Abstract
Effective nursing management strategies for adults with severe traumatic brain injury (STBI) are still a remarkable issue and a difficult task for neurologists, neurosurgeons, and neuronurses. A list of justified indications and scientific rationale for nursing management of these patients are continuously evolving. The objectives of the study are to analyze the pertinently available research and clinical studies that demonstrate the nursing management strategies for adults with STBI and to synthesize the available evidence based on the review. A comprehensive literature search was made in following databases such as Google Scholar, Cochrane, J-Gate, ProQuest, and ScienceDirect for retrieving the related studies. In the included studies, data were extracted and evaluated according to the objective. Narrative analysis was adopted to write this review. Patients with STBI have poor prognosis and require quality care for maximizing patients' survival. With a thorough knowledge and discernment of care of such patients, nurses can improve these patients' neurological outcomes.
Keywords: Narrative review, nursing management, severe traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is an injury which results from trauma to head due to external physical forces. The estimated annual burden of TBI on the United States economy is >$76 billion, with the costs for disability and lost productivity outweighing the costs for acute medical care.[1] The CDC approximates that in the US, around 52,000 people die every year due to severe TBI (STBI). Falls is the major cause of TBI, with maximum rates in children with age 0–4 and adults with age 75 and older. The second most common injury is motor vehicle related with the excessive rates in adults of age 20–24 years. Adults of 75 years of age and above have the elevated rates of TBI-related hospitalization and death. Males are higher rates than females.[2]
Each year, India produces approximately 1.5–1.7 million individuals who are neurologically disabled due to TBI.[3] The primary injury in the brain can give rise to severe disability due to neuronal destruction. Further deterioration results due to cerebral ischemia from brain swelling, hematoma formation, hypoxia, and hypotension and then leads to secondary brain injury.[4]
Nurses are the health professionals who see the full impact of TBI and have the skills that can alter the course of a patient's recovery; it is important for nurses to have a valuable resource with evidence-based recommendations on nursing activities to help them achieve the best possible outcomes. Nurses require knowledge and skills to provide quality care to the adults with STBI. Nurses play a vital role in the management of patients with STBI.
SIGNIFICANCE OF THIS REVIEW
Guidelines that promote interventions of proved benefit and discourage ineffective ones have the potential to reduce morbidity and mortality and improve quality of life, at least for some conditions. Guidelines can also improve the consistency of care.[5] Clinical management has become much more structured and evidence based since the publication of guidelines covers many aspects of care. Over the past 10 years, much of the treatment of TBI has evolved toward standardized approaches that follow international and national guidelines.[6] However, there are many recommendations on the management of STBI; there is an insufficiency of adequate facts regarding the nursing management of patients with STBIs. Even though all recommendations and activities are not individually performed by nurses, they are accountable for implementing and evaluating the results of these activities. In spite of the several existing published guidelines on the care of patients with STBI, new data and recommendations regarding the role of nursing management of STBI patients are limited.[7]
An understanding of medical complications during the care from injury to rehabilitation and discharge is important for the care of patients, for healthcare planning, and for formulating interventions that could improve outcome.[8] The most commonly encountered medical conditions in patients with TBI are eyes, ears, nose, and throat problems, psychiatric or behavioral disturbances, hypertension, and musculoskeletal injury at mild-to-moderate severity.[9] If left untreated, patients can develop certain complications such as posttraumatic seizures, hydrocephalus, deep vein thrombosis (DVT), heterotopic ossification, spasticity, gait abnormalities, agitation, and chronic traumatic encephalopathy.[10] This article outlines the nursing approaches for patients with STBI.
METHODS
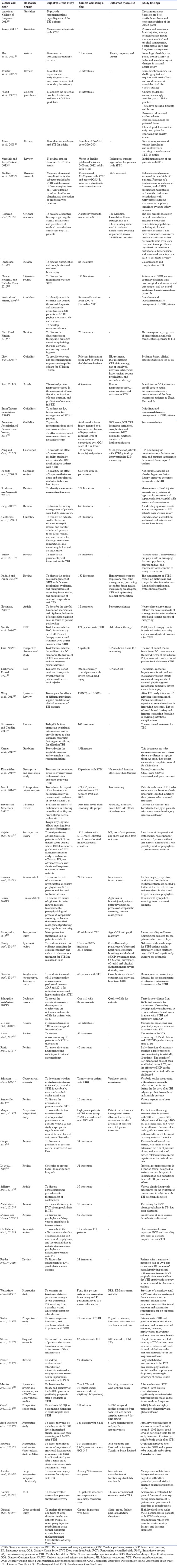
A comprehensive literature search was made in following databases: Google Scholar, Cochrane, J-Gate, ProQuest, and ScienceDirect from 2000 to 2016 for retrieving the related studies. In the included studies, data were extracted and evaluated as per the objective, and narrative analysis was adopted to write this review. The eligibility of the papers to be included was determined by a reviewer. The inclusion criteria included publication in peer-reviewed journals, guidelines, pathways, studies published in the last 16 years, clinical trials, studies with full-version available, and studies written in English only [Table 1]. Different combinations of search terms were used to collect the available literature and relevant data were retrieved. Search terms included nursing management, severe traumatic brain injury, severe brain injury, head injury, and management.
