Abstract
Introduction
N-terminal propeptide of procollagen type III (PIIINP) is generated during the synthesis of type III collagen. PIIINP can be measured in the serum as an indicator of liver fibrosis and cirrhosis.
Aim
To evaluate the effect of liver diseases of different aetiologies and clinical severity of liver cirrhosis on the serum level of PIIINP.
Material and methods
Patients with alcoholic cirrhosis (AC) – 63 subjects, non-alcoholic cirrhosis (NAC) – 31 and toxic hepatitis (HT) – 33 were studied. Cirrhotic patients were classified according to the Child-Pugh scale. The samples were analysed using the ELISA method.
Results
The level of PIIINP was significantly higher in patients with alcoholic cirrhosis, non-alcoholic cirrhosis, and toxic hepatitis in comparison to the control group. There were no significant differences in the serum PIIINP levels between liver diseases and according to the severity of liver cirrhosis. PIIINP has the highest diagnostic power for the diagnosis of toxic hepatitis. The highest sensitivity was reached in alcoholic cirrhosis, but other diagnostic values (specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy (ACC)) in alcoholic cirrhosis were lower than that in toxic hepatitis. In the diagnosis of non-alcoholic cirrhosis PIIINP has low sensitivity, specificity, PPV, NPV, and ACC.
Conclusions
The serum PIIINP shows the alterations in liver diseases in comparison to healthy controls, but not between diseases. Taking the above into account we can suggest that PIIINP may be a useful test for the detection of liver diseases.
Keywords: N-terminal propeptide of procollagen type III, liver cirrhosis, toxic hepatitis, non-invasive markers
Introduction
Liver biopsy is recommended as the “gold standard” for the detection of liver diseases. However, liver biopsy is an invasive procedure with a risk of complications, and its sensitivity in diagnosing of cirrhosis is not absolute and sampling error is very common [1, 2]. Therefore, the laboratory tests (non-invasive) would be useful in the diagnosis of liver fibrosis and cirrhosis. The process of liver fibrosis is assumed to be caused by the excess production of extracellular matrix (ECM) in hepatic stellate cells (HSCs) [3]. The increase of ECM deposited fragments (approximately six times more) in the Disse’s space leads to liver injury [4, 5]. The largest component of the ECM is collagen [6]. In the liver type III collagen mainly occurs [7, 8]. During the synthesis of type III collagen, the N-terminal propeptide of procollagen type III (PIIINP) is detached from procollagen type III [9], so the fibrogenesis results in release of ECM fragments into the blood [9]. Hence the quantity of propeptide of procollagen type III can be a direct indicator of collagen synthesis and its deposition in the extracellular space [9]. The most common causative agents of fibrosis are: alcoholic liver disease, viral hepatitis B and C, iron or copper overload, and autoimmune states [5]. The increased amount of propeptide of type III procollagen may indicate the transformation of normal liver tissue into connective tissue [5], but PIIINP is not a liver-specific marker. Increased concentrations of PIIINP were also observed in other pathological conditions associated with abnormal production of collagen, such as congestive heart failure, hypertension, and coronary heart disease. It is probably caused by ongoing inflammation [7].
Aim
The aim of this study was to evaluate the effect of liver diseases of different aetiologies (alcoholic and non-alcoholic) and clinical severity of liver cirrhosis on the serum level of PIIINP.
Material and methods
The tested group consisted of 127 consecutive patients admitted to the Department of Infectious Diseases and Hepatology (Medical University of Bialystok). The tested patients were both males (n = 85) and females (n = 42) of ages between 24 and 88 years. They were divided into three subgroups according to the clinical diagnosis of disease: alcoholic cirrhosis (AC) – 63 patients, non-alcoholic cirrhosis (NAC) – 31 patients, and toxic hepatitis (HT) – 33 patients. The diagnosis was based on the signs, symptoms, physical exams, and laboratory liver panel: platelet count (PLT), mean corpuscular volume (MCV), normalised ratio value (INR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), g-glutamyl transferase (GGT), albumin, and bilirubin. Data on age, gender, and levels of serum liver enzymes are presented in Table I.
Table I.
