Abstract
Background:
Historically, to achieve higher pregnancy rates, multiple embryos were transferred after an in-vitro fertilisation (IVF). However, this practice is being reassessed, because it leads to multiple pregnancies that is known to cause adverse maternal and fetal outcomes.
Aim:
To compare the pregnancy outcomes in fresh IVF or intracytoplasmic sperm injection (ICSI) cycles among women undergoing elective single blastocyst transfer (eSBT) vs. those undergoing double blastocyst transfer (DBT).
Settings and Design:
It is a retrospective data analysis of 582 patients undergoing fresh IVF/ICSI cycles performed from January 2012 to June 2015.
Materials and Methods:
Patients, who underwent IVF/ICSI and developed more than one blastocyst, were included in the study. Donor cycles were excluded from the study. All the embryos were cultured to blastocyst stage in sequential media followed by transfer of two blastocysts (DBT) or eSBT and cryopreservation of the remaining.
Statistical Analysis:
Statistical analysis was performed using chi square test.
Results:
Out of 582 patients, in 149 patients one blastocyst was transferred and in 433 patients two blastocysts were transferred. There was no statistical difference in the biochemical pregnancy rate, clinical pregnancy rate and live birth rate in both the groups. Statistics demonstrated a significant drop in miscarriage rate in eSBT group. There was no incidence of twins in eSBT group, whereas twin birth rate per clinical pregnancy was 29.02% in DBT group.
Conclusion:
Single blastocyst transfer is an effective method to reduce the risk of multiple births without compromising the pregnancy outcomes. Given the promising potential of vitrification; the remaining blastocyst can be cryopreserved.
KEYWORDS: Cryopreservation, double blastocyst transfer, elective single blastocyst transfer, in vitro fertilisation, miscarriage, multiple pregnancy
INTRODUCTION
In 1978, the world’s first in vitro fertilisation (IVF) baby was born, since then IVF has accounted for more than five million births until 2013.[1] Earlier to compensate for low implantation rates and achieve acceptable pregnancy rates, multiple embryos were transferred in most of the IVF set-ups. Extensive body of literature has demonstrated that multiple gestations carry an increased risk of adverse outcomes. As documented in the ASRM Practice Committee Document titled ’Multiple pregnancy association with infertility therapy’, multiple gestation leads to adverse maternal outcomes such as miscarriage, premature labour, gestational diabetes and preeclampsia.[2] It also has negative impact on fetal outcomes such as low birth weight, fetal death and long-term disabilities such as cerebral palsy.[2] A study published by European Society of Human Reproduction and Embryology on 50,258 births following IVF and intracytoplasmic sperm injection (ICSI) pregnancies reported that twins accounted for half of the total neonatal deaths and one-third of the perinatal deaths.[3] Apart from these medical conditions, delivery of multiple babies and their long-term care incurs heavy economic costs on the community. Assisted reproductive technology (ART) still accounts for 33% of all twins and 77% higher order multiple gestations in USA.[4] As a result, annual expenditures for iatrogenic preterm deliveries total twenty-six billion dollars on healthcare costs.[5,6]
With the continuous development in embryo culture media and embryo culture systems, it is possible to successfully culture human embryos till the blastocyst stage.[7] Several studies have shown that higher pregnancy rates can be achieved with blastocyst transfer compared to cleavage stage embryo transfer. Superior implantation rates can be achieved in blastocyst transfer due to better embryo selection.[8]
The pregnancy rates in IVF have reportedly doubled between 1994 and 2003 despite decrease in the mean number of embryos transferred.[9] These advances can be attributed to better understanding of embryo selection process, embryo transfer technique, ovarian stimulation and cryopreservation. We can restrict the risks of multiple pregnancies and increase the cumulative pregnancy rates by the optimum use of embryos formed and cryopreservation of supernumerary embryos.
Elective single embryo transfer is defined in Society for Assisted Reproductive Technologies as ’an embryo transfer in which more than one high quality embryo exists but it was decided to transfer only one embryo’.[10] eSET rates are comparatively higher in Europe, with Sweden (69%) reporting highest rate of eSET.[10]
Aim of our study was to compare the pregnancy outcomes between elective single blastocyst transfer (eSBT) and double blastocyst transfer (DBT) in non-donor cycles irrespective of the maternal age.
