Abstract
Introduction
The biosimilar product Inflectra® has been approved by the European Medicine Agency (EMA) for the same indications as its reference drug, infliximab, based on studies in patients with rheumatic diseases. Thus far, there have not been enough data regarding its efficacy and safety in ulcerative colitis (UC).
Aim
To assess the efficacy and safety of the biosimilar product Inflectra® in comparison with its reference biological agent (Remicade®) in rescue therapy in adult patients presenting with severe exacerbation of UC, as well as to evaluate recurrence rate during a 6-month observation after finish of treatment.
Material and methods
In a single-centre retrospective study, a cohort of 83 adult patients with severe UC treated at the Department of Gastroenterology with Inflammatory Bowel Diseases Subdivision of the Central Clinical Hospital of MSWiA, Warsaw was investigated. All patients received three induction doses of Remicade® (28 individuals) or Inflectra® (55 individuals) based on the same criteria of the National Health Fund (NFZ) Therapeutic Program (total Mayo score > 6). Activity of the disease was evaluated on the Mayo scale at qualification, after finishing the rescue treatment (after 14 weeks), and after a 6-month observation period. In all patients, sigmoidoscopy was performed at qualification and after induction (after three doses).
Results
The studied groups were similar with respect to age and sex distribution, duration of the disease, extent of the disease (left-sided type, pancolitis), additional pharmacotherapy, and smoking. Clinical response following three induction doses was noted in 81% of patients receiving Remicade® compared to 77% receiving the biosimilar product, Inflectra® (NS); while clinical remission was observed in 42% receiving Remicade® and 32% receiving Inflectra® (NS), respectively. Endoscopic remission assessed as 0 on the Mayo scale was achieved in 4 (15%) patients on Remicade® and in 7 (13%) patients on Inflectra® (p = 0.45). Relapse occurred in 68% of all patients, while 51% presented with exacerbation of the disease 3 months after finishing biological treatment. In 93%, exacerbation occurred within 12 months. The recurrence rate was similar in both groups (75% with Remicade®, 64% with Inflectra®, respectively). Side effects occurred with similar frequency in both groups.
Conclusions
In the study, it was established that the biosimilar drug (Inflectra®) has a similar efficacy and safety as the reference biological agent (Remicade®), not only in rescue therapy, but also during a 6-month observation period in adult patients with severe UC. Low mucosal healing rate in both groups and high recurrence rate of the disease soon after finishing induction treatment indicate the need for prolonged therapy with infliximab in patients with severe UC.
Keywords: inflammatory bowel disease, ulcerative colitis, biological treatment, biosimilar drug, endoscopic remission
Introduction
Ulcerative colitis (UC) is classified as an inflammatory bowel disease (IBD) together with Crohn’s disease and indeterminate colitis. This group of diseases is characterised by multifactorial etiopathogenesis – including genetic predisposition, immunological disorders, and environmental factors; however, the relationships between them are still unknown [1, 2]. Ulcerative colitis incidence in Europe is about 10 new cases per 100 thousand people annually and still rising, especially in young adults [1–3]. In UC, chronic inflammation of the mucous membrane of the large intestine can be observed. It is characteristic that the lesions are continuous, starting from the rectum, and that there are alternating periods of exacerbation and remission [1, 4]. Depending on the extent of lesions, three types of UC can be distinguished: proctitis (E1 according to the Montreal classification), left-sided UC (E2 – lesions extending from the rectum to the splenic flexure), or full involvement of the large intestine (E3 – extending from the rectum to the colon proximally to the splenic flexure) [5, 6]. The activity of the diseases is assessed based on both clinical and endoscopic criteria. The most commonly used scale is the Mayo scale, which includes both elements [5]. According to the ECCO criteria, severe UC is defined as the presence of six or more loose stools with blood per day (obligatory criterion) and tachycardia (> 90/min) or high temperature (> 37.8°C), or anaemia (haemoglobin < 10.5 g/dl), or elevated erythrocyte sedimentation rate – ESR > 30 mm/h. Early recurrence is defined as symptoms occurring less than 3 months since the last remission of UC [5].