Table 1.
The literature from 2000 to 2016 retrieved for the review on nursing management of adults with severe traumatic brain injury
RESULTS AND DISCUSSIONS
The evidence obtained out of 38 reviewed literature on the management of patients with STBI is beneficial for all the neuronurses who care for the STBI patients. This descriptive synthesis adds to the scientific evidence in the field of nursing care. The nursing care of TBI patients starts from the initial management to the rehabilitative care.
Prehospital management
The main goals of prehospital management are to prevent hypoxia and hypotension because these systemic insults lead to secondary brain damage. When assessed before hospital admission, oxygen saturation <90% is found in 44%–55% of cases and hypotension in 20%–30% of cases. Hypoxia and hypotension are strongly associated with poor outcome. In various settings, the introduction of a prehospital system capable of normalizing oxygenation and blood pressure (BP) has been associated with improved outcome. However, ensuring adequate training of paramedics is vital because intubation by poorly trained paramedics has been associated with worse outcome. Arterial hypotension is best prevented by early and adequate fluid resuscitation with normotonic crystalloids and colloids. No benefits have yet been shown for hypertonic solutions, or for albumin, which has been associated with worse outcome.[6]
Emergency room management
Initial management in the emergency room starts with recognizing TBI and involves assessment of the level of consciousness of the patient, securing the airway with an endotracheal tube for patients with Glasgow Coma Scale (GCS) score of ≤8,[11,12] ensuring adequate oxygenation (PaO2>60 mmHg) and BP (systolic BP >90 mmHg),[11] inserting peripheral intravenous (I.V) canulas, cardiac monitoring, pulse oximetry, and continuous waveform capnography if needed. A neurologic examination should be done as soon as possible,[12] and a GCS score ≤8 is considered an STBI.[11,13] A complete blood count, electrolytes, glucose, coagulation parameters, blood alcohol level, and urine toxicology should be checked.[11,14] In STBI, noncontrast head computed tomography (CT) scan is the right tool of choice. Proton neurospectroscopy is a safe, noninvasive, and fast method of predicting the end results after a TBI.[15] Intracranial pressure (ICP) monitoring is advised for all patients with STBI, patients at risk of ICH,[16] GCS score <9, and an abnormal CT scan. The target should be to maintain ICP <20 mmHg[17] and cerebral perfusion pressure (CPP) range is 60–70 mmHg.[2] A ventricular catheter connected to an external strain gauge transducer is the most appropriate method for monitoring ICP.[16,18] Draining cerebrospinal fluid (CSF) decreases ICP.[17] Hyperventilation therapy can reduce ICP,[11] but there is no strong evidence which indicates whether this improves outcomes.[19] Correction of hypotension[20] and hypoxia helps in improving patient outcomes.[16]
The management of patients with head trauma should always consider C-spine motion restriction.[21] Hold the neck immobile in line with the body, apply a rigid or semirigid cervical collar, and (unless the patient is very restless) secure the head to the trolley with sandbags and tape. Cervical spine injury can be difficult to diagnose in the unconscious patient and should be assumed to be present until it can confidently be excluded. The priority in TBI must always be to secure, maintain, and protect a clear airway. Remove secretions and foreign bodies by manual extraction or suction, giving oxygen by mask (10–12 1/min). The adequacy of ventilation can be assessed clinically and by arterial blood gas analysis. A tension pneumothorax is a life-threatening emergency which should be diagnosed clinically and treated promptly. An indwelling arterial cannula allows serial blood gas measurement and continuous recording of BP. Pulse oximetry is valuable for indirect measurement of how well the patient is being oxygenated. Hypotension is a late sign of hypovolemic shock. Pulse rate, respiratory rate, and capillary refill time are the more useful ways of assessing the circulation after injury. Peripheral I.V infusions should be considered for decreased blood volumes. Early direct monitoring of arterial pressure and central venous pressure is helpful for assessing the adequacy of resuscitation.[22] When managing the immediate and long-term consequences of TBI, many pharmacological options, including psychostimulants, antidepressants, antiparkinsonian agents, and anticonvulsants can be used. These can play a role in managing the neuropsychiatric, neurocognitive, and neurobehavioral sequelae of injury to the brain.[23]