Changes of blood liver parameters
| Parameter | C (n = 20) | AC (n = 63) | NAC (n = 31) | HT (n = 33) |
|---|---|---|---|---|
| PLT [×103/µl] | 235.35 ±51.37 | 125.31 ±104.40 | 101.09 ±49.18 | 186.48 ±85.37 |
| MCV [fl] | 87.06 ±4.49 | 97.02 ±8.84 | 90.10 ±7.77 | 96.31 ±6.85 |
| INR | 0.93 ±0.04 | 1.42 ±0.39 | 1.15 ±0.20 | 1.19 ±0.53 |
| AST [IU/l] | 23.25 ±5.31 | 103.93 ±112.78 | 75.43 ±59.78 | 149.13 ±126.13 |
| ALT [IU/l] | 17.65 ±8.29 | 44.76 ±47.91 | 58.63 ±70.09 | 177.12 ±191.41 |
| GGT [IU/l] | 23.30 ±7.33 | 390.97 ±505.18 | 100.32 ±83.33 | 914.25 ±868.51 |
| Total bilirubin [µM/l] | 17.27 ±7.52 | 110.12 ±128.93 | 21.55 ±12.83 | 102.09 ±137.14 |
| Albumin [g/dl] | 3.98 ±1.12 | 3.06 ±0.65 | 3.43 ±0.60 | 3.58 ±0.57 |
Data are mean ± standard deviation. C – controls, AC – alcoholic cirrhosis, NAC – non-alcoholic cirrhosis, HT – toxic hepatitis, PLT – platelet count, MCV – mean corpuscular volume, INR – international normalised ratio, AST – aspartate aminotransferase, ALT – alanine aminotransferase, GGT – γ-glutamyl transferase.
Blood specimen
Venous blood samples were collected from every patient by peripheral vein puncture. The sera were separated by centrifugation at 1500 × g for 10 min at room temperature and stored at –86ºC until assayed. PIIINP was measured with enzyme-linked immunosorbent assay – ELISA (EIAab, China) according to the manufacturer’s protocols. Duplicate samples were assessed for each patient. The ALT, AST, GGT, and bilirubin were measured on an ARCHITECT ci8200 (Abbott Laboratories) according to the spectrophotometric method and using Abbott reagents. The PLT count and MCV were determined on a Sysmex XS800i (Sysmex Corporation), and INR on an STA Compact Max analyser (Diagnostica Stago, Inc.) by the viscometric method.
Control group
The control group consisted of 30 healthy volunteers (16 men and 14 women). All subject individuals (healthy and sick) gave written informed consent to participate in our study. The study was approved by the Bioethical Committee at the Medical University in Bialystok, Poland.
Statistical analysis
Results were expressed as mean and standard deviation (SD). The significance of differences between groups (tested and control) was estimated by Mann-Whitney U test. The analysis of PIIINP according to the aetiology and severity of liver cirrhosis were performed by the ANOVA rank Kruskal-Wallis test. The differences were considered statistically significant at p < 0.05.
Results
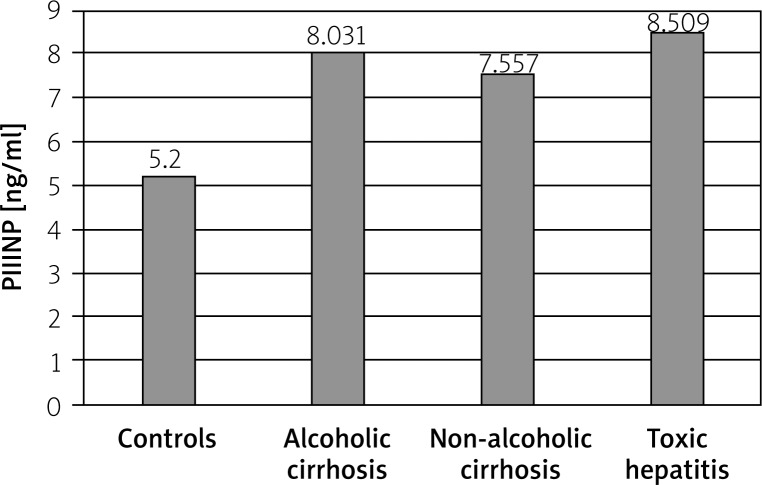
The mean level of PIIINP was significantly higher in patients with alcoholic cirrhosis (8.031 ±2.494 ng/ml), non-alcoholic cirrhosis (7.557 ±3.306 ng/ml), and toxic hepatitis (8.509 ±2.476 ng/ml) in comparison to the control group (5.200 ±0.861 ng/ml) (p < 0.001, p = 0.001, p < 0.001, respectively) (Figure 1), but there were no significant differences in the serum PIIINP levels between liver diseases (ANOVA: H = 1.558, p = 0.459). The values of PIIINP in Child-Pugh class A, B, and C were similar (8.219 ±3.609, 8.222 ±2.500, 7.306 ±2.108, respectively). The analysis of variance revealed that severity of liver cirrhosis did not affect the value of PIIINP (H = 5.926, p = 0.051).