The main goal of therapy is to reach clinical remission, confirmed on endoscopy and not requiring steroids. In patients receiving basic treatment (mesalazine, steroids, thiopurines), in whom steroid-free remission has not been achieved despite therapeutic doses of thiopurines, as well as in patients who do not tolerate immunosuppressive treatment or in whom it is contraindicated, biological therapy with infliximab, an anti-TNF-a agent, should be considered [2]. Biological treatment in rescue therapy reimbursed by the NFZ in Poland included three doses of infliximab. After approval of a biosimilar drug to infliximab by the EMA, the reference drug, Remicade®, and biosimilar drugs, Inflectra® or Remsima, have been interchangeably used at various facilities [2].
Aim
The aim of the study was to investigate the efficacy and safety of the biosimilar product (Inflectra®) compared to the reference drug (Remicade®) in rescue therapy in adult patients with acute severe ulcerative colitis, and to assess the recurrence rate during a 6-month observation period. The following were evaluated in both groups:
- Reaching:
- clinical response,
- clinical remission,
- mucosal healing.
Assessment of relapse rate and need for repeated biological treatment.
Side effects of biological treatment.
Material and methods
The studied group consisted of 83 patients with UC hospitalised in the Department of Internal Medicine and Gastroenterology of the Central Clinical Hospital of the MSWiA in Warsaw between March 2013 and September 2015. All patients were enrolled into the NFZ Therapeutic Program, according to the same criteria for rescue therapy. For the study, patients older than 18 years were enrolled presenting with severe ulcerative colitis, in whom cyclosporine was not indicated or even contraindicated: with insufficient response to standard treatment including corticosteroids with 6-mercaptopurine (6-MP) or azathioprine (AZA) (score > 6 points on the Mayo score), or not tolerating treatment with corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA), or having contraindications for treatment with corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA). Ineffectiveness of standard treatment of severe UC was defined as failure of a 3- to 5-day course of intravenous steroids.
Patients were given either of two medications: Remicade® (28 patients) or Inflectra® (55 patients). The activity of the disease was assessed at the qualification for treatment, after the finish of rescue treatment, and after 6 and 12 months of observation.
In each patient, sigmoidoscopy was performed at the moment of qualification, as well as after finishing the induction treatment (after three doses). For endoscopic and clinical evaluation, the Mayo score was applied.
Four patients (two from each group) were excluded from the analysis due to lack of evaluation of mucosal healing after the finish of therapy.
Clinical remission was defined as obtaining a maximum of two points in the Mayo score after treatment; however, no component could exceed one point. Clinical response was defined as a three-point decrease, compared to initial rating in the Mayo score (at least 30% overall), with at least one point reduction for rectal bleeding, which at the same time could not exceed one point after treatment. Endoscopic remission was defined as reaching the physiological image of the mucous membrane – zero points in the Mayo score.
Statistical analysis
For statistical analysis, descriptive statistics were used including the median, 25th and 75th percentiles, and mean values with standard deviation and range, Student’s t-test for differences between groups for quantitative variables with normal distribution, U-Mann-Whitney test for variables with normal distribution, and c2 test. Statistical significance was assumed for p < 0.05.
Results
No statistically significant differences between the two groups (Remicade® or Inflectra®) were noted with respect to age, gender, disease duration, extension of lesions (pancolitis or left-sided colitis), additional drugs, and tobacco smoking (Table I).
Table I.
Characteristics of the studied group
| Parameter | % | N |
|---|---|---|
| Female | 37 | 31 |
| Male | 63 | 52 |
| Disease duration < 10 years | 76 | 63 |
| History of biological treatment | 24 | 20 |
| Pancolitis ulcerative colitis | 69 | 57 |
| Left-sided ulcerative colitis | 31 | 26 |
| Extraintestinal manifestations | 33 | 27 |
| Tobacco smoking | 5 | 4 |
| Steroid-dependence | 96 | 80 |
| Steroid-resistance | 4 | 3 |
| Steroids during biological treatment | 76 | 63 |
In the patients, clinical response was observed in 78%, while clinical remission was observed in 35% (Table II). Mucosal healing, defined as 0–1 Mayo score, was reported in 36 patients, which was 46% of the total number of patients. Full endoscopic remission, defined as 0 in Mayo score was observed only in 11 patients, which was 13.9% of all patients.
