, and D28 if they survived in the ICU. Trial drug was discontinued if drug-induced intolerance, hypersensitivity reaction, hepatitis, or rhabdomyolysis occurred. The definition in our study for suspected drug-induced intolerance such as hepatitis and rhabdomyolysis was as follows: Drug-induced hepatitis was considered if there was rise in serum ALT level to more than twice the initial value and drug-induced rhabdomyolysis was considered if after starting the trial drug, serum CK levels increased equal to 10 times the normal value. Trial drug was also discontinued in the case of gastrointestinal intolerance as suggested by a gastric residual volume >50% of enteral intake per feed or >500 ml in previous 24 h despite use of prokinetic medications.
Septic shock management
Septic shock management was similar in the two groups, except for the use of trial drug. Fluids, vasopressors, inotropes, steroids, blood transfusions, antimicrobials and source control for sepsis, mechanical ventilation, RRT, sedation, nutrition and glycemic control, and thromboprophylaxis were in accordance with the Surviving Sepsis Campaign Guidelines of 2012.[7] Detailed description of this management is provided as a Supplementary File (257.8KB, pdf) .
Study outcomes
The primary endpoint of this study was mortality at 28 days of study inclusion. Secondary outcomes included length of ICU stay, event-free (vasopressor-free, ventilation-free, and renal replacement-free) 28 days, effect on inflammatory biomarkers, lipid profile, and adverse effects of the trial drug.
Sample size and statistical analysis
Sample size
Group sample size of 28 each in drug and placebo group was needed to achieve a power of 80%, with the aim to determine an absolute difference between group proportions of 0.30. The proportion in trial drug group was assumed to be 0.50 under the null hypothesis and 0.80 under the alternative hypothesis. The proportion in placebo group was 0.50. The test statistic used was the one-sided Z-test with unpooled variance. The significance level of the test was targeted at 0.05. We assumed a follow-up loss of about 10%, so finally the sample size for each group was 31. Power analysis and sample size (NCSS (2008) PASS, Kaysville, UT) was used to estimate the sample size for this study.
Statistical analysis
Normality of continuous data was tested using Shapiro–Wilk test. Nonnormal, continuous data were expressed as median (interquartile range), while categorical data were expressed as frequency and percentage. Mann–Whitney U-test was used to compare the medians between the drug and placebo group. Chi-square test was used to compare the proportions/test the association between groups. For repeated observations over time, Friedman analysis of variance (ANOVA) was used to estimate the significance level among the time points. If, in Friedman ANOVA, the P value was observed to be significant, then the difference in medians between individual groups was further assessed using the Wilcoxon signed rank test. A two-tailed P < 0.05 was considered statistically significant. IBM, SPSS version 23 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
The study was conducted over 24 months from January 2012 to December 2014. Five hundred and forty-five patients were admitted to the ICU during this period. Approximately 59%, 37%, and 23% patients were in sepsis, severe sepsis, and septic shock, respectively. The flow diagram of randomization of the study population is as depicted in Figure 1. Sixteen percent (20/124) prior statin users were excluded from the study. On account of sampling errors, four included patients had to be deleted from the final analysis. These sampling errors included those samples which were inadequate in volume or improperly collected, labeled, stored, transported, or misplaced. Finally, there were 36 and 37 patients in septic shock who were randomly allocated to receive atorvastatin or placebo, respectively. As D1 represents results from baseline (i.e., no trial drug treatment), then D4 represents a maximum of three possible doses and D7 represents a maximum of six possible doses. The atorvastatin and placebo group received a median of 4 (1–7) and 3 (1–7) doses of atorvastatin and placebo group, respectively, during the trial week. All patients included received the drug and placebo successfully enterally.