Conservative and operative management
Positioning
The patient should be positioned properly with the neck in neutral position and the head end of the bed elevated to 30°. This facilitates cerebral venous drainage.[2,24] Head end of the bed should be elevated for patients with CSF, rhinorrhea, and otorrhea.[25] Rigid cervical collars should be loosened or removed to decrease ICP.[17]
Brain tissue oxygen-directed management
Patients who receive brain tissue oxygen therapy to maintain brain tissue oxygen tension ≥20 mmHg and treated with ICP- or CPP-guided therapy to keep ICP <20 mmHg and CPP > 60 mmHg are recognized to have a better outcome and decreased mortality. These patients should be resuscitated and managed by the following methods such as (1) earlier recognition and removal of hematomas; (2) intubation and ventilation with FiO2 and minute ventilation adjusted to set SaO2>93% and to evade PaO2<60 mmHg; (3) PaCO2 set at 35–45 mmHg unless ICP is increased when PaCO2 is maintained between 30 and 40 mmHg; (4) normothermia (~35°C–37°C); (5) sedation by administering propofol during the initial 24 h, succeeded by sedation and analgesia with lorazepam, morphine, or fentanyl; (6) head end elevated to 15°–30° and knee elevated; (7) if seizures are present, administer anticonvulsants (phenytoin) for 1 week or more; and (8) euvolemia by administering a crystalloid infusion (0.9% normal saline, 20 mEq/L KCl; 80–100 ml/h).[26] The use of both an ICP and a brain tissue PO2 monitor and therapy directed at brain oxygen reduces the mortality rate after STBI.[27]
Temperature management
Hypothermia reduces ICP (40%) and cerebral blood flow (CBF, 60%), has positive effects on cerebral metabolism, and improves outcome for 3 months after injury. Thus, it limits secondary brain injury.[28] Normothermia should be maintained with the use of antipyretic medications, surface cooling devices, or even endovascular temperature management catheters.[11]
Stress ulcer prophylaxis
Stress ulcers (Cushing's ulcer) are a very common risk factor of patients in the Intensive Care Unit (ICU). Early enteral feeding, H2-blockers, proton-pump inhibitors, and sucralfate are recommended for the prophylaxis of stress ulcers.[24]
Nutrition
Patients immediately after injury may experience a systemic and cerebral hypermetabolic state.[13] Early enteral feeding should be initiated within 72 h of injury.[2] By day 7 of postinjury, these patients should be given full caloric replacement.[11] After TBI, early initiation of nutrition is recommended. Parenteral nutrition is superior to enteral nutrition in improving outcomes.[29] Evidence-based guidelines include the provision of early (within 24 h of injury) nutrition (>50% of total energy expenditure and 1–1.5 g/kg protein) for the first 2 weeks after the injury.[30] Feeding patients to attain basal caloric replacement at least by the 5th day and at most by the 7th day postinjury is recommended to decrease mortality. Transgastric jejunal feeding is recommended to reduce the incidence of ventilator-associated pneumonia.[31]
Fluid therapy
Fluid therapy helps in restoring vascular capacity, tissue perfusion, and cardiac flow rate. Hypertonic saline can be used for patients with complications of STBI and systemic shock.[14] Euvolemia can be maintained using isotonic fluids such as normal saline.[11]
Hyperventilation
Hyperventilation reduces PaCO2, CBF, and ICP by the cerebral autoregulation. It can be used only if ICP >30 mmHg and CPP <70 mmHg; CPP >70 mmHg but higher ICP >40 mmHg.[14]
Transport of patients
These patients should be transported with caution and care with suitable protection. It should be done by trained and suitably equipped personnel with careful supervision, support to the vital organs, continuous monitoring, prevention of damage to the spine, and complete documentation.[12]
Hemostatic therapy
Patients with STBI develop coagulopathies. Prothrombin complex concentrate, fresh frozen plasma, and/or Vitamin K should be given for patients with warfarin-associated intracerebral hemorrhage (ICH). Platelet count should be maintained >75,000 with platelet transfusions if necessary for patients with thrombocytopenia.[11]
Glucose management
Extremes of very high or low blood glucose levels should be managed accordingly. A target range of up to 140 mg/dL or possibly even 180 mg/dL may be appropriate.[11] Patients with hyperglycemia should be managed insulin protocol in cases with value >200 mg/dl for improving the outcome.[32]
Tracheostomy
In patients with severe isolated TBI, tracheostomy might be favorable if it is performed in the 2nd or 3rd week after admission.[33]
Medical management
