Abstract
Amyloidosis is a disease characterized by the extracellular deposition of the protein amyloid. It is a multiorgan disease, and cardiac involvement is not uncommon, generally in the form of a restrictive cardiomyopathy. Typical aspects of cardiac amyloidosis have been described at echocardiography and magnetic resonance imaging (MRI). In particular, the relative apical sparing at two-dimensional speckle-tracking echocardiography has been reported to be specific for cardiac amyloidosis. In our case, we report for the first time that this echocardiographic sign is related to lack of hyperenhancement at late gadolinium enhancement imaging in cardiac MRI.
Keywords: Apical sparing, magnetic resonance imaging, speckle-tracking echocardiography, strain
INTRODUCTION
Cardiac amyloidosis is generally characterized by an infiltrative restrictive cardiomyopathy and may be suspected using conventional echocardiography. Contrast cardiac magnetic resonance imaging (MRI) is generally used to confirm this diagnosis showing myocardial enhancement related to interstitial amyloid deposition. Recently, two-dimensional speckle-tracking echocardiography (2D-STE) has been shown to increase the diagnostic capability of ultrasound in cardiac amyloidosis, evidencing preservation of longitudinal systolic shortening at the left ventricle (LV) apex.[1,2] However, a relationship between the 2D-STE relative apical sparing and lack of late enhancement at contrast MRI has poorly been documented so far.
CASE REPORT
A 70-year-old male patient, with a history of pharmacologically treated arterial hypertension, was evaluated by echocardiography because of effort dyspnea. The patient had a recent diagnosis of multiple myeloma and was waiting for chemotherapy. Furthermore, the biopsy of a lip nodule showed amyloid deposition in soft tissues. Standard echocardiography showed symmetric increase in LV wall thickness with a sparkling appearance, diffuse hypokinesia, and moderate-to-severe reduction of ejection fraction. These signs indicated suspected cardiac amyloidosis, so further investigations with 2D-STE and cardiac MRI were undertaken for diagnostic confirmation. The 2D-STE examination showed a relatively preserved longitudinal systolic function at the LV apex in contrast with reduced longitudinal shortening at basal level [Figure 1]. Global longitudinal strain was − 8.3%. Cardiac MRI confirmed the diffuse symmetric increased thickness of the LV walls and the moderate-to-severe impairment of LV systolic function. After contrast administration, diffuse hyperenhancement was observed except than at the LV apical level, where subepicardial enhancement was relatively absent [Figure 2]. Therefore, the final diagnosis of cardiac amyloidosis with relative sparing of the LV apex was made.
Figure 1.
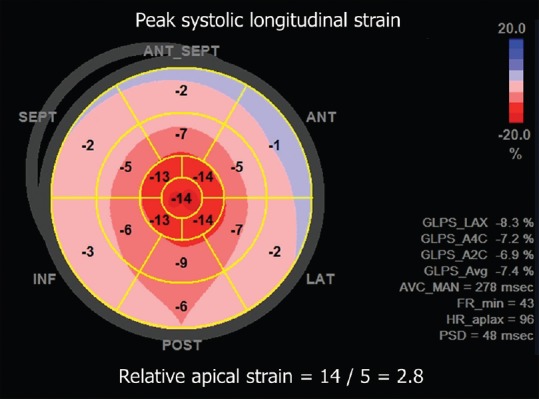
Color-coded polar diagram obtained by speckle-tracking echocardiography showing the distribution of peak systolic longitudinal strain values throughout the myocardium. There is progressive impairment of longitudinal shortening from the apex (central area, red color) to the basal left ventricular segments (external areas, pink color). This pattern is known as relative apical sparing
Figure 2.
