Abstract
Background:
The members of the transforming growth factor-B superfamily, as the bone morphogenetic proteins (BMPs) subfamily and anti-Müllerian hormone (AMH), play a role during follicular development, and the bone morphogenetic protein-2 (BMP2), AMH, and THY1 are expressed in ovaries.
Aim:
This study was designed to define whether or not the expressions of these proteins in human cumulus cells (CCs) can be used as predictors of the oocyte and embryo competence.
Settings and Design:
The study included nine female patients who were diagnosed as idiopathic infertility, aged 25–33 years (median 30 years) and underwent Assisted Reproductive Technologies.
Materials and Methods:
The CCs from 60 oocyte–cumulus complexes obtained from the nine patients were evaluated with immunofluorescence staining in respect of BMPs, AMH and THY1 markers. The CCs surrounding the same oocytes were evaluated separately according to the oocyte and embryo quality.
Statistical Analysis:
Quantitative data were statistically analyzed for differences using the two-sided Mann–Whitney U test (P < 0.05).
Results and Conclusions:
Significant differences in immunofluorescence staining were observed in oocyte quality and embryo quality for the BMP2 only (P < 0.05). No significant differences were observed for AMH or CD90/THY1.
Conclusion:
These results demonstrated that there is a significant difference in the expression of BMP2 in the CCs of good quality oocytes and subsequently a good embryo.
KEYWORDS: AMH, BMP2, cumulus cell, embryo, granulosa cell, infertility, oocyte, THY1
INTRODUCTION
The cumulus–oocyte complex (COC) in a mammalian ovarian follicle is consistent with an oocyte and neighboring follicular cells, known as cumulus cells (CCs), and there is a close interaction between the oocyte and CCs that is shown in Figure 1. This paracrine communication has very important roles in the oocyte development.[1] Ovarian follicular cells and oocytes express many growth factors which are members of the transforming growth factor-B (TGFB) superfamily, and these have different roles in female reproduction in mammals. The TGFB family has an active role in folliculogenesis and oocyte development, and these factors mediate the primary follicle differentiation to secondary follicle and the late preantral/early antral stage follicular growth.[2,3] TGFB signaling uses both Smad and non-Smad pathways by gene regulation in the nucleus.[4] Bone morphogenetic proteins (BMPs) and activin are members of TGFB. Activin, BMP2, BMP5, and BMP6 are expressed by granulosa cells (GCs), and these factors support proliferation and prevent early luteinization.[3] BMP2, BMP3, BMP4, and BMP7 have been shown to be expressed by theca cells, and BMP6, BMP15 and GDF9 have been shown to be expressed by oocytes. BMP2 and BMP6 have been reported to prevent luteinization of theca and GCs in porcine follicles.[5] Anti-Müllerian hormone (AMH; also known as Müllerian-inhibiting substance) is a peptide belonging to the transforming growth factor β (TGF-β) family. AMH is a member of the TGFB superfamily and also plays a role during follicular development. AMH reduces the response to follicle stimulating hormone (FSH) in developing follicles in females through a signal transduction pathway similar to BMPs.[6] AMH was identified firstly as the hormone produced by Sertoli cells, and this hormone has been shown to have an inhibitory role on Müllerian ducts in testis in the fetus during the male reproductive tract development.[3,7] More recently, it also has been shown that GCs express AMH in the female gonads.[6,8] Ovarian AMH has inhibitor roles on primordial follicles to growing follicles translation in AMH null mice. AMH and AMH receptor type 2 expressed in the GCs of small follicles and AMH may stimulate the small follicles to produce paracrine factors that may have an inhibitory effect on primordial follicles.[9] AMH has been observed on growing follicles of neonatal mouse ovaries and has been defined as inhibiting folicullar development.[6] AMH has been shown to be expressed in human CCs of the late stages of foliculles and still remains relatively highly expressed in CCs. AMH expressions in CCs are relevant to the maturational status of the follicle and to the follicular diameter.[10] Greater AMH gene expression has been described in the cumulus of incompetent mouse oocytes compared to in the cumulus of competent mouse oocytes[11] THY1, also known as CD90, which is a cell surface glycoprotein and a member of the immunoglobulin gene superfamily. THY1 has an important role together with the src-family protein kinases in cell signaling through the modulation of adhesion and migration processes.[12] CD90 is also expressed in ovarian cells.[13] Theca cells and GCs have different THY1 localization patterns in mouse ovarian follicles. THY1 is rarely localized in GCs but is always present in the theca cell layer of all adult mice. IL1 stimulation in the THY1+ myometrial subset increases PGF2 production by COX2, and high COX1 expression in THY1+ fibroblasts has been associated with sustained PGE2 synthesis.[14] Cyclooxygenase 2 (COX2; PTGS2) gene expression in CCs is related to embryo quality.[15]
Figure 1.
