Abstract
Background:
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in reproductive age women and is associated with both reproductive and metabolic abnormalities. Recent studies have demonstrated an early onset of abnormal cardiovascular risk profile in women with PCOS. Abnormal lipid profile patterns are common in women with PCOS, and these abnormalities are not uniform in all populations. Anthropometry is a simple and commonly used research tool for assessing metabolic risk in women with PCOS. Therefore, this study examined the correlations between anthropometric parameters and lipid profile in women with PCOS.
Objectives:
The objectives of the study were (1) To study the anthropometric profile of women with PCOS, (2) To examine the lipid profile pattern of these women with PCOS and (3) To see whether there exists any correlation between these anthropometric parameters and lipid profile.
Materials and Methods:
This observational cross-sectional study examined anthropometry and lipid profile in 86 married women with PCOS in the age group of 18–35 years and correlated them by using Pearson’s correlation coefficient.
Results:
More than 80% of the women with PCOS demonstrated abnormal anthropometric parameters, and in more than 70% women, lipid abnormalities such as low levels of high-density lipoprotein (HDL) cholesterol and high levels of triglycerides and low-density lipoprotein cholesterol were observed. Significant positive correlations were seen between body mass index (BMI) and triglycerides (P ≤ 0.001) and waist circumference (WC) and triglycerides (P ≤ 0.029). Negative correlations were observed between BMI and HDL cholesterol (P ≤ 0.013).
Conclusion:
This study revealed that BMI and WC are the most important anthropometric parameters correlated to dyslipidemia in the south Indian women with PCOS.
KEYWORDS: Anthropometry, lipid profile, PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrinopathy characterized by both reproductive and metabolic abnormalities. In 2003, the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine redefined PCOS as the presence of two or more of the following features: (1) oligo- or anovulation, (2) signs of clinical or biochemical hyperandrogenism and (3) ultrasonographic evidence of polycystic ovaries once other related endocrine and gynaecological disorders are excluded.[1] Recent studies have shown an early onset of abnormal cardiovascular risk profile in women with PCOS. Many of these women develop abnormal glucose and lipid metabolism, hypertension, obesity, insulin resistance and other features suggestive of systemic inflammatory response.[2] Because of the higher rates of prevalence of these risk factors, the symptoms of majority of the women with PCOS also fit into metabolic syndrome, which is a known risk factor for cardiovascular disorders.[3] The elevation of risk factors in young women with PCOS may, therefore, put them at an increased risk of developing accelerated atherosclerosis resulting in an early onset of coronary artery disease (CAD).[4,5] A close correlation was observed between adiposity and the severity of symptoms in women with PCOS. The android type of body fat distribution, which is more commonly associated with metabolic disturbances, was found to be more common in women with PCOS.[6,7] In fact, studies that emphasized on anthropometric parameters in women with PCOS have revealed higher body mass index (BMI) and increased waist circumference (WC) in women with PCOS.[8,9]
Anthropometry is commonly used as a research tool in assessing non-communicable disease risk factors in the populations because they are inexpensive and easy to monitor at the community level. These anthropometric parameters are not uniform and vary from population to population. In some populations, they have high predictive value for assessing non-communicable disease risk factors such as diabetes, hypertension and dyslipidemia.[10] Various studies have demonstrated abnormal lipid profile patterns in women with PCOS, and these abnormalities were not uniform in all populations.[11,12] Few studies are available on the lipid profile pattern and their anthropometric correlates in women with PCOS. Because PCOS shares many of the metabolic risk factors of cardiovascular disorders (CVD), this study was undertaken to examine the correlations between anthropometry and lipid profile in women with PCOS. The objectives of the study were (1) to study the anthropometric profile of women with PCOS, (2) to examine the lipid profile pattern of these women with PCOS and (3) to see whether there exists any correlation between these anthropometric parameters and lipid profile.