An increase in ICP can be prevented by administering sedation.[17] The foremost therapies after pain and agitation are mannitol or hypertonic sodium chloride solution. Propofol, I.V dexmedetomidine, and fentanyl are commonly used in mechanically ventilated patients.[2] Steroids are not recommended in TBI.[11,16] Barbiturates are commonly used to treat ICP. There is no affirmation that barbiturates reduce mortality; it also causes low BP.[34,35] Mannitol can be used to reduce ICP[16,17] and it also helps in improving CBF.[11] Phenytoin is recommended to reduce posttraumatic seizures.[16] Levetiracetam can be used as an alternative.[11,36] Sympathetic storming which includes posturing, dystonia, hypertension, tachycardia, dilatation of the pupils, sweating, hyperthermia, and tachypnea can occur within the first 24 h after injury till several weeks. This can be caused after the cessation of sedatives and narcotics in the ICUs and should be treated based on their signs and symptoms by initiating planned medications to reduce the activities of the sympathetic nervous system.[37] The patients who receive erythropoietin show lower mortality and better neurological outcome and limit neuronal damage induced by TBI.[38] Naloxone effectively reduce mortality and control ICP in TBI.[39]
Surgical management
A surgical evacuation is done on patients having GCS score ≤8 with a huge lesion on noncontrast head CT scan. Depressed skull fractures those are open or complicated need surgical repair. Decompressive craniectomy helps in positive patient outcome.[2,24,40] Still, there is no evidence to support whether decompressive craniectomy improves mortality and quality of life, but it may improve neurological outcomes in pediatric patients.[41] An epidural hematoma larger than 30 mL in volume despite a patient's GCS score should be evacuated immediately. Acute subdural hematomas greater than 10 mm in thickness or associated with midline shift greater than 5 mm on CT also should be should be surgically evacuated. If there is an evident mass effect, then a surgical evacuation is recommended in traumatic ICH. Superficial debridement and dural closure are indicated in a penetrating injury to prevent CSF leak. For depressed skull fractures, elevation and debridement are recommended.[11]
Monitoring
The primary aim of neuromonitoring in patients with TBI is early detection of secondary brain insults so that timely interventions can be instituted to prevent or treat secondary brain injury. ICP monitoring has been a stalwart in neuromonitoring.[42] Measurement of ICP and arterial BP is used to derive CPP and to guide targeted therapy of STBI necessitating ICU admission.[43,44] Cerebral oxygenation and near-infrared spectroscopy are also established as an important parameter for monitoring. Multimodal monitoring allows different parameters of brain physiology and function to be monitored and can improve identification and prediction of secondary cerebral insults.[42] Cerebral microdialysis is an invasive laboratory device for analyzing brain tissue biochemistry. It is used to measure biochemical changes in the area of brain which are at higher risk to secondary insults and its use is very limited.[24] Vestibulo-ocular monitoring is an indicator of brainstem function. It helps identify brainstem lesions by imaging techniques.[45]
On-going management and prevention of complications
The prevention of ventilator-associated pneumonia in patients with STBI is a central challenge. In patients with trauma, persistent systemic inflammatory response syndrome increases the risk of nosocomial sepsis, and low-dose hydrocortisone might exert beneficial immunomodulatory effects rather than inducing an immunosuppressive state. The use of stress-dose steroids to prevent ICU-acquired infections is still an emerging concept.[46] Patients with STBI are prone to develop pressure ulcer. The factors influencing pressure ulcer in patients with TBI are poorer GCS, delayed enteral feeding, >10% fall in hemoglobin, and >10% fall in albumin.[47] A risk identification scale can be used each shift to identify which patients are at risk, and it should be ensured that tubing and devices are not placed between skin surfaces. It should be made sure that ventilator tubing is not causing tension on tracheostomy tube and faceplate and pressure-relief devices including specialty mattress surfaces, padded cervical collars, heel lift devices, and pillows, skin barrier creams, topical or indwelling fecal containment devices can be used to prevent the pressure ulcer.