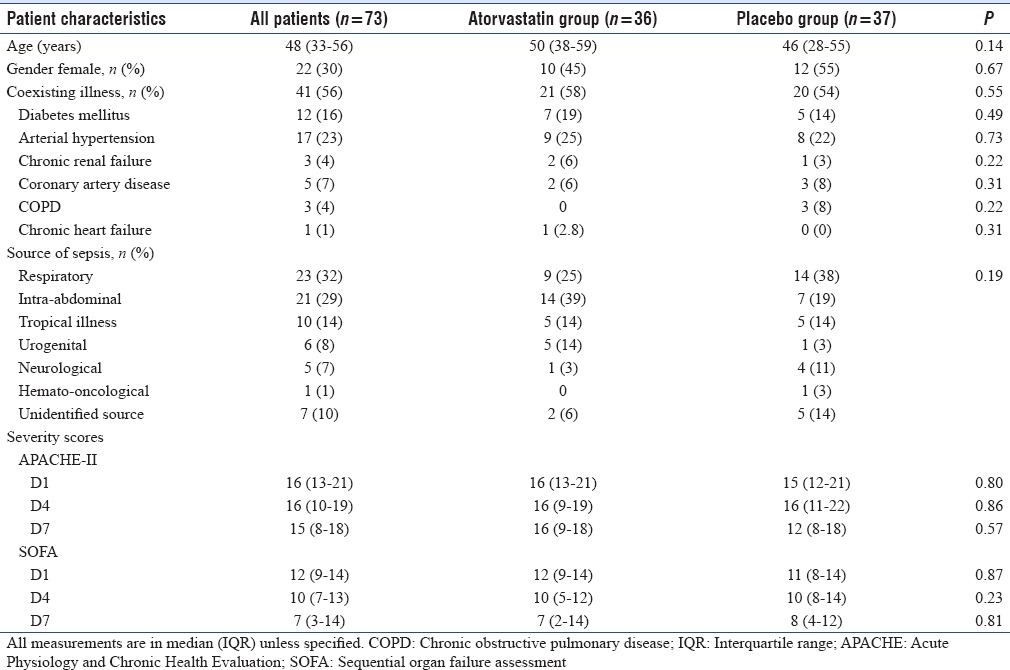
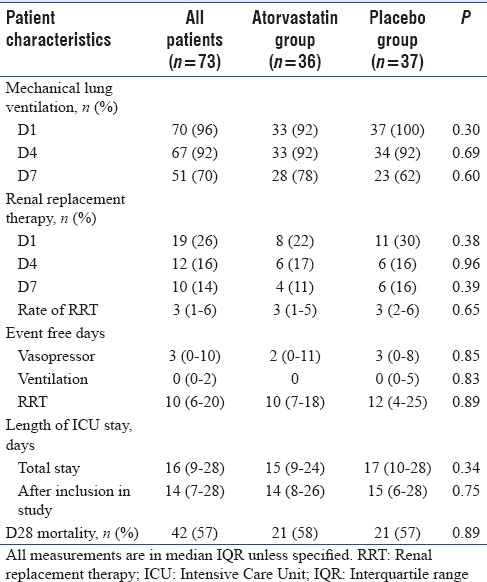
Baseline patient characteristics
Both atorvastatin and placebo group were equally matched in terms of age, gender, coexisting illnesses, source of sepsis, and severity of illness (APACHE-II and SOFA) [Table 1].
Table 1.
Baseline characteristics of septic shock patients
Laboratory and physiological indices
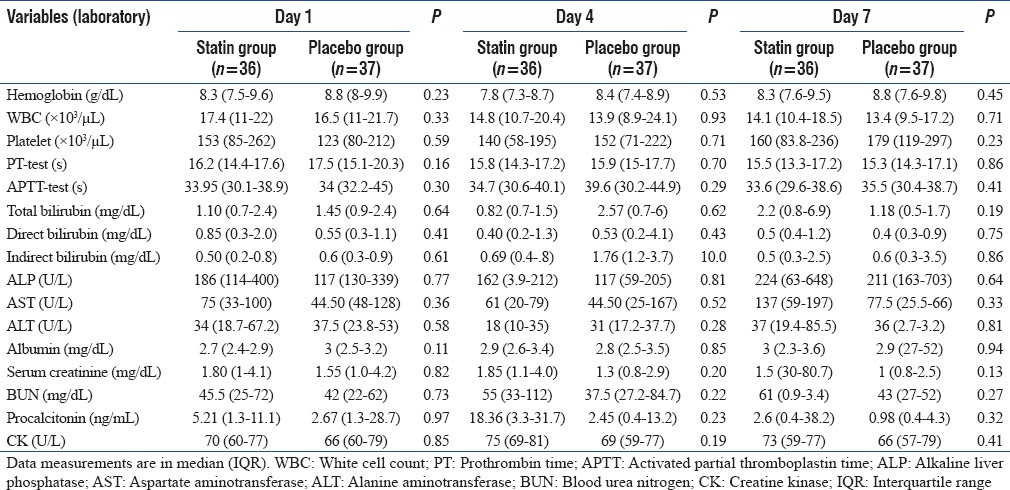
The hematological, coagulation, and liver and renal function laboratory parameters are as depicted in Table 2. Hemoglobin, whole blood cell, platelet, prothrombin time, activated partial thromboplastin time, liver function, Scr, and BUN were all comparable between statin and placebo on D1 and did not differ significantly on D4 and D7 either [Table 2].