Table II.
Efficacy of induction with infliximab in the entire population of patients
| Parameter | % of patients (n) |
|---|---|
| Clinical response | 78 (62) |
| Clinical remission | 35 (28) |
| Mucosal healing – Mayo 0–1 | 46 (36) |
| Mucosal healing – Mayo 0 | 14 (11) |
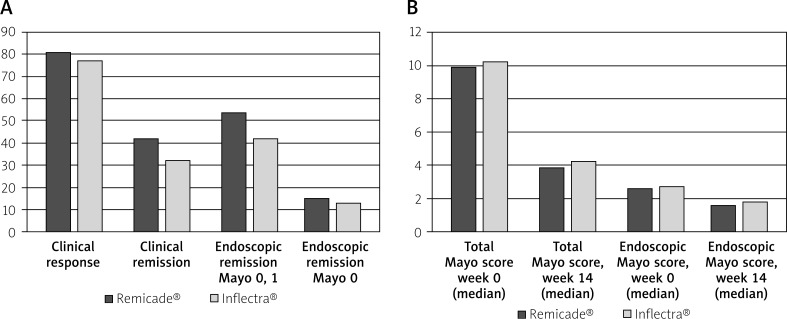
Statistically significant differences between groups treated with Remicade® vs. Inflectra® (Figures 1 A–B), regarding: clinical response (81% vs. 77%), p > 0.05; clinical remission (42% vs. 32%), p > 0.05; mucosal healing (15% vs. 13%), p > 0.05; endoscopic remission (13.8% vs. 13.2%), p > 0.05 were not observed.
Figure 1.
A – Efficacy of induction treatment – comparison of both drugs, B – efficacy of induction treatment – comparison of both drugs according to Mayo score criteria
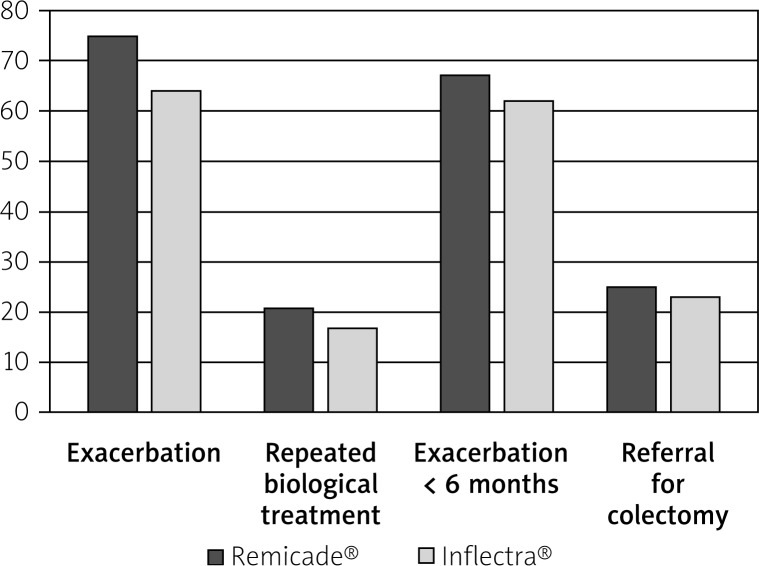
Relapse occurred in 68% of patients (Table III A). It must he highlighted that about half of the patients (51%) reported relapse very fast – within 3 months of finishing treatment. In the long-term perspective, exacerbation occurred in almost all (91%) of the studied patients within 12 months after finishing biological treatment. In both groups, the relapse rate was similar (75% Remicade®, 64% Inflectra®) (Table III A, Figure 2).
Table III.