MATERIALS AND METHODS
This observational cross-sectional study was conducted in the gynaecology department of a tertiary care teaching hospital and included 86 married women with PCOS in the age group of 18–35 years who satisfied the inclusion criteria.
Inclusion criteria
All participants with PCOS who satisfied the criteria of European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine were included.
Exclusion criteria
Participants with hypothyroidism, diabetes, congenital adrenal hyperplasia hyperprolactinemia and Cushing syndrome were excluded from the study.
Written informed consent was obtained from all the participants, and institutional ethics review committee approval was obtained before commencing the study. After taking a detailed medical history and conducting a thorough clinical examination, the participants underwent transvaginal ultrasound examination. The diagnosis of PCOS was made when any two of the following three criteria were present: (1) a history of oligo-/anovulation, (2) hirsutism, acne, and acanthosis nigricans in few cases and (3) when the number of follicles measuring 2–9 mm in both ovaries exceeded twelve in number, and the volume of the ovaries exceeded 11 ml. Weight, height, WC and hip circumference (HC) were measured, and anthropometric parameters such as BMI, waist–hip ratio (WHIPR) and waist–height ratio (WHTR) were calculated. The threshold cut-off values adapted for BMI were ≥25 kg/m2 for overweight and ≥30 kg/m2 for obesity, WC ≥80 cm, WHIPR ≥0.85 and WHTR ≥0.55. Venous samples were taken after an overnight fast for fasting lipid profile. Serum lipids (total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) plasma cholesterol concentrations) were measured. Cholesterol and triglyceride levels were determined in the serum by commercially available kits on a Chem 7 semiauto analyzer. HDL cholesterol was measured by using the direct HDL method. LDL and very low-density lipoprotein (VLDL) cholesterol were calculated according to the formula of Friedewald et al.: LDL cholesterol = cholesterol−[HDL cholesterol + (0.46 × triglycerides)]. Statistical analysis was performed using the Statistical Package for the Social Sciences trial version 18.0 software (SPSS Inc., Chicago, IL, United States) and MS Excel 2007 spreadsheet. Pearson’s correlation coefficient was calculated to see the correlation between anthropometric parameters and lipid profile. For all statistical analyses, P value <0.05 was considered statistically significant.
RESULTS
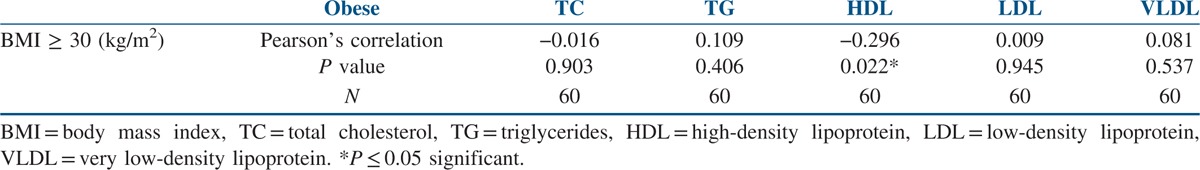