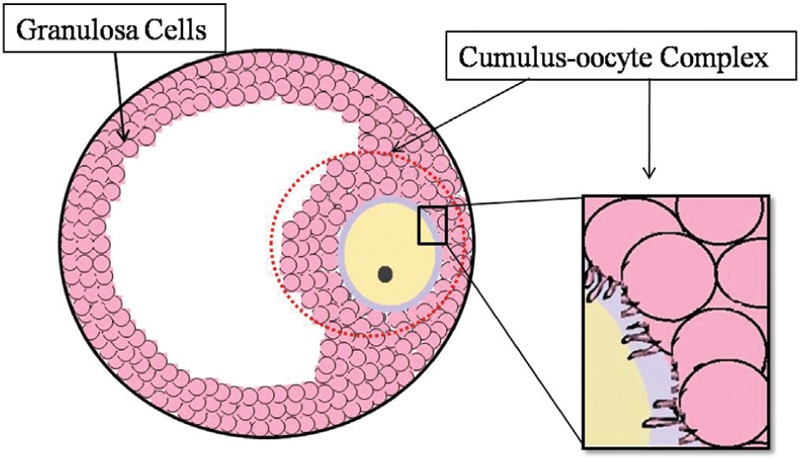
Cumulus–oocyte complex schematic figure
This study was designed with the thought that all the oocytes and neighboring CCs in an ovarian follicle might have different protein expressions, and these differences of protein expressions in CCs might reflect the oocyte and embryo quality, and therefore, CCs might be used to determine oocyte and/or embryo quality for embryo transfers in the intracytoplasmic sperm injection (ICSI).
MATERIALS AND METHODS
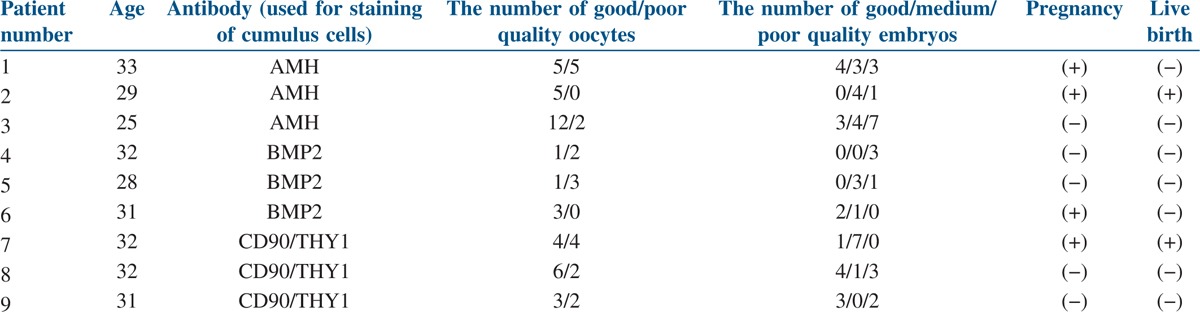
This prospective case study included nine women (aged 25–35 years) who were diagnosed with idiopathic infertility and underwent Assisted Reproductive Technologies with the technique of ICSI between October 2011 and December 2012. Cases with infertility based on male factors and other female factors were excluded from the study. The CCs from 60 oocyte–cumulus complexes obtained from the patients were stained with BMP2, AMH, and THY1 immunofluorescence markers [Table 1]. The results were reported according to each oocyte (good or poor quality) that developed good, medium, or poor quality embryo. Patients were included in the study after providing oral and written consent. This study was approved by the Ethics Committee for Human Research of University (11-2, 9; 28.03.2011) and was performed in accordance with the ethical standards described in the 1964 Declaration of Helsinki and its later amendments.
Table 1.