Table 2.
Day-wise comparison of laboratory indices between groups
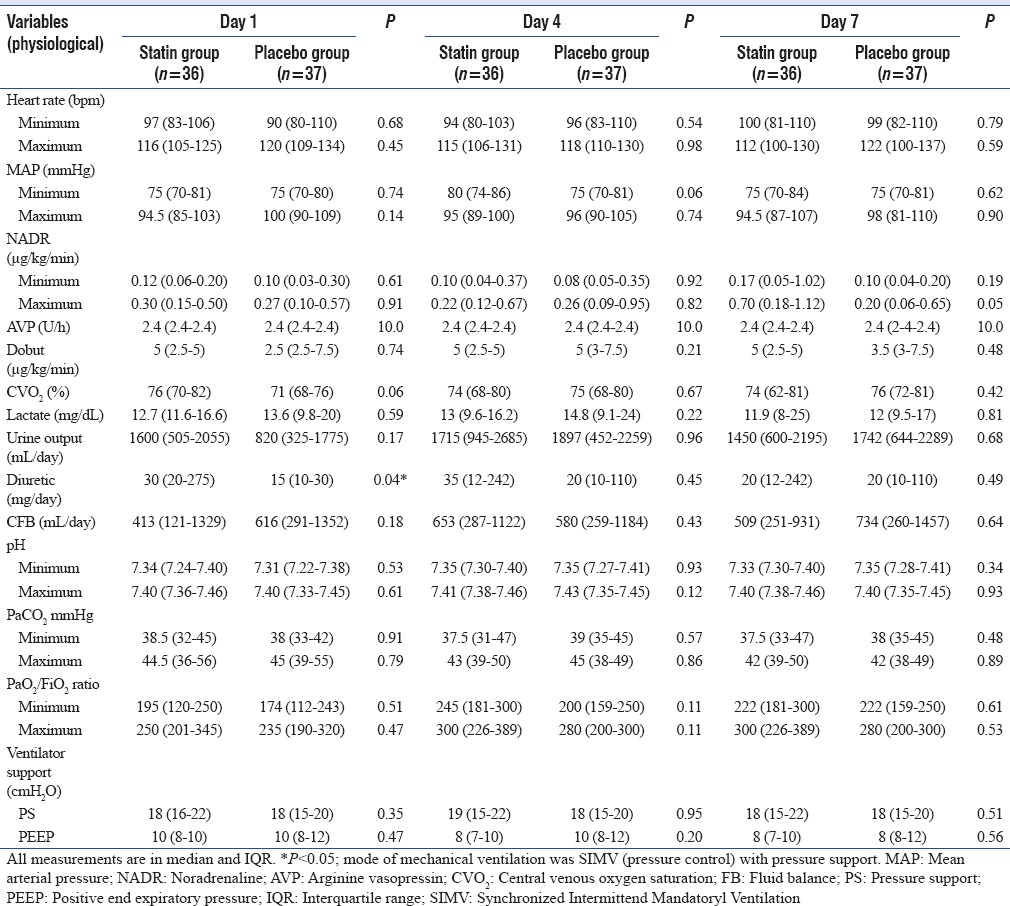
Physiological parameters were as depicted in Table 3. Hemodynamics, vasopressor and inotrope dose, perfusion, and metabolic parameters were comparable at start and during the trial week [Table 3]. However, on D7, the maximum noradrenaline dose in statin group was much higher than any other day, albeit nonsignificantly. Furthermore, the lactate levels were neither raised at baseline or during the trial week, nor did they differ significantly between groups. The atorvastatin group also received higher dose (mg/day) of diuretics (30 [20–275] vs. 15 [10–30] in placebo; P = 0.04) and consequently also had lower fluid balance on D1.