The data of the 86 study participants were analysed. The mean age of the study’ participants was 24.28 ± 3.82 years, and the mean BMI was 27.93 ± 4.64 kg/m2. The mean WC was 91.70 ± 9.45 cm, and the mean WHIPR was 0.89 ± 0.05. The mean WHTR was 0.60 ± 0.058. Similarly, the mean of total cholesterol (TC) was 164.69 ± 34.06 mg/dl, and for total LDL cholesterol it was 96.97 ± 27.18 mg/dl. The mean of HDL cholesterol was 44.83 ± 14.62 mg/dl, and for VLDL it was 30.01 ± 18.36 mg/dl. The mean of triglycerides was 123.39 ± 56.10 mg/dl [Table 1]. Out of 86 participants with PCOS, 16 (18.6%) were normal weight, 10 (11.62%) were overweight and 60 (69.76%) were obese. Seventy-six participants (88.37%) with PCOS had WC above the normal threshold value of 80 cm. Seventy participants (81.39%) with PCOS had WHIPR above the normal threshold value of 85. Similarly, 75 (87.21%) participants with PCOS had WHTR above the normal threshold of 0.55. TC levels were above the threshold value of 200 mg/dl in eight participants (9.3%). Total LDL cholesterol levels were above the threshold value of 130 mg/dl in 46 participants (53.48 %). Total HDL cholesterol levels were below the threshold value of 50 mg/dl in 56 participants (65.11%). Total VLDL cholesterol levels were above the threshold value of 40 mg/dl in 12 participants (14%). Total triglyceride levels were above the threshold value of 150 mg/dl in 46 participants (53.5%). When Pearson’s correlation coefficient was calculated to see the correlation between anthropometric parameters and lipid profile in the total study participants, positive correlations were seen between BMI and triglycerides (P ≤ 0.001) and WC and triglycerides (P ≤ 0.029). Significant negative correlations were seen between BMI and HDL cholesterol (P ≤ 0.013) [Table 2]. When Pearson’s correlation coefficient was calculated to see the correlation between anthropometric parameters and lipid profile in the normal weight participants, significant negative correlations were seen between BMI and HDL cholesterol (P ≤ 0.005) [Table 3]. When Pearson’s correlation coefficient was calculated to see the correlation between anthropometric parameters and lipid profile in overweight participants, positive correlations were seen between BMI and triglycerides (P ≤ 0.012), and negative correlations were seen between BMI and HDL cholesterol (P ≤ 0.013) [Table 4]. When Pearson’s correlation coefficient was calculated to see the correlation between anthropometric parameters and lipid profile in obese participants, negative correlations were seen between BMI and HDL cholesterol (P ≤ 0.022) [Table 5].
Table 1.
Descriptive statistics of the study parameters in the total study population
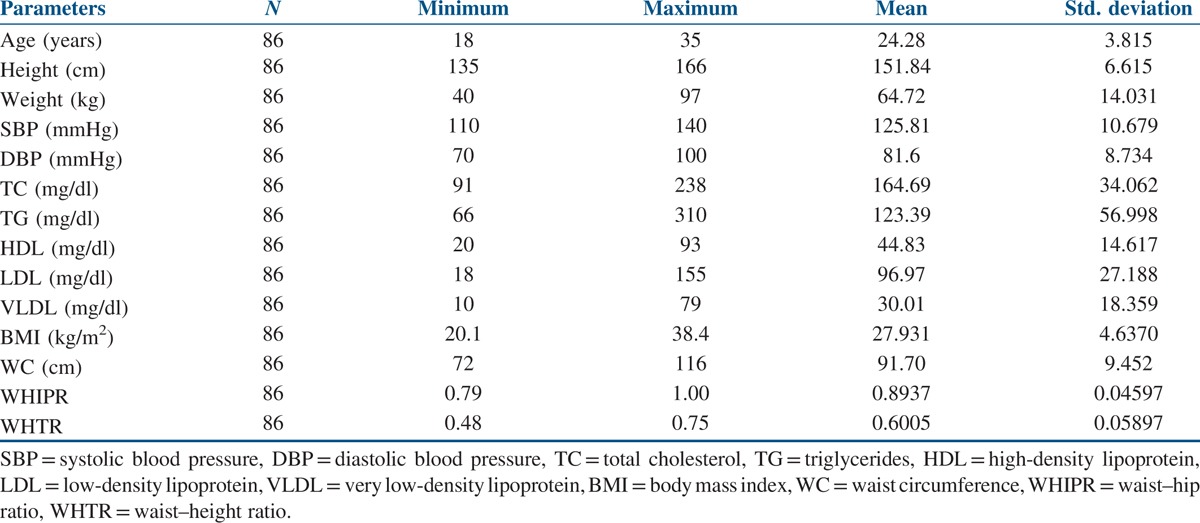
Table 2.