The laboratory and clinical outcomes of nine patients
Ovarian stimulation protocol and oocyte pick-up
Gonadotropin-releasing hormone therapy was administered to all the women prior to ICSI. Recombinant FSH (Gonal F, Serono International SA, Geneva 75-225 IU) was administered according to the patient’s ovarian response. 0.1 mg GnRH analog (Decapeptyl, Ferring GmbhH Kiel, Germany) was administered on the 21st day of the preceding menstrual cycle. Blood estradiol and progesterone levels were measured, and vaginal ultrasonography was performed at regular intervals during the induction. Human chorionic gonadotropin (hCG, Ovitrelle, Merck-Serono S.p.A. Rome, Italy, 5000–10,000 IU) was conducted when the diameters of follicles reached a competent size. After 34–36 h, the follicles were aspirated transvaginally under anesthesia and transferred into sterile tubes.[16]
Collection of cumulus cells and immunofluorescence staining protocol
The COCs were incubated at 37°C in an atmosphere with 5% CO2. CCs were removed from oocytes with mechanical methods using a sterile pipette. The CCs surrounding the same oocyte were transferred into a sterile tube individually, not pooled. Then the remaining CCs were removed from oocytes with hyaluronidase enzyme (Hyase, Vitrolife).
CCs were incubated overnight on cover slips in six-well plates in a medium containing Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were fixed with 4% paraformaldehyde for 15 min and then washed with phosphate-buffered saline (PBS) three times for 5 min each wash. Cells were incubated in a solution of 0.1% TritonX100 in PBS for 1 h and then in blocking serum (1% bovine serum albumin in PBS) at room temperature for 30 min. The cells were washed with PBS three times for 5 min each wash. Primary antibodies targeting AMH, BMP2, and CD90 (ab84415, ab14933, and ab133350, respectively; Abcam, USA) were diluted 1:100 to 1:200 with PBS, and cells were incubated with antibodies overnight at 4°C or for 1 h at room temperature. The cells were incubated with appropriate secondary antibodies diluted 1:200 to 1:300 with PBS [Alexa Fluor 488 (green), Alexa Fluor 594 (red), (Invitrogen, Carlsbad, CA, USA)] at room temperature for 30 min and washed with PBS three times for 5 min each wash.[17] Negative controls were treated with the same procedure, without the incubation with the primary antibody. Finally, cells were stained with DAPI to visualize nuclei and were viewed under a fluorescent microscope (Olympus BX 50, Olympus, Tokyo, Japan).
Design of the oocyte and embryo competence groups
The microscopically evaluated oocytes were divided into two groups morphologically and according to nuclear maturation. Group 1, considered “good quality oocytes”, included only mature oocytes in metaphase II with good morphology and Group 2, considered “poor quality oocytes”, included degenerated oocytes with poor morphology and immature metaphase I oocytes. Approximately 30–60 min after denudation, the microinjections were performed. After this process, the oocytes were individually incubated in a fertilization medium in an incubator at 37°C with an atmosphere containing 5% CO2. The microinjected oocytes were monitored individually, not pooled. After 18 h, the fertilization was evaluated microscopically by the presence of two pronuclei, with the first and second polar bodies and cytoplasmic localization of the polar bodies. Then the first mitotic division began and two-cell embryos were formed, which were evaluated according to the shape, size, and number of blastomeres, rate of fragmentation, rate of multinucleation, and degree of compaction. On day 4 after fertilization, microscopic evaluation was made of the compaction, morulation, blastocoel formation, the inner cell mass, and trophectoderm cell mass of developing embryos.[18,19] The embryos were divided in 1–4 grades considering all these criteria. The embryos were classified into three groups: Group 1, considered “good quality embryos”, included grades 1 and 1/2 embryos, Group 2, considered “medium quality embryos”, included grades 2 and 2/3 embryos and Group 3, considered “poor quality embryos”, included grades 3 and 4 embryos. Laser-assisted hatching was applied to the embryos, and the embryos were then transferred to the patients. At 12–14 days after the transfers, b-hCG levels were measured in the blood of the patients. Ultrasound was applied to the patients to determine the gestational sac and to measure the fetal heart rate.
Statistical analysis
Quantitative data were statistically analyzed for differences using the two-sided Mann–Whitney U test (P < 0.05).