[48] Around 80% of the urinary tract infections are attributable to an indwelling urethral catheter. Limiting catheter use and minimizing the duration the catheter remains in situ are primary strategies for catheter-associated urinary tract infection prevention. Urinary catheters must be inserted only when necessary for patient care and leave them in place only if indications persist. For its prevention, hand hygiene must be practiced immediately before insertion of the catheter and before and after any manipulation of the catheter site or apparatus. Nurses should make sure that catheters are inserted by use of aseptic technique and sterile equipment.[49] Contractures are a common complication of TBI and may occur in up to 84% of cases. The most commonly affected joints are hip, shoulder, ankle, elbow, and knee, with a significant percentage of patients developing contractures in five or more joints. Stretch is one of the most widely used techniques for the treatment and prevention of contractures. Splints, positioning programs, or casts changed at regular intervals (serial casting) can also be used. All methods involve mechanical elongation of soft tissues during varying lengths of time.[50]
Postoperative care
Postoperatively, any change in ICP, circulation of the CSF, CBF should be monitored continuously. Mechanical ventilation should be provided to maintain PaCO2 between 35 and 45 mmHg, maintain normal temperature, correct cerebral perfusion pressure, and prevent secondary brain injury. Patients with STBI are at an alarming risk for DVT. This can be minimized with the range-of-motion exercises, pneumatic compression devices,[51] and drugs such as low-molecular-weight heparin if needed.[2,16,31,52,53] The detection of DVT is difficult; therefore, it is good to concentrate on preventing their development using mechanical or pharmacological methods.[54] Ventriculostomies and other ICP monitors should be placed under sterile conditions to prevent CSF infections.[16]
Initiation of early in-hospital rehabilitation
STBI is the most debilitating of all injuries and has very poor outcome.[55] The survivors of STBI suffer from multiple problems such as physical deformity, memory disturbances, functional disability, cognitive dysfunction, and difficulty in performing various activities.[56] The strategy of early rehabilitation results in better short- and long-term outcomes. To optimize and accelerate the treatment process, patients should receive rehabilitative treatment as soon as possible.[57] Potential benefits for patients participating in early rehabilitation in the ICU include improved muscle strength, physical function, and quality of life and reduced hospital and ICU length of stay, duration of mechanical ventilation, and hospital costs.[58] As a part of long-term management, cranioplasty can be done after 2–6 months of the initial injury to replace the patient's bone flap or restore the area with mesh or plastic. Patients with decompressive craniectomy need neuropsychological, physical, speech, and occupational therapy. Patients require weeks to months of TBI rehabilitation.[2] Measuring the S-100β protein could be convenient in determining the long-term prognosis in patients with severe traumatic injury.[59,60,61] The cognition in STBI is always related with the patient outcome. Cognition improves over time and will be stable from 3 months to 1 year; thus, early screening of cognitive function is recommended for rehabilitation planning in a clinical setting (Stenberg et al., 2015).[62] Amantadine is proved to be effective in accelerating the pace of recovery during acute rehabilitation.[64] Sleep problems along with anxiety, depression, daytime sleepiness, and fatigue are common in patients with STBI during rehabilitation. Nurses should use actigraphy, sleep charts, sleep diaries for the assessment and diagnosis of sleep problems.[65]
CONCLUSION
TBI is a major cause of death and disability throughout the world. Injury can be divided into primary and secondary injuries. For patients with TBI admitted to the ICU, the management and prevention of secondary injury are most important. There is much debate surrounding many treatments in these patients. Management of TBI patients requires multidisciplinary approach, frequent close monitoring, and judicious use of multiple treatments to lessen secondary brain injury and improve outcomes. The management of STBI includes different approaches, which clearly requires the efforts of bedside nurses along with the other healthcare team in the hospital. While such management can be challenging, nurses should have enough knowledge and skill to provide quality care and to be competent in the healthcare sector.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American College of Surgeons. Best Practices in the Management of Traumatic Brain Injury. 2015. pp. 1–29. Available from: https://www.east.org/content/documents/45_podcast_acstqip_tbi_guidelines_2.pdf .