Correlations between anthropometry and lipid profile in the total study population
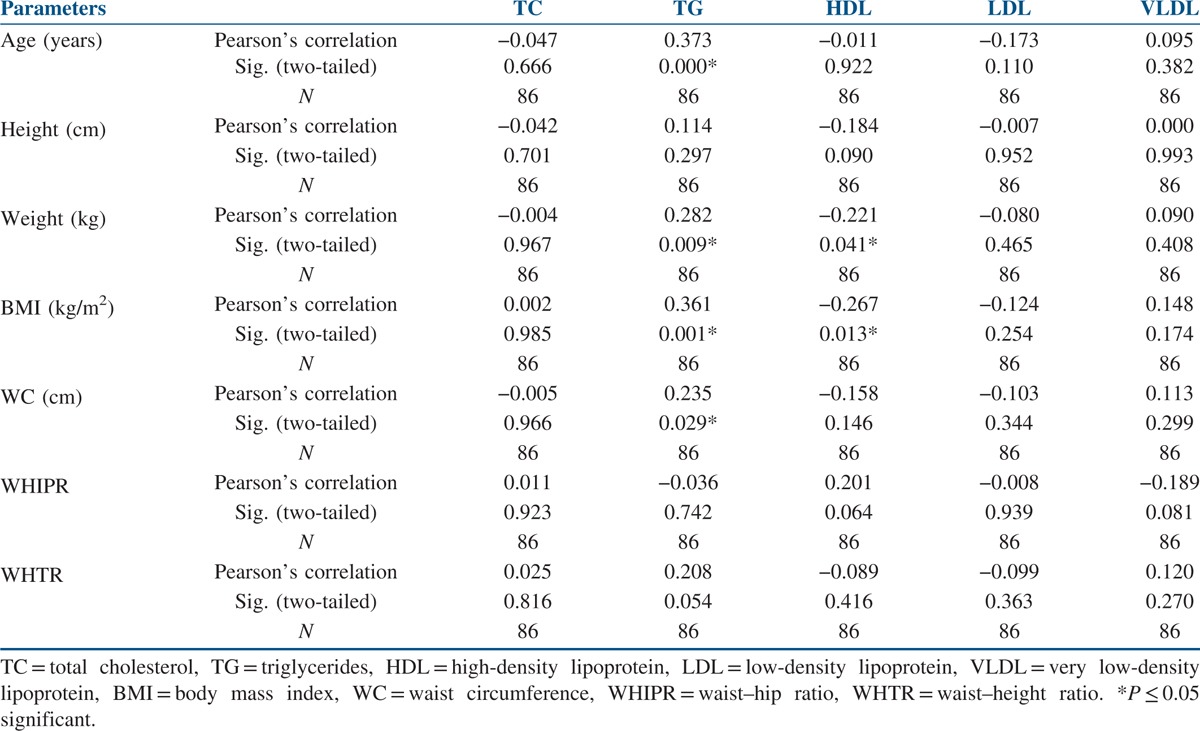
Table 3.
Correlations between anthropometry and lipid profile in normal weight women with PCOS
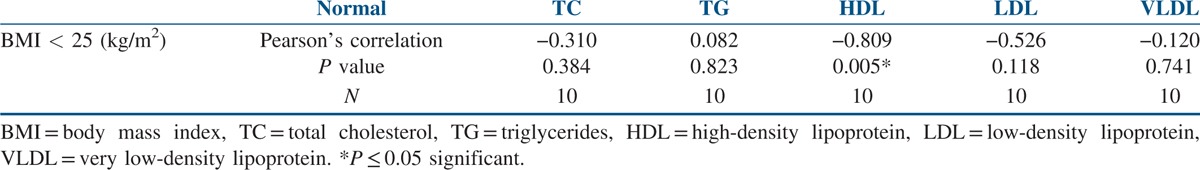
Table 4.