RESULTS
A total of 60 COCs were studied from nine patients. The 60 oocytes were examined according to quality and the CCs of these oocytes were grouped. These 60 clusters of CCs were stained by immunofluorescence antibodies for AMH, BMP2, and CD90/THY1, as shown in Figures 2–4, respectively. All of the CCs in all areas were double-blind counted according to positive and negative staining and the average of two results was analyzed statistically. The statistical analyses of differences in immunofluorescence staining according to the quality of oocyte and embryo are shown in Figures 5 and 6. Significant differences in immunofluorescence staining were observed between good and poor quality oocytes for BMP2 only (P < 0.05). No significant differences were observed for AMH or CD90/THY1. A significant difference was observed between different quality embryos for BMP2 only (P < 0.05) and no significant differences were observed for AMH or CD90/THY1 [Table 2].
Figure 2.
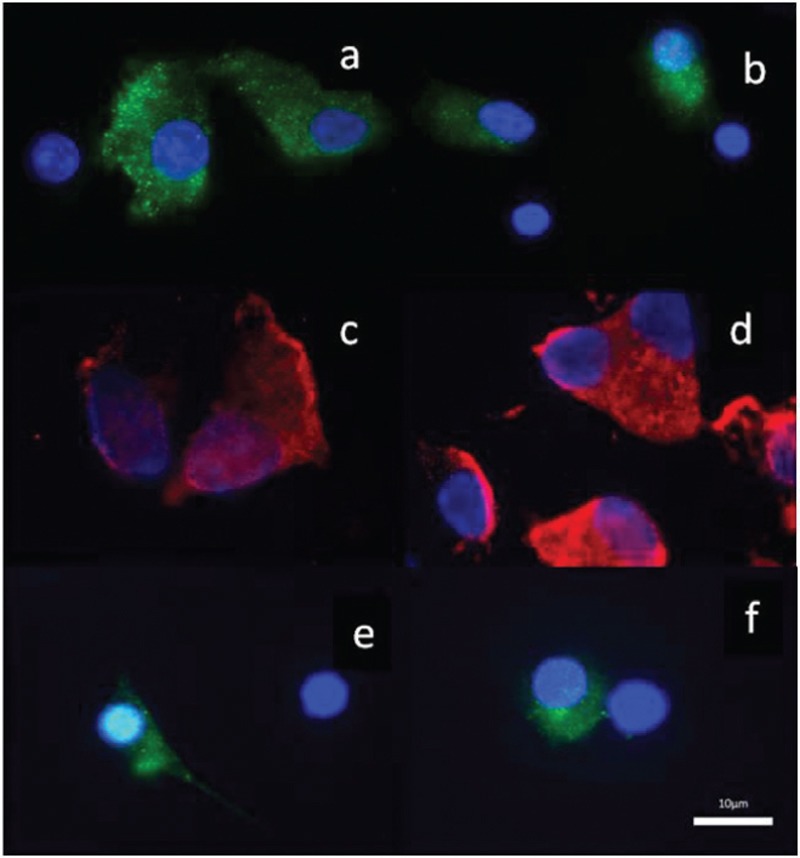
The photographic images of the cumulus cells showing AMH immunofluorescence staining for three patients. a and b: Patient 1, c and d: Patient 2, e and f: Patient 3. (×100)
Figure 4.
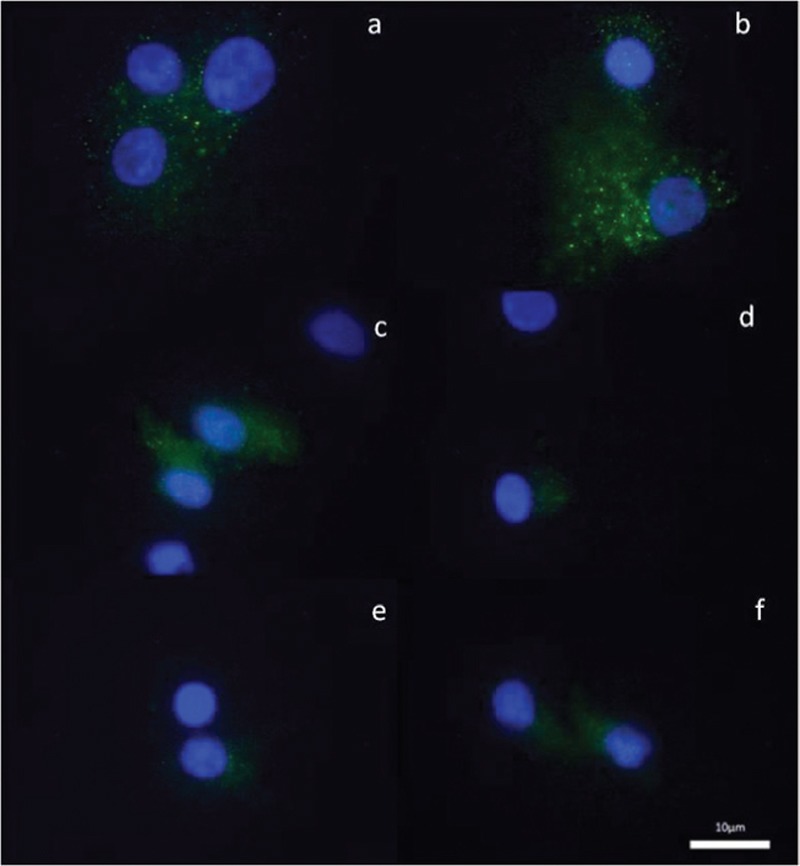
The photographic images of the cumulus cells showing CD 90/THY1 immunofluorescence staining for three patients. a and b: Patient 1, c and d: Patient 2, e and f: Patient 3. (×100)
Figure 5.