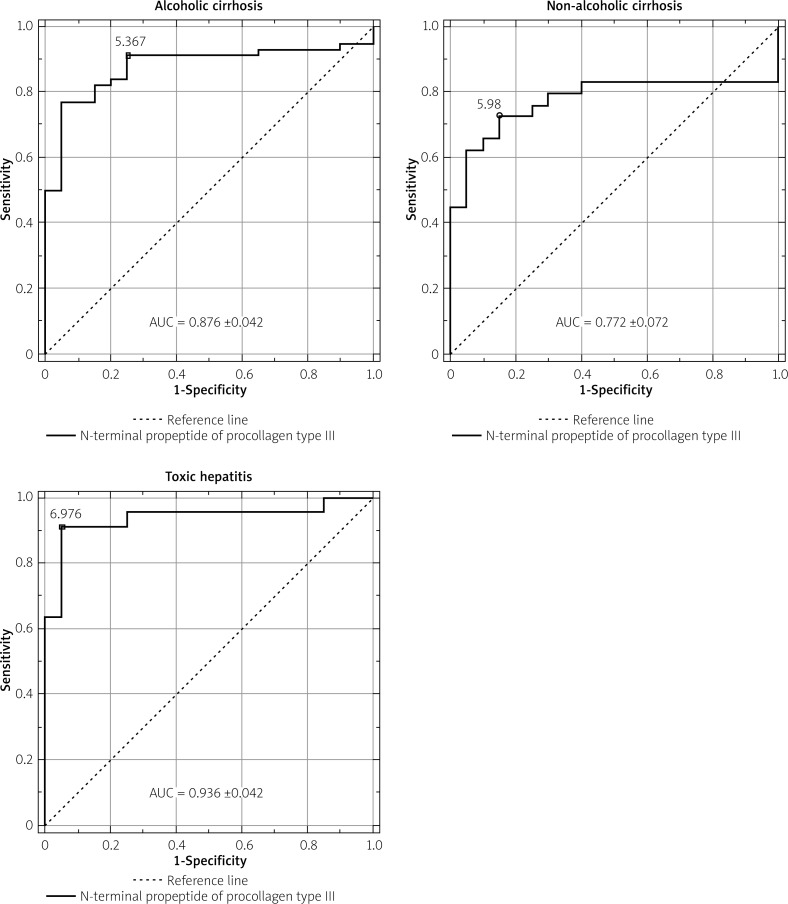
Figure 1.
The ROC curves of N-terminal propeptide of procollagen type III in liver diseases
The ROC curves of serum PIIINP in liver diseases are presented in Figure 2. PIIINP has the highest diagnostic power for the diagnosis of toxic hepatitis at the cut-off point of 6.976 ng/ml. The diagnostic sensitivity of PIIINP in toxic hepatitis is equal to 90.9%, specificity – 95%, positive predictive value (PPV) – 95.2%, negative predictive value (NPV) – 90.5%, and diagnostic accuracy (ACC) – 92.9%. PIIINP has the highest sensitivity (91.1%) in alcoholic cirrhosis at the cut-off point of 5.367 ng/ml, but other diagnostic values (specificity – 75%, PPV – 91.1%, NPV – 75%, ACC – 86.8%) in alcoholic cirrhosis were lower in comparison with toxic hepatitis. In diagnosis of non-alcoholic cirrhosis PIIINP has low sensitivity (72.%), specificity (85%), PPV (87.5%), NPV (68%), and ACC (77.6%).
Figure 2.
The levels of serum N-terminal propeptide of procollagen (PIIINP) type III in liver diseases
Discussion
Studies based on PIIINP as a liver damage indicator suggest that tissues with high fibrogenic activity or non-mature fibrotic tissue consist mostly of type III collagen, whereas in mature fibrotic tissue the levels of collagen type III are lower. Also, tissues undergoing healing have increased levels of PIIINP [4]. So it is problematic to distinguish mild stages of liver fibrosis from the process of healing and regeneration. Therefore, we tried to examine if the level of serum PIIINP reflects the liver injury and process of fibrogenesis.