Follow-up of all patients – 6-month observation after induction treatment (A), follow-up with respect to the drug used – 6-month observation after induction treatment (B)
| A | ||
|---|---|---|
| Patient with flare after treatment | % | N = 55/81 |
| Within 3 months | 51 | 28/55 |
| Within 6 months | 64 | 35/55 |
| Within 12 months | 93 | 51/55 |
| Treatment of flare | ||
| Repeated steroids | 73 | 40/55 |
| Surgery | 9 | 7/55 |
| Repeated biological treatment | 19 | 15/55 |
| Clinical study | 11 | 9/55 |
Figure 2.
Follow-up with respect to the drug used – 6-month observation after induction treatment
| B | |||||
|---|---|---|---|---|---|
| Variable | Remicade® (n = 28) | Inflectra® (n = 55) | P-value | ||
| Exacerbation | 75% | 21 | 64% | 34 | 0.64 |
| Repeated biological treatment | 21% | 6 | 17% | 9 | 0.83 |
| Exacerbation < 6 months | 67% | 14 | 62% | 21 | 0.86 |
| Referral for colectomy | 25% | 7 | 23% | 12 | 0.90 |
Statistically significant differences concerning the safety of both drugs have not been proven. Complications during induction treatment were observed in 7 patients from both groups, and they occurred with similar rates in both groups (Table IV). Allergic reactions occurred in 3 patients from the group treated with Inflectra® (5.5% of the treated patients) and in 1 patient the from the group treated with Remicade® (3.5% of the treated patients). All four patients presenting with allergic reaction were treated with anti-TNF drug at least once in the past. Psoriasis induced by Infliximab occurred in 1 patient treated with Inflectra and 1 patient treated with Remicade®. One patient treated with Inflectra® was diagnosed with serum sickness. Statistically significant differences between the drugs have not been proven.
Table IV.
Adverse reactions during induction treatment
| Parameter | Remicade® (n = 28) | Inflectra® (n = 55) |
|---|---|---|
| Allergic reactions | 3.5% | 5.5% |
| Psoriasis | 3.5% | 1.8% |
| Serum sickness | 0 | 1.8% |
Discussion
Biosimilar drugs, after being accepted by the EMA, were introduced to the Polish market in 2015 for both children and adults. The first study evaluating their efficacy and safety was published by Sieczkowska et al. in JCC [7].
The pharmacology of biopharmaceuticals is definitely more complicated than other drugs routinely used in IBD. They are complex, three-dimensional molecules with high molecular weight produced by living cells. The production is complex and multi-stage, and furthermore, it is not possible to produce a drug that is 100% identical to the reference agent [8, 9]. Therefore, biosimilars should not be thought of as simple generic drugs – each drug has different biological properties, particularly immunogenicity [8–10]. For that reason, immunological issues were raised before introduction to clinical practice, especially considering the safety – rate of side-effects, particularly of severe allergic reactions. Another aspect is the potential to produce antibodies against the drug, which prove important in the case of supportive therapy (secondary loss of response). All of the above-mentioned aspects resulted in physicians being anxious to use them in everyday practice, which has been proven in a study on German gastroenterologists [11]. On the other hand, biosimilar products have been successfully used in other fields of medicine, especially in rheumatology. During registration of the biosimilar in Europe, the indications have been extrapolated from positive study results on patients with rheumatic diseases.
The results of our study prove that the biosimilar product is as effective in inducing remission of severe ulcerative colitis. The results of the most recent observational studies have been presented in the meta-analysis published in Alimentary Pharmacology & Therapeutics in 2017 [12], which included a dozen studies on the efficacy of biosimilar drugs both in UC and CD. The analysis covered over 800 patients, and the safety profile was investigated. The clinical response in week 14 was obtained in 74% of patients, and our result was very similar (77%). In other studies from the last year, a slightly higher rate of clinical remission in week 14 was reported when using the biosimilar (56%, Jahnsen et al.), although in a small group of 32 patients [13], and 47% (Farkas et al.) in a group of 63 patients [14]. An important parameter is also full healing of the mucosa in week 13, based on which the effectiveness of treatment is more objective and the prognosis is made as to the development of the disease. In the last study, the parameter was higher compared to our results (27%, Farkas et al.), at 13 and 15%, respectively (assuming mucosal healing equivalent to a Mayo score of 0 pts). Also, Czech researchers assessed a smaller group of patients (n = 29) twice and proved a higher mucosal healing rate – 27.7% [15].