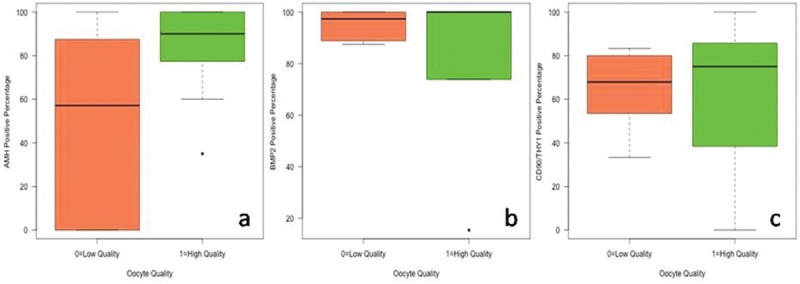
AMH, BMP 2, and CD 90/THY1 positive percentage distribution between oocyte quality groups
Figure 6.
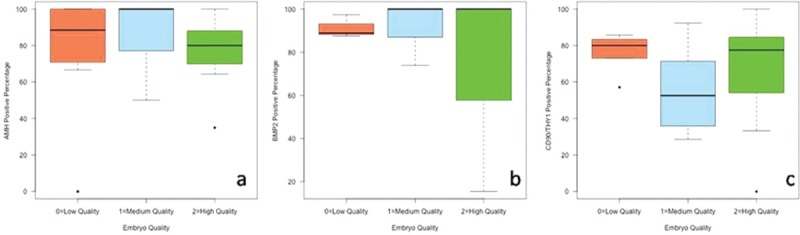
AMH, BMP 2, and CD 90/THY1 positive percentage distribution between embryo quality groups
Table 2.
The results of the statistical analysis

Figure 3.
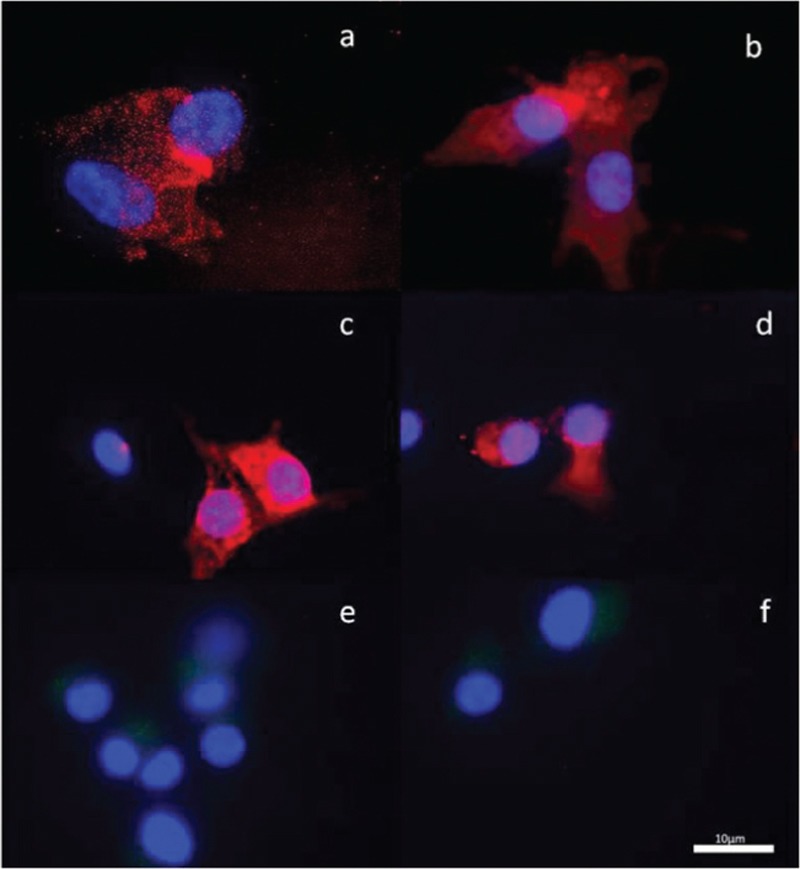
The photographic images of the cumulus cells showing BMP2 immunofluorescence staining for three patients. a and b: Patient 1, c and d: Patient 2, e and f: Patient 3. (×100)
DISCUSSION
In this analysis of the human CCs, the results indicate significant changes in BMP2 protein expression related to the oocyte and embryo quality. The results of this study showed no correlation between AMH, CD90/THY1, and the oocyte or embryo quality. Granulosa and CCs, follicular fluid and blood serum levels are the most studied tissues in the comparison of developmentally incompetent or competent antral oocytes or clinical outcomes. Because there are few granulosa and CCs surrounding the same oocytes, previous studies using these cells have been designed as groups according to the morphology, maturity, and competence of the oocyte or the embryo and the clinical outcomes. In this study, it was assumed that each oocyte and each group of CCs surrounding different oocytes might have different protein expressions that might reflect oocyte and embryo quality and CCs surrounding the same oocyte were evaluated according to the oocyte and embryo quality, separately. Immunofluorescence expression for AMH, BMP2, and THY1 was used on the CCs to reveal the relationship between these markers and oocyte and/or embryo competence. BMP2 has been shown to have diverse functions in the formation of the oocyte and other follicular cells in hamster fetal ovaries in vitro. It also organizes meiosis and anti-apoptosis on germ cells.[20] BMPR2 (bone morphogenetic protein receptor-2) is very important for follicle stimulating hormone (FSH) mediated-follicular development in the pre-ovulatory period in human GCs.[21] However, in another study, BMP2, 4, and 7 gene expressions were not described in any follicular cells or oocyte in non-atretic follicles in sheep.[22] BMP2, BMP4, and type I–II receptors have been demonstrated in bovine antral follicles, and these seem to be important for the follicular development.[23] BMP2 and BMP-15 have been seen to be expressed in the GCs, the theca cells and at higher levels in the oocyte in swine, and the level of BMP2 mRNA changes in response to the luteinizing hormone (LH) surge. Expression of BMP2, −6, −15, and GDF-9 mRNA has differed in swine follicular development stage.[24] In this human CC immunofluorescence analysis, it was demonstrated that BMP2 was expressed in CCs and the expression was related to oocyte and the embryo quality.