In this study we have shown that the mean serum level of procollagen III N-terminal propeptide is elevated in patients with liver diseases. Our findings are similar to the results reported by Nojgaard et al., who determined samples from patients using radioimmunoassay (RIA) method. They revealed an increased expression of PIIINP in patients suffering from different alcoholic liver diseases compared to controls [4]. Additionally, Parsian et al. showed that the mean serum PIIINP levels are higher in HBV and immune hepatitis than that in the control group [10]. It is probably the result of the increased hepatic synthesis and turnover of ECM [11], and also a diminishing hepatic clearance [12]. On the other hand, we observed that PIIINP has the highest diagnostic power for the diagnosis of toxic hepatitis (good sensitivity, specificity, PPV, and NPV). Therefore, we can speculate that PIIINP is a better indicator of inflammation than cirrhosis [13].
In this study we did not find any differences in PIIINP serum levels between alcoholic cirrhosis, non-alcoholic cirrhosis, and toxic hepatitis. These findings are different to those obtained in the study of Nojgaard et al., who observed higher levels of serum PIIINP in non-cirrhotic patients with alcoholic hepatitis than non-cirrhotic patients with hepatitis [6]. Collazos and Diaz reported the association between PIIINP and portal hypertension, which was probably due to the fact that the most patients with this disease has also cirrhosis [11]. Similar results were obtained by Jansen et al., who observed the correlation of PIIINP in HIV-HCV co-infected patients with hepatic venous pressure gradient [14]. They also reported that PIIINP level is associated with the stage of fibrosis stratified by FIB-4 score [14]. Also, Nielsen et al. revealed that PIIINP level is higher in patients with chronic hepatitis C in higher stage of fibrosis (Ishak stage 4 compared to stage 2), and it is a good indicator to differentiate advanced from mild fibrosis [15]. The one possible explanation for increased PIIINP levels in advanced fibrosis is the fact that ECM remodelling occurs as an attempt to repair damaged tissue in response to liver injury. If the liver damage is extensive the synthesis of ECM components, and collagens, is greater. However, there are no studies comparing PIIINP concentration with severity of liver damage expressed in Child-Pugh scale. Our study revealed that the values of PIIINP are similar independently of the severity of liver cirrhosis.
Conclusions
We can state that the levels of the PIIINP were elevated in the sera of patients with liver diseases compared to healthy patients. However, PIIINP levels were similar in liver diseases of different aetiologies and according to the severity of liver cirrhosis. Therefore, we can suggest that PIIINP may be a useful test for liver diseases detection, but it does not reflect the stage of liver damage.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007;381:107–13. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Muiznieks LD, Keeley FW. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim Biophys Acta. 2013;1832:866–75. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Wu H, Byrne M, et al. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94:1852–6. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarah D, Berry SD, Vasan S, et al. Procollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Fraility Aging. 2013;2:129–34. [Google Scholar]
- 6.Nojgaard C, Johansen JS, Christensen E, et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–86. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 7.Baranova A, Lal P, Birerdinc A, et al. Non-Invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91–6. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colli A, Colucci A, Paggi S, et al. Accuracy of a predictive model for severe hepatic fibrosis or cirrhosis in chronic hepatitis C. World J Gastroenterol. 2005;11:7318–22. doi: 10.3748/wjg.v11.i46.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross TJ, Calvaruso V, Maimone S, et al. Prospective comparison of Fibroscan, King’s score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. J Viral Hepat. 2010;17:546–54. doi: 10.1111/j.1365-2893.2009.01210.x. [DOI] [PubMed] [Google Scholar]
- 10.Parsian H, Nouri M, Rahimipour A, et al. Liver Biopsy. Intech; 2011. Comparison of five liver fibrosis indexes with serum levels of laminin and N terminal peptide of procollagen type III in chronic hepatitis patients; pp. 343–60. [Google Scholar]
- 11.Collazos J, Diaz F. Role of measurement of serum procollagen III N-terminal peptide in the evaluation of liver diseases. Clin Chim Acta. 1994;227:37–43. doi: 10.1016/0009-8981(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 12.Jarcuska P, Janicko M, Veseliny E, et al. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009–17. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Smedsr B, Perotoft H, Gustsfson S, et al. Scavenger functions of the liver endothelial cell. Biochem J. 1990;266:313–27. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen C, Leeming DJ, Mandorfer M, et al. PRO-C3-levels in patients with HIV/HCV-co-Infection reflect fibrosis stage and degree of portal hypertension. PLoS One. 2014;9:e108544. doi: 10.1371/journal.pone.0108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–37. doi: 10.1111/liv.12700. [DOI] [PubMed] [Google Scholar]