Correlations between anthropometry and lipid profile in overweight women with PCOS
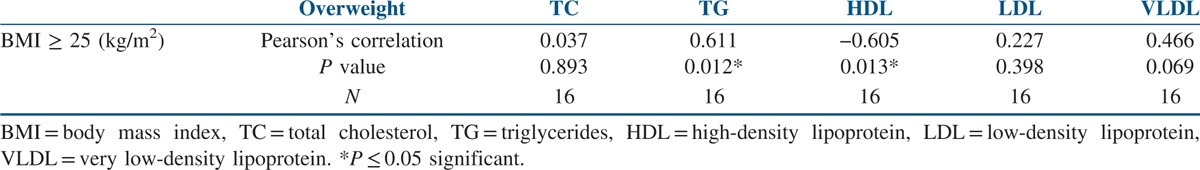
Table 5.
Correlations between anthropometry and lipid profile in obese women with PCOS
DISCUSSION
This study examined the anthropometric parameters and lipid profile patterns in 86 women with PCOS. Majority of the women with PCOS had anthropometric parameters above the threshold cut-off value. The most common anthropometric abnormality observed was WC (88.37%) followed by WHTR (87.21%), BMI (81.4%) and WHIPR (81.39%). The common lipid parameter abnormalities were low HDL levels in 65.11% of the participants and high levels for triglycerides (53.5%) and LDL cholesterol (53.48%). VLDL and TC levels were above the threshold cut-off value in only 14 and 9.3% of the study participants, respectively. The lipid abnormalities in this study were similar to the observations made by El-Mazny et al.,[3] Rocha et al.,[13] Hussain et al.[14] and Wild et al.[15] in their PCOS participants. In studies conducted by Clark et al.[16] women with PCOS had higher BMI and lower HDL levels. Ahmadi et al.[6] reported higher WC in women with PCOS. In studies conducted by Kar,[11] BMI, WC, WHIPR and triglyceride levels were higher in women with PCOS. Iuhas et al.[17] reported lower HDL levels and high total and LDL cholesterol levels in women with PCOS. Different types of dyslipidemia in women with PCOS was attributed to the influence of hyper-androgenism and insulin resistance that is commonly seen in women with PCOS along with diet, exercise and genetic predisposition. The atherogenic dyslipidemia (low HDL cholesterol along with raised LDL cholesterol and triglycerides) that was observed in this study was also seen in various other studies conducted across the world. This PCOS-associated dyslipidemia may predispose these women to increased risk of premature atherosclerosis, and early preventive interventions may be required to halt the progress of atherosclerosis in these women.
This study also explored the relationship between anthropometry and lipid parameters. There were significant positive correlations between BMI, WC and triglycerides and negative correlations between BMI and HDL cholesterol in the total study population [Table 2]. Similar observations were made in overweight women with PCOS between BMI, triglycerides and HDL cholesterol [Table 4]. In normal, overweight and obese women with PCOS, significant negative correlations were observed between BMI and HDL cholesterol [Tables 3–5]. No relationship was seen between WHIPR, WHTR and lipid parameters. This study reveals that decreased HDL levels were a common lipid abnormality seen in women with PCOS independent of their BMI status. Elevated levels of triglycerides and LDL cholesterol were the other lipid abnormalities seen in this study in women with PCOS. Only few studies examined the relationship between anthropometry and lipid profile in women with PCOS. In studies conducted by Maryam et al.,[12] positive correlations were seen between BMI, WC, HC, WHTR and TC and LDL cholesterol. In addition, HC was associated with triglycerides. In a meta-analysis study, Wild et al.[15] found significant association between BMI, LDL and non-HDL cholesterol in women with PCOS. BMI had significant impact on HDL levels in women with PCOS in studies conducted by Rocha et al.[13] In Brazilian women with PCOS, WC and WHTR were found to be the predictors of lipid abnormalities and metabolic syndrome.[18] In a comparative study, American women with PCOS had higher BMI and triglyceride levels compared to Italian women.[19] In young Korean women with PCOS, substantial increase in the prevalence of dyslipidemia was seen in the absence of obesity.[20] WC and WHTR were associated with composite CV risk factors in women with PCOS in studies conducted by Gateva et al.