AMH has been studied as a reliable biomarker of oocyte quality in in vitro fertilization (IVF) in several studies. Greater expression of AMH mRNA signals has been observed in the GCs closest to the oocyte of preantral and small antral follicles in rats.[25] Increased AMH serum concentration has not been associated with oocyte quality, but with negative pregnancy outcome.[26] In a recent study, CCs associated either with developmentally incompetent or competent antral oocytes were grouped or compared by immunofluorescence staining, and AMH protein showed very bright fluorescence in CCs associated with developmentally incompetent antral oocytes compared to CCs associated with developmentally competent antral oocytes.[11] In the current study, no significant differences were determined in the AMH expression in the CCs according to the oocyte and embryo quality.
THY1, or CD90, is a type of surface glycoprotein and its expression patterns change during development within the cell types of the species.[27,28] THY 1 expression is mostly seen in some hematopoietic and stromal stem cells and also in fibroblasts, mesangial cells and the follicular basement membrane and has a particular role on intercellular interactions.[27,29] THY1 differentiation protein and macrophages under the basement membrane of follicles are important for the development of preantral and antral follicles.[29] THY1 differentiation protein has been observed to be secreted from vascular pericytes in growing follicles.[30] The expression of THY1 has been demonstrated in theca cells from secondary to preantral follicles during mouse folliculogenesis, and its expression might be stimulated with follicle stimulating hormone (FSH).[13] In the current study, CD90/THY1 were determined to be expressed in CCs, but there was no significant correlation with the oocyte or embryo quality. BMPs have been already shown to have an effective role in reproduction in recent studies, and therefore, the results obtained were expected. However, an unexpected finding was that the important role of AMH on reproduction was unrelated to the oocyte or embryo quality in the results of this study. This could be explained by the different roles of AMH on follicular development as activation and inhibition or that CCs have different oocyte-dependent protein expressions. The role of THY1 in reproduction is still a subject of ongoing research.