- 2.Lump D. Managing patients with severe traumatic brain injury. Nursing. 2014;44:30–7. doi: 10.1097/01.NURSE.0000443311.50737.a8. [DOI] [PubMed] [Google Scholar]
- 3.Das A, Botticello AL, Wylie GR, Radhakrishnan K. Neurologic disability: A hidden epidemic for India. Neurology. 2012;79:2146–7. doi: 10.1212/WNL.0b013e3182752cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy T, Bhatia P, Sandhu K, Prabhakar T, Gogna R. Secondary brain injury: Prevention and intensive care management. Indian J Neurotrauma. 2005;2:7–12. [Google Scholar]
- 5.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–30. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 7.Özztekin SD, Yüksel S. Initial nursing management of patient with severe traumatic brain injury. J Neurol Sci Turk. 2013;30:461–8. [Google Scholar]
- 8.Godbolt AK, Stenberg M, Jakobsson J, Sorjonen K, Krakau K, Stalnacke BM, et al. Subacute complications during recovery from severe traumatic brain injury: Frequency and associations with outcome. BMJ Open. 2015;5:e007208. doi: 10.1136/bmjopen-2014-007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb EM, Millis SR, Hanks RA. Comorbid disease in persons with traumatic brain injury: Descriptive findings using the modified cumulative illness rating scale. Arch Phys Med Rehabil. 2012;93:1338–42. doi: 10.1016/j.apmr.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Pangilinan PH, Kishner S. Classification and Complications of Traumatic Brain Injury. J Chem Inf Model. 2013;53:1–30. [Google Scholar]
- 11.Hemphill JC, Phan N. Management of acute severe traumatic brain injury. Uptodate. 2016:1–27. [Google Scholar]
- 12.Rusticali B, Villani R. Working Group. Treatment of minor and severe traumatic brain injury. National reference guidelines. Minerva Anestesiol. 2008;74:583–616. [PubMed] [Google Scholar]
- 13.Sheriff FG, Hinson HE. Pathophysiology and clinical management of moderate and severe traumatic brain injury in the ICU. Semin Neurol. 2015;35:42–9. doi: 10.1055/s-0035-1544238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao KH, Chang CK, Chang HC, Chang KC, Chen CF, Chen TY, et al. Clinical practice guidelines in severe traumatic brain injury in Taiwan. Surg Neurol. 2009;72(Suppl 2):S66–73. doi: 10.1016/j.surneu.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Puri BK. The role of proton neurospectroscopy in the assessment of brain function, estimation of coma duration, and prediction of outcome in severe traumatic brain injury. Neurol India. 2011;59:657–8. doi: 10.4103/0028-3886.86535. [DOI] [PubMed] [Google Scholar]
- 16.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 17.Nursing management of adults with severe traumatic brain injury. Glenview (IL): American Association of Neuroscience Nurses; 2008. American Association of Neuroscience Nurses; p. 20. [Google Scholar]
- 18.Zeng T, Gao L. Management of patients with severe traumatic brain injury guided by intraventricular intracranial pressure monitoring: A report of 136 cases. Chin J Traumatol. 2010;13:146–51. [PubMed] [Google Scholar]
- 19.Roberts I, Schierhout G. Hyper ventilation therapy for acute traumatic brain injury. Cochrane Database Syst Rev. 1997;4:CD000566. doi: 10.1002/14651858.CD000566. DOI: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protheroe RT, Gwinnutt CL. Early hospital care of severe traumatic brain injury. J Assoc Anaesth Gt Britain Irel. 2011;66:1035–47. doi: 10.1111/j.1365-2044.2011.06874.x. [DOI] [PubMed] [Google Scholar]
- 21.Jung JY. Airway management of patients with traumatic brain injury/C-spine injury. Korean J Anesthesiol. 2015;68:213–9. doi: 10.4097/kjae.2015.68.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentleman D, Dearden M, Midgley S, Maclean D. Guidelines for resuscitation and transfer of patients with serious head injury. BMJ. 1993;307:547–52. doi: 10.1136/bmj.307.6903.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talsky A, Pacione LR, Shaw T, Wasserman L, Lenny AM, Verma A, et al. Pharmacological interventions for traumatic brain injury. BC Med J. 2011;53:26–31. [Google Scholar]
- 24.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. doi: 10.1186/1757-7241-20-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachman T. Patient positioning: More than just “turn every 2h.”. Barrow Q. 2006;22:16–24. [Google Scholar]
- 26.Spiotta AM, Stiefel MF, Gracias VH, Garuffe AM, Kofke WA, Maloney-Wilensky E, et al. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010;113:571–80. doi: 10.3171/2010.1.JNS09506. [DOI] [PubMed] [Google Scholar]