Table 3.
Day-wise comparison of physiological indices between groups
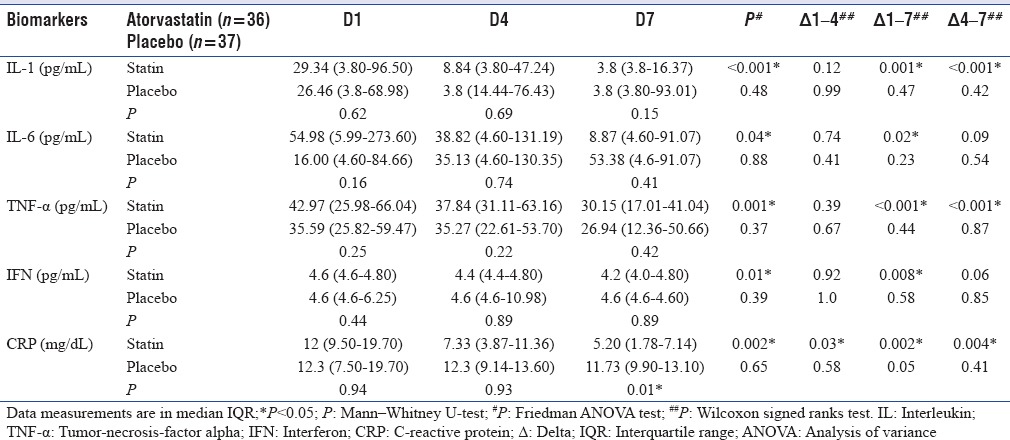
Inflammatory biomarkers
IL-1, IL-6, TNF-α, IFN, and CRP, all showed a significant decreasing trend, during the trial week, in atorvastatin group as against the placebo group. However, these differences were comparable on D1, D4, and D7. The delta (Δ) biomarker levels differed significantly [Table 4]. The Δ 1–4 for CRP (P = 0.002), Δ1–7 for all biomarkers, and Δ 4–7 for IL-1 (P < 0.001), TNF-α (P < 0.001), and CRP (P = 0.004) differed significantly. IL-6 was insignificantly higher at baseline in the atorvastatin group.
Table 4.
Day-wise comparison of trends in biomarkers between groups
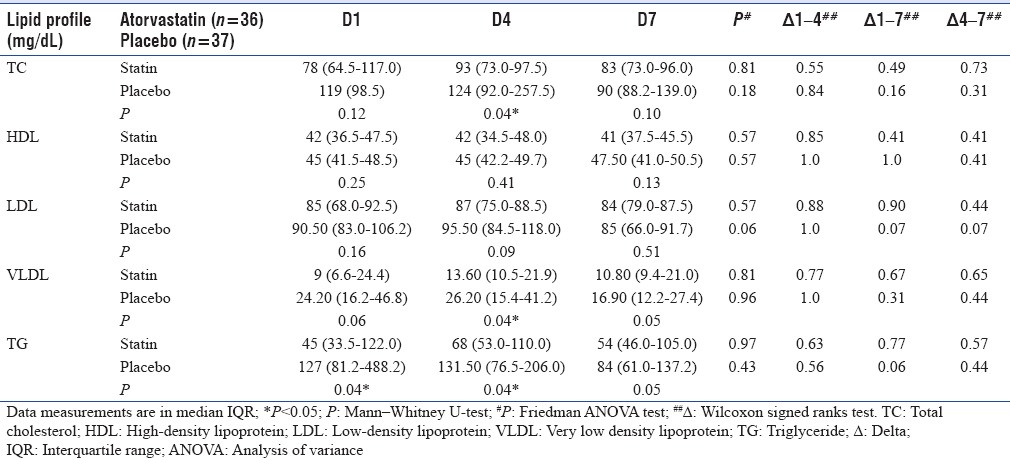
Lipid profile
Atorvastatin group had insignificantly lower lipid profile levels both before and during the trial week as compared to the placebo group. Furthermore, these and the day-wise Δ lipid profile were also observed to be statistically insignificant [Table 5].
Table 5.
Day-wise comparison of trends in lipid profile between groups
Outcome
There was no significant difference in primary outcome of mortality at D28 after inclusion in study as calculated by Kaplan–Meier estimate and compared by application of the log-rank [Figure 2]. Secondary outcomes estimated by event-free days and length of ICU stay were also comparable [Table 6].