In conclusion, the expression of BMP2 in human CCs is very important to reveal the molecular pathway taken by quality oocytes and embryos, and if the results of this study are supported by further studies, BMP2 seems to be a predictor for oocyte and embryo selection in the future.
Financial support and sponsorship
The authors thank the financial support rendered by the Ege University Scientific Research Project Fund (Ege-BAP 2011 Tip-00014).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This study was approved by the Ethics Committee for Human Research of Ege University (11-2, 9; 28.03.2011).
REFERENCES
- 1.Assidi M, Montag M, Van der Ven K, Sirard MA. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: A preliminary study. J Assist Reprod Genet. 2011;28:173–88. doi: 10.1007/s10815-010-9491-7. doi: 10.1007/s10815-010-9491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: Actions of BMP-4, −6 and −7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction. 2004;127:239–54. doi: 10.1530/rep.1.00090. doi: 10.1530/rep.1.00090. [DOI] [PubMed] [Google Scholar]
- 3.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 4.Kaivo-oja N, Jeffery LA, Ritvos O, Mottershead DG. Smad signalling in the ovary. Reprod Biol Endocrinol. 2006;4:21. doi: 10.1186/1477-7827- 4- [Google Scholar]
- 5.Brankin V, Quinn RL, Webb R, Hunter MG. BMP-2 and −6 modulate porcine theca cell function alone and co-cultured with granulosa cells. Domest Anim Endocrinol. 2005;29:593–604. doi: 10.1016/j.domaniend.2005.04.001. doi: 10.1016/j.domaniend.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction. 2002;124:601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 7.Tran D, Picard JY, Campargue J, Josso N. Immunocytochemical detection of anti-Müllerian hormone in Sertoli cells of various mammalian species including human. J Histochem Cytochem. 1987;35:733–43. doi: 10.1177/35.7.3295030. [DOI] [PubMed] [Google Scholar]
- 8.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 9.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96. doi: 10.1210/endo.140.12.7204. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 10.Grondahl ML, Nielsen ME, Dal Canto MB, Fadini R, Rasmussen IA, Westergaard LG, et al. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod Biomed Online. 2011;22:389–98. doi: 10.1016/j.rbmo.2010.12.005. doi: 10.1016/j.rbmo.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Vigone G, Merico V, Prigione A, Mulas F, Sacchi L, Gabetta M, et al. Transcriptome based identification of mouse cumulus cell markers that predict the developmental competence of their enclosed antral oocytes. BMC Genomics. 2013;14:380. doi: 10.1186/1471-2164-14-380. doi: 10.1186/1471-2164- 14- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, et al. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–96. doi: 10.1016/j.yexcr.2004.01.026. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Itami S, Tamotsu S, Sakai A, Yasuda K. The roles of THY1 and integrin beta3 in cell adhesion during theca cell layer formation and the effect of follicle-stimulating hormone on THY1 and integrin beta3 localization in mouse ovarian follicles. Biol Reprod. 2011;84:986–95. doi: 10.1095/biolreprod.110.087429. doi: 10.1095/biolreprod.110.087429. [DOI] [PubMed] [Google Scholar]
- 14.Koumas L, Phipps RP. Differential COX localization and PG release in Thy-1(+) and Thy-1(−) human female reproductive tract fibroblasts. Am J Physiol Cell Physiol. 2002;283:C599–608. doi: 10.1152/ajpcell.00065.2002. doi: 10.1152/ajpcell.00065.2002. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: A predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–74. doi: 10.1093/humrep/deh535. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 16.Fenwick J, Platteau P, Murdoch AP, Herbert M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum Reprod. 2002;17:407–12. doi: 10.1093/humrep/17.2.407. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Hiradate Y, Hoshino Y, Tanemura K, Sato E. Quantitative analysis in LC3-II protein in vitro maturation of porcine oocyte. Zygote. 2014;22:404–10. doi: 10.1017/S0967199413000269. doi: 10.1017/S0967199413000269. [DOI] [PubMed] [Google Scholar]