MATERIALS AND METHODS
Period and patients
The study involved a total of 582 fresh IVF cycles performed over a period of three and half years, from January 2012 to June 2015. Patients underwent either standard insemination or ICSI depending on whichever was clinically appropriate. Maternal age was between 21 and 44 years of age with the mean age of 31.27 years. One of the inclusion criteria for the patients was that they should have developed more than one blastocyst. All the donor cycles were excluded from this study. Patients were divided into two groups depending on the number of blastocyst transferred. eSBT included the patients who transferred only one blastocyst and cryopreserved the remaining blastocyst, whereas DBT group included patients who transferred two blastocyst and may or may not have cryopreserved remaining blastocyst. Cryopreservation was performed using vitrification protocol.
Ethical clearance was obtained from institutional scientific committee.
Ovarian stimulation protocol
Controlled ovarian stimulation was performed using antagonist protocol along with administration of gonadotropin for 8–14 days. Patients received recombinant follicular stimulating hormone (FSH) or highly purified human menopausal gonadotropin. Daily dosages were adjusted based on the anticipated follicular response. Follicular development was monitored by transvaginal ultrasonography. When majority of follicles reached 18 mm size, human chorionic gonadotropin (hCG) was administered. The oocytes were collected 35 h post-hCG administration. Oocyte retrieval was performed under general anaesthesia with ultrasound guidance.
Embryo culture
According to the indication of a patient, conventional insemination or ICSI was performed 2–3 h post-oocyte retrieval. Oocytes were checked for fertilisation 18–20 h post IVF/ICSI by presence of two pronuclei. All the embryos were cultured in sequential media (G1 PLUS/G2 PLUS, Vitrolife, Sweden) till day 5. Blastocyst grading was performed according to Gardener and Schoolcraft blastocyst grading system.[11] Blastocyst were graded based on the level of expansion, inner cell mass (ICM) and outer trophectoderm (TE). Degree of the expansion was graded from 1 to 6, where 1 refers to an early blastocyst, wherein blastocoel cavity occupies less than half the volume of the embryo, 2 refers to a blastocyst with cavity more than half the volume of the embryo, 3 refers to a full blastocyst with cavity filling more than half the volume of the embryo with slight expansion in overall size, 4 refers to fully expanded blastocyst with thinning of zona pellucida, 5 refers to hatching blastocyst and 6 refers to a hatched blastocyst. The quality of ICM was assessed from A to C, where A stands for many tightly packed cells, B stands for several loosely packed cells and C stands for very few loosely packed cells. The outer TE quality was also assessed from A to B, wherein A stands for many cells forming a cohesive layer, B stands for few cells forming a loose epithelium and C stands for very few large cells.
Patient counselling
Patients were counselled before the embryo transfer by their infertility specialist about the advantages and disadvantages of single and DBT. Ultimately the final decision regarding how many blastocyst to be transferred was made by the patient.
Patient preparation
After the oocyte retrieval, patient was instructed to take progesterone vaginal suppository 400 mg twice a day till the pregnancy test. Embryo transfer (ET) was performed on day 5 after oocyte retrieval. Patient was asked to take blood test for the detection of beta-human chorionic gonadotrophin (βhCG) on 11th day after ET. βhCG value ≥100 mIU/mL was considered positive, if βhCG value was between 10 and 100 mIU/mL, the patient was asked to repeat the test after 48 h. Transvaginal ultrasound examination was performed 1 week after the positive βhCG to confirm the presence of gestational sac.