Figure 2.
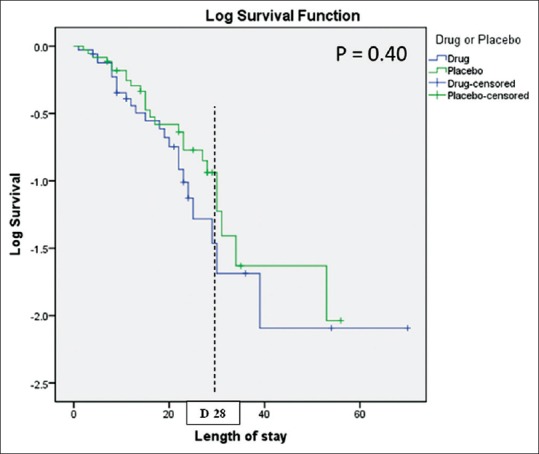
Kaplan–Meier survival analysis
Table 6.
Comparison of outcomes between groups in septic shock patients
Adverse effects
Serial CK levels did not differ significantly between groups before and during trial week [Table 2]. None of the patients in atorvastatin group had a rise of CK ≥10 times the normal value. ALT levels also did not rise significantly in the atorvastatin group. Furthermore, no deterioration in renal profile was observed with atorvastatin.
DISCUSSION
Mortality in septic shock remains high despite advances in management. Pleiotropic impact of statins, discordance between observational and randomized studies, variability among existing RCTs, and limited inclusion of septic shock patients was the stimulus for this RCT. The main findings from our study were that 40 mg of atorvastatin for 7 days at onset of septic shock did not decrease mortality or vasopressor-, ventilation-, RRT-free days. However, it did result in decreased levels of inflammatory biomarkers, IL-1, IL-6, TNF-α, IFN, and CRP levels without any detectable drug-related adverse effects or toxicities.
Atorvastatin is the most widely prescribed statin with well-established clinical safety profile even at variable strengths and duration of use. Previous studies of statins in sepsis have used both higher and lower doses of same or different statins for a longer duration than in our study. An unacceptably high mortality of 30%–70% was observed in a retrospective audit of septic shock patients admitted to our ICU (not reported). Length of ICU stay was also observed to be very unpredictable. Fear of adverse drug effects or toxicities, multiple drug interactions, multiorgan dysfunction/failure in septic shock, nonavailability of therapeutic drug monitoring in our setting, and already available negative previous studies of statins in sepsis wherein smaller doses for longer duration had been used prompted us to use higher atorvastatin dose but for a shorter duration.
In spite of the profound variability seen in previous RCTs, mortality benefit of statins in sepsis has been universally rejected by all. A table of previous double-blinded RCTs of statins in sepsis is provided in the Supplementary File (257.8KB, pdf) . Varying proportions (0%–79%) of septic shock patients have been included in previous RCTs.[10,11,12,13,14,15,16] The present RCT, an exclusive cohort of septic shock patients using atorvastatin (higher dose albeit shorter course), is in concordance with previous RCTs.
Significant earlier hemodynamic stabilization at both lower dose and shorter duration of vasopressor was reportedly achieved with rosuvastatin in the study by El Gendy and Elsharnouby.[15] In contrast to this, we did not observe any significant difference in either hemodynamics or vasopressor dose or vasopressor-free days between statin and placebo group. The reasons for these differences could be multifactorial. Inclusion of nonseptic shock patients, severity of illness, source of sepsis, coexisting illness, fluid status, choice of antimicrobials, and timing of onset of sepsis and start of therapy may all be plausible explanations. A higher dose of noradrenaline ≥0.7 (0.18–1.12) was used specifically in 10 patients in the statin group on D7 in our study [Table 3]. Higher severity of illness and nonsurvival explains the higher vasopressor requirements in this select group of patients. Truwit et al. in their study on sepsis-associated acute respiratory distress syndrome had reported that rosuvastatin group had lesser free days of ventilation, cardiovascular, coagulation, and renal and hepatic failure.[16] However, only renal and hepatic failure-free days were significantly lesser in the statin group. Similarly, in our study too, there were insignificantly lesser free days of vasopressor, ventilation, and RRT in the atorvastatin group.