- 18.della Ragione T, Verheyen G, Papanikolaou EG, Van Landuyt L, Devroey P, Van Steirteghem A. Developmental stage on day-5 and fragmentation rate on day-3 can influence the implantation potential of top-quality blastocysts in IVF cycles with single embryo transfer. Reprod Biol Endocrinol. 2007;5:2. doi: 10.1186/1477-7827-5-2. doi: 10.1186/1477-7827-5- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Montfoort AP, Geraedts JP, Dumoulin JC, Stassen AP, Evers JL, Ayoubi TA. Differential gene expression in cumulus cells as a prognostic indicator of embryo viability: A microarray analysis. Mol Hum Reprod. 2008;14:157–68. doi: 10.1093/molehr/gam088. doi: 10.1093/molehr/gam088. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty P, Roy SK. Bone morphogenetic protein 2 promotes primordial follicle formation in the ovary. Sci Rep. 2015;5:12664. doi: 10.1038/srep12664. doi: 10.1038/srep12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatzirodos N, Hummitzsch K, Irving-Rodgers HF, Harland ML, Morris SE, Rodgers RJ. Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genomics. 2014;15:40. doi: 10.1186/1471-2164-15-40. doi: 10.1186/1471-2164- 15- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juengel JL, Reader KL, Bibby AH, Lun S, Ross I, Haydon LJ, et al. The role of bone morphogenetic proteins 2, 4, 6 and 7 during ovarian follicular development in sheep: Contrast to rat. Reproduction. 2006;131:501–13. doi: 10.1530/rep.1.00958. doi: 10.1530/rep.1.00958. [DOI] [PubMed] [Google Scholar]
- 23.Fatehi AN, van den Hurk R, Colenbrander B, Daemen AJ, van Tol HT, Monteiro RM, et al. Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but absence of effects of BMP2 and BMP4 during IVM on bovine oocyte nuclear maturation and subsequent embryo development. Theriogenology. 2005;63:872–89. doi: 10.1016/j.theriogenology.2004.05.013. doi: 10.1016/j.theriogenology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Foxcroft GR, Vinsky MD, Paradis F, Tse WY, Town SC, Putman CT, et al. Macroenvironment effects on oocytes and embryos in swine. Theriogenology. 2007;68(Suppl 1):S30–9. doi: 10.1016/j.theriogenology.2007.04.032. doi: 10.1016/j.theriogenology.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Hirobe S, He WW, Gustafson ML, MacLaughlin DT, Donahoe PK. Müllerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod. 1994;50:1238–43. doi: 10.1095/biolreprod50.6.1238. [DOI] [PubMed] [Google Scholar]
- 26.Szafarowska M, Molinska-Glura M, Jerzak MM. Anti-Müllerian hormone concentration as a biomarker of pregnancy success or failure. Neuro Endocrinol Lett. 2014;35:322–6. [PubMed] [Google Scholar]
- 27.Leyton L, Hagood JS. Thy-1 modulates neurological cell-cell and cell-matrix interactions through multiple molecular interactions. Adv Neurobiol. 2014;8:3–20. doi: 10.1007/978-1-4614-8090-7_1. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Molina R, Valdivia A, Kong M, Alvarez A, Cardenas A, Quest AF, et al. Thy-1-interacting molecules and cellular signaling in cis and trans. Int Rev Cell Mol Biol. 2013;305:163–216. doi: 10.1016/B978-0-12-407695-2.00004-4. doi: 10.1016/b978-0-12- 407695-2. 00004-4. [DOI] [PubMed] [Google Scholar]
- 29.Bukovsky A, Caudle MR, Keenan JA, Wimalasena J, Foster JS, Van Meter SE. Quantitative evaluation of the cell cycle-related retinoblastoma protein and localization of Thy-1 differentiation protein and macrophages during follicular development and atresia, and in human corpora lutea. Biol Reprod. 1995;52:776–92. doi: 10.1095/biolreprod52.4.776. [DOI] [PubMed] [Google Scholar]
- 30.Bukovsky A, Caudle MR, Svetlikova M, Upadhyaya NB. Origin of germ cells and formation of new primary follicles in adult human ovaries. Reprod Biol Endocrinol. 2004;2:20. doi: 10.1186/1477-7827-2-20. doi: 10.1186/1477-7827- 2- [DOI] [PMC free article] [PubMed] [Google Scholar]