Statistical analysis
Number of blastocyst transferred was the primary explanatory variable. Patient parameters, IVF outcomes and pregnancy outcomes were compared between patients undergoing eSBT and DBT. Live birth rate was the primary outcome parameter and biochemical pregnancy rate, clinical pregnancy rate, implantation rate, miscarriage rate, and twins per pregnancy rate were the secondary outcome parameters and were calculated using standard formulas. Patient parameters such as age, days of stimulation, total dose of gonadotropins, estradiol and progesterone levels on the day of hCG trigger and endometrial thickness on hCG were considered as explanatory parameters. IVF outcomes such as fertilisation rate, cleavage rate and blastocyst formation rate were also calculated using standard formulas. Descriptive analysis of all the explanatory and outcome parameters was performed using mean and standard deviation for quantitative variables, frequency and proportion for categorical variables. Data were checked for compliance with normal distribution by Shapiro Wilk test and normality plots. The statistical significance of the differences in rates of various events between both the groups was assessed using chi square test. P value <0.05 was considered as statistically significant. IBM Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 21.0 software was used for statistical analysis.
RESULTS
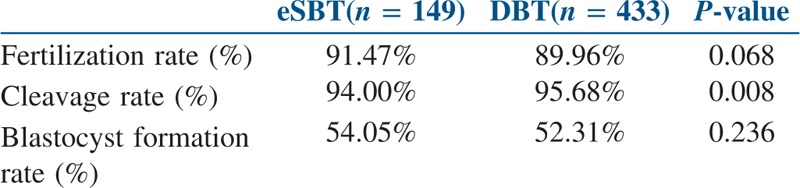
A total of 582 blastocyst transfers were undertaken in the study period. Out of which, 149 patients underwent fresh eSBT, and 433 patients underwent fresh DBT. As shown in Table 1, both the groups showed no statistical difference in mean patient age, progesterone on the day of hCG trigger, estradiol on the day of hCG trigger, oocyte number and endometrial thickness. However, the total days of stimulation and the total dose of gonadotropins were statistically higher in DBT group (P value 0.007 and 0.022 respectively). As summarised in Table 2, statistically significant differences were not observed in the fertilisation rate of mature oocytes and blastocyst formation rate between both the study groups. The cleavage rate was statistically higher in DBT group as compared to eSBT group (P value 0.008). All the 149 patients undergoing eSBT cryopreserved supernumerary blastocyst. In DBT group, 253 patients cryopreserved their remaining blastocyst, whereas 180 patients exhausted all their blastocyst in the fresh transfer itself, thus, having nothing to freeze (eSBT − 100% and DBT − 58.42%).
Table 1.
Patient parameters in both the groups
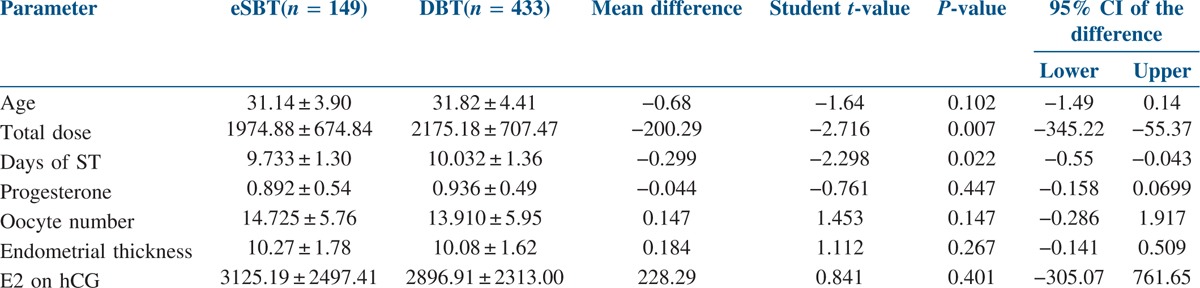
Table 2.