- 27.Care SC. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805–11. doi: 10.3171/jns.2005.103.5.0805. [DOI] [PubMed] [Google Scholar]
- 28.Carlier P, Penrod LE. The use of moderate therapeutic hypothermia for patients with severe head injuries: A preliminary report. J Neurosurg. 1993;79:354–62. doi: 10.3171/jns.1993.79.3.0354. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Dong Y, Han X, Qi XQ, Huang CG, Hou LJ, et al. Nutritional support for patients sustaining traumatic brain injury: A systematic review and meta-analysis of prospective studies. PLoS One. 2013;8:e58838. doi: 10.1371/journal.pone.0058838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scrimgeour AG, Condlin ML. Nutritional treatment for traumatic brain injury. J Neurotrauma. 2014;31:989–99. doi: 10.1089/neu.2013.3234. [DOI] [PubMed] [Google Scholar]
- 31.Carney N, Totten AM, Reilly CO, Ullman JS, Bell MJ, Bratton SL. Brain Trauma Foundation TBI Guidelines. 4th ed. 2016. Guidelines for the Management of Severe Traumatic Brain Injury; pp. 1–10. [Google Scholar]
- 32.Khajavikhan J, Vasigh A, Kokhazade T, Khani A. Association between hyperglycaemia with neurological outcomes following severe head trauma. J Clin Diagn Res. 2016;10:11–3. doi: 10.7860/JCDR/2016/17208.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marek D, Helene B, Philipp H, Walter GH. Tracheostomy is associated with decreased hospital mortality after moderate or severe isolated traumatic brain injury. Wien Klin Wochenschr. 2016;128:397–403. doi: 10.1007/s00508-016-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev. 2012;12:CD000033. doi: 10.1002/14651858.CD000033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majdan M, Mauritz W, Wilbacher I, Brazinova A, Rusnak M, Leitgeb J, et al. Barbiturates use and its effects in patients with severe traumatic brain injury in five European Countries. J Neurotrauma. 2013;30:23–9. doi: 10.1089/neu.2012.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirmani BF, Mungall D, Ling G. Role of intravenous levetiracetam in seizure prophylaxis of severe traumatic brain injury patients. Front Neurol. 2013;4:170. doi: 10.3389/fneur.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemke DM. Sympathetic storming after severe traumatic brain injury. Crit Care Nurse. 2007;27:30–7. [PubMed] [Google Scholar]
- 38.Baltopoulos GJ. Neuroprotective effects of erythropoietin in patients with severe closed brain injury. Turk Neurosurg. 2015;25:552–8. doi: 10.5137/1019-5149.JTN.9685-14.4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Wang X, Li Y, Du R, Xu E, Dong L, et al. Naloxone for severe traumatic brain injury: A meta-analysis. PLoS One. 2014;9:e113093. doi: 10.1371/journal.pone.0113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouello G, Hamel O, Asehnoune K, Bord E, Robert R, Buffenoir K, et al. Study of the long-term results of decompressive craniectomy after severe traumatic brain injury based on a series of 60 consecutive cases. ScientificWorldJournal. 2014;2014:207585. doi: 10.1155/2014/207585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahuquillo J. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006:CD003983. doi: 10.1002/14651858.CD003983.pub2. DOI: 10.1002/14651858.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Lee SK, Goh JP. Neuromonitoring for traumatic brain injury in neurosurgical intensive care. Proc Singapore Healthc. 2010;19:319–33. [Google Scholar]
- 43.Aries MJ, van der Naalt J, van den Bergh WB, van Dijk M, Elting JW, Czosnyka M, et al. Neuromonitoring of patients with severe traumatic brain injury at the bedside. Netherlands J Crit Care. 2015;20:6–12. [Google Scholar]
- 44.Ristic A, Sutter R, Steiner LA. Current neuromonitoring techniques in critical care. J Neuroanaesth Crit Care. 2015;2:97–103. [Google Scholar]
- 45.Schlosser HG, Lindemann JN, Vajkoczy P, Clarke AH. Vestibulo-ocular monitoring as a predictor of outcome after severe traumatic brain injury. Crit Care. 2009;13:R192. doi: 10.1186/cc8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonneville R. Prevention of pneumonia after severe traumatic brain injury. Lancet Respir Med. 2014;2:10–2. doi: 10.1016/S2213-2600(14)70123-7. [DOI] [PubMed] [Google Scholar]
- 47.Manju D, Dhandapani S, Agarwal M. Pressure ulcer in patients with severe traumatic brain injury: Significant factors and association with neurological outcome. J Clin Nurs. 2013;23:3–17. doi: 10.1111/jocn.12396. [DOI] [PubMed] [Google Scholar]