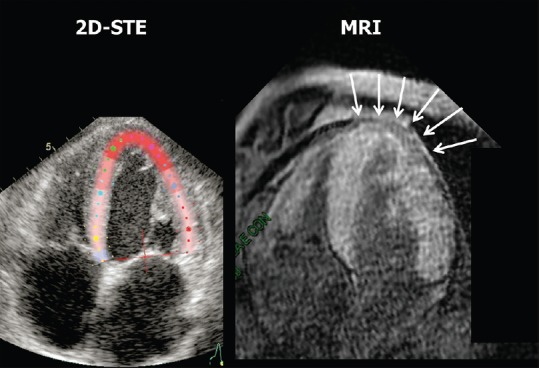
Comparison between the two-dimensional speckle-tracking echocardiography functional image of the left ventricle and contrast magnetic resonance imaging. The magnetic resonance imaging T1 late gadolinium enhancement (fast-gradient-echo inversion-recovery) image was acquired 10 min after bolus intravenous injection of 0.15 mmol/kg body weight of gadolinium-diethylenetriamine pentaacetic acid (gadobutrol, Schering, Germany), followed by saline flush. The inversion time was adapted to null normal myocardium. The arrows on the magnetic resonance imaging image indicate an apical area with relative absence of contrast uptake, which corresponds to the red color at the apex of the left ventricle on the two-dimensional speckle-tracking echocardiography functional image
DISCUSSION
The noninvasive diagnosis of cardiac amyloidosis has been recently improved with the introduction of 2D-STE.[1,2] In particular, this technique may help to differentiate cardiac amyloidosis from other causes of myocardial hypertrophy such as hypertension, hypertrophic cardiomyopathy, Fabry disease, and aortic stenosis, evidencing a much greater restriction of basal compared to apical LV longitudinal systolic deformation.[1,3] This peculiar pattern of LV myocardial longitudinal shortening has been called “relative apical sparing.”[1] Phelan et al.[1] also provided a quantitative index of the relative apical longitudinal deformation, which is calculated as the ratio between the average apical longitudinal systolic strain and the average of basal and mid-ventricle longitudinal systolic strain. A cutoff value of 1 is capable to differentiate cardiac amyloidosis from other LV hypertrophic phenotypes with good sensitivity and specificity.[1] Cardiac MRI is also helpful for the diagnosis of cardiac amyloidosis, especially with administration of gadolinium. This is a paramagnetic contrast that has a late washout in tissues with augmented interstitial space, as in the case of cardiac amyloidosis. A diffuse subendocardial late enhancement at cardiac MRI after gadolinium injection is highly characteristic of cardiac amyloidosis and correlates with prognosis.[4,5]
Our case confirms the fundamental role of multimodality imaging for diagnosis and functional evaluation of cardiac amyloidosis, as also pointed out by other authors.[1] Furthermore, this case demonstrates, for the first time, a relationship between the pattern of gadolinium distribution at cardiac MRI and the functional relative apical sparing at 2D-STE. Both techniques, in fact, showed a similar nonhomogeneous behavior of regional longitudinal systolic function and contrast uptake at the apical and basal LV segments. Future studies are indicated to expand this observation and clarify the reason of the relative apical sparing in cardiac amyloidosis. Furthermore, differentiation of cardiac amyloidosis from LV hypertrophy with preserved LV ejection fraction on the basis of 2D-STE findings[6,7] is an additional challenging task to be further explored.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–8. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 2.Schiano-Lomoriello V, Galderisi M, Mele D, Esposito R, Cerciello G, Buonauro A, et al. Longitudinal strain of left ventricular basal segments and E/e’ ratio differentiate primary cardiac amyloidosis at presentation from hypertensive hypertrophy: An automated function imaging study. Echocardiography. 2016;33:1335–43. doi: 10.1111/echo.13278. [DOI] [PubMed] [Google Scholar]
- 3.D’Andrea A, Radmilovic J, Ballo P, Mele D, Agricola E, Cameli M, et al. Left ventricular hypertrophy or storage disease? the incremental value of speckle tracking strain bull's-eye. Echocardiography. 2017;34:746–59. doi: 10.1111/echo.13506. [DOI] [PubMed] [Google Scholar]
- 4.Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 5.Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2:1369–77. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Di Bella G, Pizzino F, Minutoli F, Zito C, Donato R, Dattilo G, et al. The mosaic of the cardiac amyloidosis diagnosis: Role of imaging in subtypes and stages of the disease. Eur Heart J Cardiovasc Imaging. 2014;15:1307–15. doi: 10.1093/ehjci/jeu158. [DOI] [PubMed] [Google Scholar]
- 7.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]