The lower efficacy was probably due to the fact that patients with more severe disease are enrolled in the NFZ Therapeutic Program. However, it is important that we did not observe differences in effectiveness between the two products. Also, Irish authors reported similar efficacy of the original drug and the biosimilar this year in an observational study in JCC [16]. An additional aspect was the safety of the biosimilar compared to the reference drug. The side effect rate was estimated to be 8% in the previously mentioned meta-analysis and 7% in our study (allergic reaction in patients who had previously been treated with anti-TNF agents). It was important that we did not observe any statistically significant differences in complication rate between the two groups. Also, the data presented by us are not sufficient to assess long-term outcomes of the biosimilar, and we did not evaluate immunogenicity, which is important for potential secondary loss of response.
Application of biosimilars in everyday practice also has a strong influence on pharmacoeconomics [17, 18]. The decision to use infliximab is, in Poland, made by intrahospital tender, while in other countries such as Norway, the tender is conducted at the national level by national institutions. Introduction of a biosimilar to the Scandinavian market heavily influenced IBD therapy in Norway, Denmark, and Sweden [19]. In Norway, the biosimilar is 60% cheaper than the reference drug. In the 3 years since its introduction, it reached 90% of Norwegian market, similarly to Denmark. In 2015, the Norwegian healthcare system managed to save 13.2 million euro due to the lowered price of the biosimilar and 3.9 million euro by lowering the cost of the reference drug. Another objective argument in favour of biosimilars is the fact that in 2016, 25% more ampules of the biosimilar were purchased compared to 2015 [19]. It definitely affected the accessibility to treatment and greater elasticity in long-term supportive treatment. We should bear in mind that those are among the richest countries in the world with immense financing of the healthcare system per capita.
Polish patients enrolled in the NFZ Therapeutic Program are characterised by severe course of the disease compared to patients in Western Europe, where the access to treatment is easier, quicker, and less regulated. Therefore, it is cost-effective to prolong treatment because only a small percentage of patients reach mucosal healing, which is one of the most important risk factors. Based on our observations, in 93% of patients the relapse occurred within 12 months of finishing induction treatment. The lack of possible long-term treatment means that, in patients with severe course of the disease, the biological treatment can be administered only after the period determined by the NFZ (16 weeks since last dose), which can lead to ineffectiveness of treatment or allergic reactions [8–10].
It should be remembered that the lack of the achievement of mucosal healing is associated with higher colectomy rate in the first year, which results in higher costs of hospitalisations, potential surgical interventions, and absence from work reaching months or sometimes leading to a lay-off [20, 21]. Considering long-term observations, costs are even higher compared to the drug itself, not even mentioning the patient’s quality of life [22–24], risk of cancer, declining fertility rate, etc. Introduction of biosimilars indirectly affects all the presented aspects, as previously shown in the Nordic countries.
Based on the data presented, we can confirm the efficacy and safety of short-term UC therapy with the biosimilar compared to the reference drug, not only in induction but also during a 6-month observation. At the same time, it must be concluded that lower mucosal healing rate in both groups and high recurrence rate shortly after finishing the induction treatment indicate the need for prolonged therapy in patients with severe UC.
Conflict of interest
This study was not supported by pharmaceutical industry. Prof. Rydzewska and Dr Kaniewska were receiving honoraria for continuing educational activities from MSD, Abbvie and Alvogen.