IVF/ICSI outcomes in both the groups
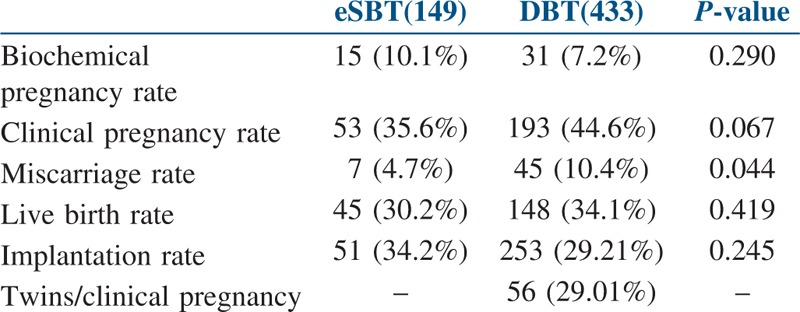
There was no statistical difference between eSBT and DBT groups with regard to biochemical pregnancy rate (15/149, 10.1% vs. 31/433, 7.2%, P value 0.290), clinical pregnancy rate (53/149, 35.6% vs. 193/433, 44.6%, P value 0.067), live birth rate (45/149, 30.2% vs. 148/433, 34.1%, P value 0.419) and implantation rate (51/149, 34.2% vs. 253/866, 29.21%, P value 0.245). Miscarriage rate was significantly lower in eSBT group (7/149, 4.7% vs. 45/433, 10.4%, P value 0.044). Significantly larger population of DBT cycles resulting in clinical pregnancy showed twin gestations, whereas there was no incidence of twin gestations in the eSBT group (0/149, 0% vs. 56/193, 29.01%) [Table 3].
Table 3.
Various pregnancy outcomes after IVF/ICSI
We performed an age-wise subgroup analysis of the data and derived the following results. Implantation rates for patients who underwent eSBT were higher in all age groups (20–30 years − 34.2%, 31–35 years − 34.48%, 36–40 years − 35.29%) except in the age group of >40 (0%), in comparison with those who underwent two blastocyst transfer (20–30 years − 32.0%, 31–35 years − 27.64%, 36–40 years − 25.73%, >40 years − 29.41%). The difference in implantation rates was statistically significant (P value <0.01) in the age groups 20–30 and 31–35 years.
There was no significant difference in clinical pregnancy rates in eSBT vs. DBT in all age groups; 20–30 years of age (37.0% vs. 46.6%, P value 0.207), 31–35 years of age (32.8% vs. 44.7%, P value 0.125), 36–40 years of age (41.2% vs. 39.7%, P value 1.000), >40 years of age (0% vs. 41.2%) [Table 4].
Table 4.
Age-wise distribution of results
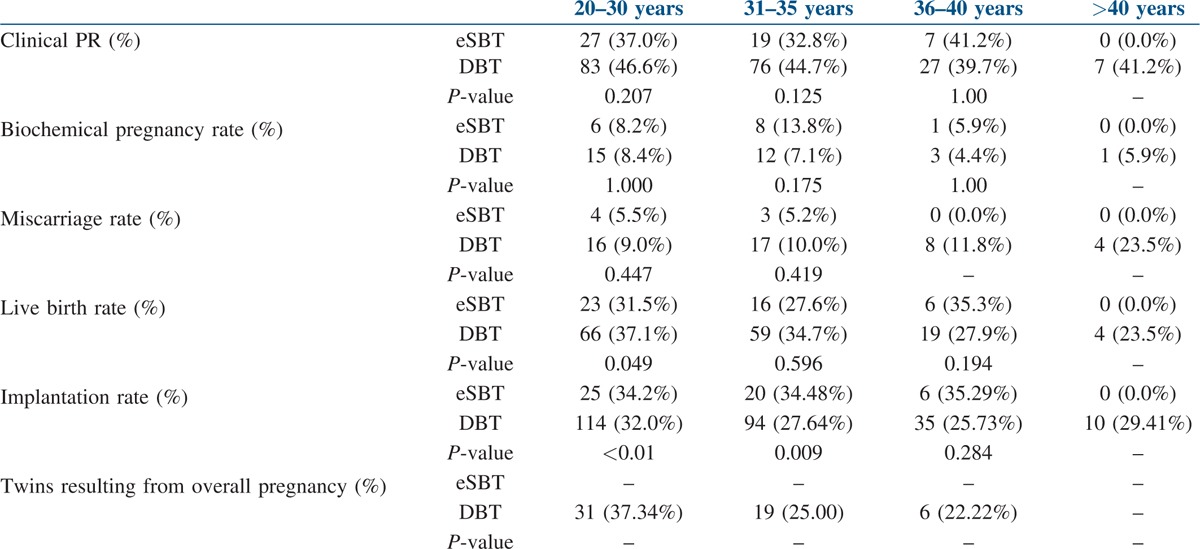
Further, there were no significant differences in live birth rate for eSBT vs. DBT in age groups, 31–35 years of age (27.6% vs. 34.7%, P value 0.596), 36–40 years of age (35.3% vs. 27.9% P value 0.194), >40 years of age (0% vs. 23.5%); however, in the age group of 20–30 years the DBT group showed statistically significant higher live birth rates (37.1%, vs. 31.5%, P value 0.049).