[21] In studies conducted by Zabulienė et al.,[22] increased skin and subcutaneous adipose tissue mass along with decreased muscle mass was observed in lean women with PCOS compared to controls. Studies conducted by Kar[11] and Sujatha et al.[23] revealed high prevalence of obesity and lipid abnormalities in women with PCOS, as in this study. Variations in body weight and composition alone may not fully explain differences in dyslipidemia in women with PCOS of diverse ethnic and geographical backgrounds. Genetic and environmental factors such as diet and activity level are likely contributors to these differences. Low calorie diet and physical exercise induced weight loss in women with PCOS were associated with significant improvement in anthropometric parameters in studies conducted by Crosignani et al.[8]
The limitations of this study are that only limited number of participants were recruited, and this study did not take into consideration the phenotypic variations in PCOS while examining anthropometry and lipid profile. The strength of the study is that all study participants had ultrasonographic evidence of polycystic ovaries, and ultrasound examination was conducted by the same sonologist. Inter-observer variability in the interpretation of result was eliminated. In studies conducted by Ahmed et al.[24] in women with PCOS, radiological evidence of polycystic ovaries had 92.04% positive predictive value and 81.25% negative predictive value in diagnosing PCOS. In studies conducted by Gilberto et al.,[25] radiological evidence of polycystic ovaries was associated with obesity and clinical manifestations of hyperandrogenism.
CONCLUSION
Various studies reveal that PCOS is frequently associated with dyslipidemia. The pattern of dyslipidemia is not the same in all populations. Different types of dyslipidemia are probably the net result of interfering influences of hyperandrogenism, insulin resistance, environmental factors and genetics. In most of the studies, atherogenic dyslipidemia was the common manifestation in women with PCOS, as in this study. This study suggests that BMI and WC are the important anthropometric parameters correlated to dyslipidemia in south Indian women with PCOS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Dokras A. Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med. 2008;26:39–44. doi: 10.1055/s-2007-992923. [DOI] [PubMed] [Google Scholar]
- 3.El-Mazny A, Abou-Salem N, El-Sherbiny W, El-Mazny A. Insulin resistance, dyslipidemia, and metabolic syndrome in women with polycystic ovary syndrome. Int J Gynecol Obstet. 2010;109:239–41. doi: 10.1016/j.ijgo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Pietro S, Ilaria D, Roberta R, Bulzis G, Dachille A, Caputo P, et al. Cardiovascular risk in women with PCOS. Int J Endocrinol Metab. 2012;10:611–8. doi: 10.5812/ijem.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karoli R, Fatima J, Siddiqi Z, Vatsal P, Sultania AR, Maini S. Study of early atherosclerotic markers in women with polycystic ovary syndrome. Indian J Endocr Metab. 2012;16:1004–8. doi: 10.4103/2230-8210.103021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadi A, Akbarzadeh M, Mohammadi F, Akbari M, Jafari B, Tolide-Ie HR. Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian J Endocr Metab. 2013;17:672–6. doi: 10.4103/2230-8210.113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandrelle K, Kamath MS, Bondu DJ, Chandy A, Aleyamma TK, George K. Prevalence of metabolic syndrome in women with polycystic ovary syndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci. 2012;5:26–31. doi: 10.4103/0974-1208.97791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18:1928–32. doi: 10.1093/humrep/deg367. [DOI] [PubMed] [Google Scholar]
- 9.Boyle JA, Cunningham J, O’Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin. Med J Aust. 2012;196:62–6. doi: 10.5694/mja11.10553. [DOI] [PubMed] [Google Scholar]