Three out of seven RCTs done previously reported levels of IL-6[10,11,13] and one reported both IL-6 and TNF-α[10] to assess the impact of statins. Novack et al. in a single-center RCT reported significant reduction in IL-6 and TNF-α after 72 h of 40 mg simvastatin in patients of suspected or documented bacterial infection not in severe sepsis.[10] Similarly, Kruger et al. in single-center study in 2011 reported a decreasing trend in IL-6 over time albeit insignificantly.[11] However, the same author in a multicenter study from 21 ICUs in 2013 reported no difference in IL-6 levels between study and placebo group.[13] Our study has utilized a more comprehensive inflammatory panel of IL-1, IL-6, TNF-α, IFN, and CRP to assess the impact of statins in septic shock. It is unclear why the atorvastatin group had a somewhat higher IL-6 levels upon enrollment even though the groups were otherwise equally matched [Table 4]. Given the small sample size, a chance occurrence is a plausible explanation. Biomarker levels, comparable at baseline, reduced significantly in all by D7 in the atorvastatin group. These variations are suggestive of the impact of atorvastatin on inflammatory biomarkers in septic shock in spite of being used for a brief period of 1 week. Potential sustenance of this effect cannot be discussed as biomarker estimations were not performed beyond the trial week. However, the positive effects in biomarkers were not replicated in laboratory or physiological parameters both during the trial week [Tables 2 and 3] and during the latter 3 weeks of observation (table not shown). Plausible reason for this discordance could be that the drug dose was just enough to alter inflammatory biomarker levels but of inadequate duration to produce any clinically meaningful effect on physiological or laboratory parameters.
In the absence of plasma statin levels, Patel et al. suggested reduction in plasma lipids as indicative of treatment compliance, absorption of statins, and surrogate marker of their pleiotropic effects.[12] Two previous RCTs studied the effect of statins on lipid profile with differing results. The study by Patel et al.[12] reported significant reduction in lipid profile (TC and LDL) after 40 mg of atorvastatin on D4, while Kruger et al.[13] reported no difference with use of 20 mg atorvastatin. In our study, we observed insignificant difference between groups at baseline or over time [Table 5] somewhat similar to Kruger et al.[13] However, our study in no-way explains a different effect with differing doses and duration of statins in septic shock. In the absence of plasma statin levels, the next best ideal parameter to assess the impact of statins (lipid profile vs. inflammatory biomarkers) is not yet clear.
Similar to previous RCTs, we did not observe any significant elevation of ALT or CK levels in the study population. In none of the patients, trial drug was withdrawn on account of rise in ALT or CK. Furthermore, no deterioration in renal profile was observed with atorvastatin. Large RCT of rosuvastatin in cardiac surgery patients reported adverse renal impact.[17] However, cardiac surgery and septic shock patients are dissimilar cohort and the insult in either cannot be equated. Although the difference in class, dose, cohort, and ethnicity can plausibly explain the discordance, caution is still advisable as ours is a small study.
Conflicting results exist for the time course of pleiotropic and lipid-lowering effects. Animal studies report that pleiotropic effects occur earlier and independent of lipid-lowering effects.[18] However, an editorial on acute effects of statins commented that that the two could occur simultaneously making it difficult to assess the relative contribution.[19] In our study, the effects of inflammation occurred while no changes were seen in the lipid profile [Tables 4 and 5]. Type, dose, route, timing, and duration of statin use along with severity of illness, drug–drug interaction, and genetic makeup of the patient have important implications in acute effect of statins. Intracellular statin concentrations, kinetics, and role of specific drug transporters are poorly understood as of now.[19] Oral application may not be the best option in septic shock, but as of now intravenous formulation is not available.[19] Benefit of intravenous statins in septic shock needs to be explored.