- 48.Cooper KL. Evidence-based prevention of pressure ulcers in the intensive care unit. Crit Care Nurse. 2013;33:57–66. doi: 10.4037/ccn2013985. [DOI] [PubMed] [Google Scholar]
- 49.Lo E, Nicolle L, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S41–50. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 50.Salierno F, Rivas ME, Etchandy P, Jarmoluk V, Cozzo D, Mattei M, et al. Physiotherapeutic procedures for the treatment of contractures in subjects with traumatic brain injury (TBI) Traumatic Brain Injury. InTechOpen. 2014:307–28. [Google Scholar]
- 51.Abdel-Aziz H, Dunham CM, Malik RJ, Hileman BM. Timing for deep vein thrombosis chemoprophylaxis in traumatic brain injury: An evidence-based review. Crit Care. 2015;19:96. doi: 10.1186/s13054-015-0814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demuro JP, Hanna AF. Prophylaxis of deep venous thrombosis in trauma patients: A review. J Blood Disord Transfus. 2013;4:151. [Google Scholar]
- 53.Chelladurai Y, Stevens KA, Haut ER, Brotman DJ, Sharma R, Shermock KM, et al. Venous thromboembolism prophylaxis in patients with traumatic brain injury: A systematic review. F1000Res. 2013;2:132. doi: 10.12688/f1000research.2-132.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paydar S, Sabetian G, Khalili HA, Fallahi J, Tahami M, Ziaian B, et al. Management of deep vein thrombosis (DVT) prophylaxis in trauma patients. Bull Emerg Trauma. 2016;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 55.Wertheimer JC, Hanks RA, Hasenau DL. Comparing functional status and community integration in severe penetrating and motor vehicle-related brain injuries. Arch Phys Med Rehabil. 2008;89:1983–90. doi: 10.1016/j.apmr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Sinha S, Gunawat P, Nehra A, Sharma BS. Cognitive, functional, and psychosocial outcome after severe traumatic brain injury: A cross-sectional study at a tertiary care trauma center. Neurol India. 2013;61:501–6. doi: 10.4103/0028-3886.121920. [DOI] [PubMed] [Google Scholar]
- 57.Steiner E, Murg-Argeny M, Steltzer H. The severe traumatic brain injury in Austria: Early rehabilitative treatment and outcome. J Trauma Manag Outcomes. 2016;10:5. doi: 10.1186/s13032-016-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker A, Sricharoenchai T, Needham DM. Early rehabilitation in the intensive care unit: Preventing physical and mental health impairments. Curr Phys Med Rehabil Rep. 2013;1:307–14. doi: 10.1007/s40141-013-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mercier E, Boutin A, Lauzier F, Fergusson DA, Simard JF, Zarychanski R, et al. Predictive value of S-100β protein for prognosis in patients with moderate and severe traumatic brain injury: Systematic review and meta-analysis. BMJ. 2013;346:f1757. doi: 10.1136/bmj.f1757. [DOI] [PubMed] [Google Scholar]
- 60.Goyal A, Failla MD, Niyonkuru C, Amin K, Fabio A, Berger RP, et al. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J Neurotrauma. 2013;30:946–57. doi: 10.1089/neu.2012.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egea-Guerrero JJ, Murillo-Cabezas F, Gordillo-Escobar E, Rodríguez-Rodríguez A, Enamorado-Enamorado J, Revuelto-Rey J, et al. S100B protein may detect brain death development after severe traumatic brain injury. J Neurotrauma. 2013;30:1762–9. doi: 10.1089/neu.2012.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stenberg M, Godbolt AK, Nygren De Boussard C, Levi R, Stalnacke BM. Cognitive impairment after severe traumatic brain injury, clinical course and impact on outcome: A Swedish-Icelandic study. Behav Neurol. 2015;2015:680308. doi: 10.1155/2015/680308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jourdan C, Bayen E, Pradat-Diehl P, Ghout I, Darnoux E, Azerad S, et al. Acomprehensive picture of 4-year outcome of severe brain injuries. Results from the pariS-TBI study. Ann Phys Rehabil Med. 2016;59:100–6. doi: 10.1016/j.rehab.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Kalmar K, Childs N, Khademi A, Eifert B, Long D, Katz DI, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–26. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 65.Gardani M, Morfiri E, Thomson A, O'Neill B, McMillan TM. Evaluation of sleep disorders in patients with severe traumatic brain injury during rehabilitation. Arch Phys Med Rehabil. 2015;96:1691–7e3. doi: 10.1016/j.apmr.2015.05.006. [DOI] [PubMed] [Google Scholar]