There was no statistically significant difference between eSBT and DBT groups with regard to miscarriage rate and biochemical pregnancy rates in different age groups. In the overall analysis without age stratification, we noticed a statistically significant higher miscarriage rate in the DBT group (10.4% vs. 4.7%, P value 0.044); after doing the age-wise sub-group analysis, the difference was found to be statistically not significant in all the groups, which might be due to small sample size in each age group.
The twin gestation rate was zero in the eSBT group, and in the DBT group, the twinning rates in all age categories (20–30, 31–35, 36–40, >40) were 37.34, 25.0, 22.2 and 0% respectively.
DISCUSSION
After its inception, the first two decades of IVF saw practice of transferring multiple embryos to diminish the impact of low success rates, which resulted in unacceptable high rates of higher order pregnancies. First case of twins due to IVF was reported in 1981, since then multiple gestations and IVF became inseparable.[12,13] Multiple gestations are associated with significant perinatal and neonatal complications such as pregnancy loss, congenital abnormalities, preterm delivery and perinatal mortality.[14] However, with the progressive success of newer techniques in the field of ART, there has been a considerable reduction in triplets and higher order pregnancies in IVF, but another persistent dilemma of twin gestation is still maintained.[15] Similarly, even the financial impact due to health care costs of multiple gestations has remained persistent through these years. Earlier, when the incidence of multiple gestations was 15–30% due to IVF, the delivery expenses were up to four times more per child for multiple gestations compared to single gestations.[16] The European Society for Human Reproduction and Embryology has emphasised that the achievement of singleton pregnancy alone should be considered the quality of care parameter in IVF program.[17]
Physicians tend to transfer more embryos in women of advanced maternal age, as age has been known to be a negative predictor of IVF success.[18] These women are already at a higher risk of developing complications such as gestational diabetes and preeclampsia. For example, women over the age of 35 with preeclampsia have three times the risk of pregnancy-related mortality as compared to their younger counterparts.[19,20] Studies available in literature compare the eSBT with the DBT in patients less than 35 years of age.[21] Few European studies have addressed the role of eSET in advanced maternal age population. One such a study found that eSET could be applied to patients aged 36–39, while drastically decreasing the multiple gestation rate and achieving similar pregnancy rates.[22]
According to data compiled by Indian Society of Assisted Reproduction (ISAR) in the year 2013, the percentage of cycles undergoing the transfer of three embryos decreased from 47.3% in the year 2007 to 43.09% in the year 2009.[23] This also reflected in decrease in triplet gestation rate from 3.8 to 2.9% in these years, whereas, the double embryo transfer rates increased from 21.4 to 24.78% from 2007 to 2009. Single embryo transfer was performed in 10% of IVF/ICSI cycles and remained the same in all the 3 years.[23] Around 74% of all the pregnancies in the three-year period (2007–2009) were singleton gestations, and 22% of all the pregnancies were twin pregnancies.[23] In general, eSET is becoming increasingly popular in certain countries where IVF treatment is covered by the states within. In India, the IVF treatment is not offered in government hospitals, ART is very expensive and only a few can afford them. As the IVF costs are typically borne by the patients themselves, multiple embryos are transferred to get higher pregnancy rates, because infertile couples do not always perceive multiple pregnancies as an unwelcome side effect; they would rather take the risk to increase their pregnancy chances in a single IVF cycle. Hence, because there is a lack of data in India as far as eSET is concerned, we decided to take up this study to evaluate the efficacy of eSET vs. DET.