- 10.Jaap CS, Henry SK, David FW, Lauren L, Rodolfo V. Report from a centers for disease control and prevention work shop on use of adult anthropometryfor public health and primary health care. Am J Clin Nutr. 2001;73:123–6. doi: 10.1093/ajcn/73.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Kar S. Anthropometric, clinical, and metabolic comparisons of the four Rotterdam PCOS phenotypes: A prospective study of PCOS women. J Hum Reprod Sci. 2013;6:194–200. doi: 10.4103/0974-1208.121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maryam SA, Saeed P, Mehranghiz EM, Mohammad AJ, Soudabeh A, Bita S. Lipid profile in relation to anthropometric indices and insulin resistance in overweight women with polycystic ovary syndrome. Health Promot Perspect. 2013;3:206–16. doi: 10.5681/hpp.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocha MP, Marcondes JA, Barcellos CR, Hayashida SA, Curi DD, da Fonseca ÂM, et al. Dyslipidemia in women with polycystic ovary syndrome: Incidence, pattern and predictors. Gynecol Endocrinol. 2011;27:814–9. doi: 10.3109/09513590.2010.508852. [DOI] [PubMed] [Google Scholar]
- 14.Hussain A, Alam JM. Dyslipidaemia in woman with polycystic ovarian syndrome: A case control study in tertiary care hospital of Karachi. J Pak Med Assoc. 2014;64:1049–52. [PubMed] [Google Scholar]
- 15.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073–9. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Clark NM, Podolski AJ, Brooks ED, Chizen DR, Pierson RA, Lehotay DC, et al. Prevalence of polycystic ovary syndrome phenotypes using updated criteria for polycystic ovarian morphology: An assessment of over 100 consecutive women self-reporting features of polycystic ovary syndrome. Reprod Sci. 2014;21:1034–43. doi: 10.1177/1933719114522525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iuhas C-I, Costin N, Mihu D. Lipid parameters in patients with polycystic ovary syndrome. Appl Med Inf. 2012;31:27–32. [Google Scholar]
- 18.Costa EC, Sá JC, Soares EM, Lemos TM, Maranhão TM, Azevedo GD. Anthropometric indices of central obesity how discriminators of metabolic syndrome in Brazilian women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:12–5. doi: 10.3109/09513590.2011.583956. [DOI] [PubMed] [Google Scholar]
- 19.Essah PA, Nestler JE, Carmina E. Differences in dyslipidemia between American and Italian women with polycystic ovary syndrome. J Endocrinol Invest. 2008;31:35–41. doi: 10.1007/BF03345564. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci. 2013;56:137–42. doi: 10.5468/ogs.2013.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gateva A, Kamenov Z. Anthropometric indices of visceral obesity and cardiovascular risk factors in patients with polycystic ovarian syndrome. Endocr Abst. 2012;29:P900. doi: 10.1016/j.ejogrb.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Zabulienė L, Urboniene J, Tutkuvienė J. Body composition of lean women with polycystic ovary syndrome. Anthropol Rev. 2013;76:183–98. [Google Scholar]
- 23.Sujatha T, Vijayalakshmi K, Jayashankar E, Anuradha K, Uma A, Qurratulain H. Anthropometric and biochemical characteristics of polycystic ovarian syndrome in south Indian women using AES-2006 criteria. Int J Endocrinol Metab. 2014;12:e12470. doi: 10.5812/ijem.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed S, Pahwa S, Das CJ, Mir FA, Nisar S, Jehangir M, et al. Comparative evaluation of sonographic ovarian morphology of Indian women with polycystic ovary syndrome versus those of normal women. Indian J Endocr Metab. 2014;18:180–4. doi: 10.4103/2230-8210.129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tena G, Moran C, Romero R, Moran S. Ovarian morphology and endocrine function in polycystic ovary syndrome. Arch Gynecol Obstet. 2011;284:1443–8. doi: 10.1007/s00404-010-1816-3. [DOI] [PubMed] [Google Scholar]