References
- 1.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;3:1606–19. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Eder P, Łodyga M, Łykowska-Szuber L, et al. Guidelines for the management of ulcerative colitis. Recommendations of the Working Group of the Polish National Consultant in Gastroenterology and the Polish Society of Gastroenterology. Prz Gastroenterol. 2013;8:1–20. doi: 10.5114/pg.2023.125882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–8. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartnik W. Wytyczne postępowania w nieswoistych chorobach zapalnych jelit. Prz Gastroenterol. 2007;2:215–29. [Google Scholar]
- 5.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 7.Sieczkowska J, Jarzębicka D, Banaszkiewicz A, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary observations. J Crohns Colitis. 2016;10:127–32. doi: 10.1093/ecco-jcc/jjv233. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann M, Covic A. The protein science of biosimilars. Nephrol Dial Transplant. 2006;21(Suppl. 5):v4–8. doi: 10.1093/ndt/gfl474. [DOI] [PubMed] [Google Scholar]
- 9.Morrow T, Felcone LH. Defining the difference: what makes biologics unique. Biotechnol Healthc. 2004;1:24–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Weise M, Kurki P, Wolff-Holz E, et al. Biosimilars: the science of extrapolation. Blood. 2014;124:3191–6. doi: 10.1182/blood-2014-06-583617. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan E, Piercy J, Waller J, et al. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One. 2017;12:e0175826. doi: 10.1371/journal.pone.0175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komaki Y, Yamada A, Komaki F, et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-alpha agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043–57. doi: 10.1111/apt.13990. [DOI] [PubMed] [Google Scholar]
- 13.Jahnsen J, Detlie TE, Vatn S, Ricanek P. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol. 2015;9(Suppl 1):45–52. doi: 10.1586/17474124.2015.1091308. [DOI] [PubMed] [Google Scholar]
- 14.Farkas K, Rutka M, Golovics PA, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016;10:1273–8. doi: 10.1093/ecco-jcc/jjw085. [DOI] [PubMed] [Google Scholar]
- 15.Kolar M, Duricova D, Bortlik M, et al. Infliximab biosimilar (Remsima™) in therapy of inflammatory bowel diseases patients: experience from one tertiary inflammatory bowel diseases centre. Dig Dis. 2017;35:91–100. doi: 10.1159/000453343. [DOI] [PubMed] [Google Scholar]
- 16.Harkin G, Keogh A, Slattery E. The effect of biosimilars [Inflectra®] in the management of acute severe ulcerative colitis. J Crohns Colitis. 2017 doi: 10.1093/ecco-jcc/jjx059. jjx059. [DOI] [PubMed] [Google Scholar]
- 17.Rydzewska G, Lipinski M. Biosimilars in gastroenterology – an important moment in the treatment of inflammatory bowel diseases. Int J Gastroenterol Disord Ther. 2016;3:124. [Google Scholar]
- 18.Parasomthy S, Cleveland NK, Zmeter N, et al. The role of biosimilars in inflammatory bowel disease. Gastroenterol Hepat. 2016;12:741–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Jahnsen J, Jorgensen KK. Experience with biosimilar Infliximab (Remsima®) in Norway. Dig Dis. 2017;35:83–90. doi: 10.1159/000449088. [DOI] [PubMed] [Google Scholar]
- 20.Meder A, Świątkowski M, Meder G, et al. Treatment costs for the group of patients with non-specific inflammatory bowel disease during acute exacerbation and further annual observation. Prz Gastroenterol. 2011;6:36–44. [Google Scholar]
- 21.Petryszyn P, Witczak I. Costs in inflammatory bowel diseases. Prz Gastroenterol. 2016;11:6–13. doi: 10.5114/pg.2016.57883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrowolska-Zachwieja A, Jakubowska-Burek L. Korzyści leczenia biologicznego u osób z nieswoistymi chorobami zapalnymi jelit. Prz Gastroenterol. 2010;5:68–76. [Google Scholar]
- 23.Stawowczyk E, Kawalec P. Cost-effectiveness of biological treatment of ulcerative colitis – a systematic review. Prz Gastroenterol. 2017;12:90–7. doi: 10.5114/pg.2017.68166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morys JM, Kaczówka A, Jeżewska M. Assessment of selected psychological factors in patients with inflammatory bowel disease. Prz Gastroenterol. 2016;11:47–53. doi: 10.5114/pg.2015.52560. [DOI] [PMC free article] [PubMed] [Google Scholar]