Our study has several limitations. Small sample size, single-center, absence of plasma atorvastatin levels, and short duration of trial drug are its major limitations. The 5-year audit of our unit revealed that the minimum mortality in our septic shock patients was approximately 30%. Whether or not introduction of statins in our septic shock management protocol would reduce the mortality was the question. The minimum absolute mortality difference between the statin and placebo group that we desired was 30% between groups. To achieve this, a sample size of 28–28 was enough to achieve 80% power with 95% confidence. However, keeping a fall-out rate of 10%–15%, we estimated the sample size of 30 in each group. Hence, the actual sample size of 36 and 37 in the drug and placebo group is larger than actually required. The sample size of our study may look small but is actually not too small to justify the mortality difference. Source of sepsis has a strong impact on prognosis. Larger sample size with subgroup analysis would have helped differentiate the impact of therapy versus disease groups. Patients were received mostly during the postresuscitation phase of septic shock resulting in lower baseline lactate levels. Furthermore, the time of onset of sepsis, septic shock, fluid resuscitation, and admission to ICU did not necessarily occur simultaneously. Lactate is a sensitive, albeit nonspecific, stand-alone indicator of cellular or metabolic stress rather than shock. Higher mortality in our study is worrisome. Some of the reasons for this high mortality are as follows: delayed inter- or intra-hospital transfer of critically ill patients to our ICU from either nonexistent or poor quality ICUs, multisite colonization, inappropriate antimicrobial use, higher prevalence of multidrug resistance, inadequately staffed ICU with nurse–patient ratio of ≥1:2 or 1:3 on most occasions, nonavailability of round the clock respiratory physiotherapist, inadequate microbiological surveillance, inclusion of only septic shock patients, and nonavailability of advanced cardiovascular support systems like extracorporeal membrane oxygenation. These reasons may make our findings not generalizable to other ICUs.
CONCLUSIONS
In statin naïve septic shock patients, atorvastatin of 40 mg/day for 7 days does impact the levels of IL-1, IL-6, TNF-α, IFN, and CRP. However, these alterations did not confer any mortality or secondary benefit though no adverse effects were seen either. Hence, as of now, evidence from our and previous RCTs questions the utility of oral atorvastatin in septic shock. However, the possibility of a future large study using intravenous statins exists.
Financial support and sponsorship
We received financial support for the research from Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow - 226 014, Uttar Pradesh, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the support provided to us in statistical analysis by the Department of Biostatistics & Health Informatics, and Department of Immunology in estimation of inflammatory biomarkers. Both Departments are in Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Raibareli Road, Lucknow-226014, Uttar Pradesh, India.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco-Colio LM, Tuñón J, Martín-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: Panacea for sepsis? Lancet Infect Dis. 2006;6:242–8. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- 4.Mekontso-Dessap A, Brun-Buisson C. Statins: The next step in adjuvant therapy for sepsis? Intensive Care Med. 2006;32:11–4. doi: 10.1007/s00134-005-2860-5. [DOI] [PubMed] [Google Scholar]
- 5.Wan YD, Sun TW, Kan QC, Guan FX, Zhang SG. Effect of statin therapy on mortality from infection and sepsis: A meta-analysis of randomized and observational studies. Crit Care. 2014;18:R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: A meta-analysis of randomized trials. Am J Med. 2015;128:410–70. doi: 10.1016/j.amjmed.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 10.Novack V, Eisinger M, Frenkel A, Terblanche M, Adhikari NK, Douvdevani A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: A randomized double-blind placebo controlled clinical trial. Intensive Care Med. 2009;35:1255–60. doi: 10.1007/s00134-009-1429-0. [DOI] [PubMed] [Google Scholar]
- 11.Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, et al. Continuation of statin therapy in patients with presumed infection: A randomized controlled trial. Am J Respir Crit Care Med. 2011;183:774–81. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 12.Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS trial) Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, et al. Amulticenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–50. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 14.Papazian L, Roch A, Charles PE, Penot-Ragon C, Perrin G, Roulier P, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA. 2013;310:1692–700. doi: 10.1001/jama.2013.280031. [DOI] [PubMed] [Google Scholar]
- 15.El Gendy HA, Elsharnouby NM. Safety and vasopressor effect of rosuvastatin in septic patients. Egypt J Anaesth. 2014;30:311–7. [Google Scholar]
- 16.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Jayaram R, Jiang L, Emberson J, Zhao Y, Li Q, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–53. doi: 10.1056/NEJMoa1507750. [DOI] [PubMed] [Google Scholar]
- 18.Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, et al. Intravenous rosuvastatin for acute stroke treatment: An animal study. Stroke. 2008;39:433–8. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- 19.Laufs U, Adam O. Acute effects of statins. J Am Coll Cardiol. 2012;59:71–3. doi: 10.1016/j.jacc.2011.08.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