Having understood the complications of multiple gestations and to reduce the number of embryos transferred, it is important to select one best quality embryo. Culturing the embryos till blastocyst stage allows the selection of more chromosomally normal embryos and increases the chance of transferring euploid embryos.[24] It has been demonstrated by many investigators that in women older than 36 years of age, 59% of the top quality cleavage stage embryos might show aneuploidy, whereas 35% of top quality blastocyst might show aneuploidy.[24,25,26] It is a known fact that the embryo physiologically reaches the endometrium only 5 days after ovulation; hence it is more logical to transfer day 5 blastocyst.[27] In support of the move towards single embryo transfer (SET), we identified nine prospective randomised trials on blastocyst transfer.[28,29,30,31,32,33,34,35,36] Five studies have reported a significant increase in the implantation rates when embryos were transferred at the blastocyst stage, whereas three trials reported no difference in the implantation rate with respect to the day of transfer.
Several randomised studies have proven the merits of single embryo transfer for reducing the twin gestation rate, while maintaining acceptable pregnancy rates[21,37] In a prospective randomised trial, Gardner et al.[38] compared pregnancy outcomes in patients undergoing either one or two blastocyst, and that there was no difference in implantation or pregnancy rates between the two groups, although there was a significant increase in multiple gestation rate, with no incidence of multiple pregnancy in patients receiving a single blastocyst. Mullin et al. compared pregnancy outcomes in eSBT vs. DBT for women aged below 40. The findings of this study suggested that eSBT was associated with a statistically significant reduction (92%) in the twinning rate (25% in DBT group vs. 2% in eSBT group) while maintaining a high clinical pregnancy rates (PR) (63% in the eSBT group vs. 61% in the DBT group).[39]
When comparing fresh eSBT and DBT cycles in this study, it was found that parameters which influence the pregnancy outcomes such as mean age, IVF characteristics namely the level of E2 and P4 on day of trigger, oocyte number, endometrium thickness and blastocyst formation rate were similar in both the groups. The characteristics such as the number of days of ovarian stimulation and the dose of gonadotropins used were significantly higher in DBT group, but it is unlikely to influence the pregnancy outcome. Our study suggests that in clinical practice, when two blastocysts are available for transfer, DBT will not increase the live birth rate per transfer cycle, while significantly increasing the multiple pregnancy rates.
Because of the retrospective nature of this study, one weakness of this study might be the relatively small sample size in eSBT group; this was undoubtedly due to the perception of the patients that single embryo transfer could result in lower pregnancy rates as compared to multiple embryos. In most of the cases, physicians are reluctant to move towards the transfer of a single embryo if the age of the woman is more than 35 years. The main strength of our study is that as our data include women up to the maximum age of 44 years. In our study, if we look into the subgroup analysis in the age group of 36–40 years, there is no statistically significant difference in the implantation rate, clinical pregnancy rate and live birth rate between eSBT and DBT. In this age group, the incidence of twin gestation is 22.22% in DBT as compared to none in eSBT. Our data are encouraging, and the option of eSBT can be considered for older patients (>35 years of age) with blastocyst available for cryopreservation, who prefer to avoid multiple gestation. The live birth rates were comparable up to age 40 years in our study. Among patients between 40 and 44 years of age, only one woman went in for eSBT, and it did not result in pregnancy. So in our study, the comparison of eSBT and DBT in this age group was not possible to arrive at a conclusion.
Among the multiple gestations resulting from ART, few studies have demonstrated an increased risk of monozygotic twinning, with some suggesting added risk for monozygotic twinning with blastocyst transfer.[40] However, it has been reported that with an increased experience in blastocyst culture and transfer, the overall blastocyst monozygotic twinning rate has decreased over the years to the range of something that is seen with the cleavage stage transfer (0.4%).[41,42] In our study, we did not see any incidence of monozygotic twinning; hence the twin rate/clinical pregnancy was 0% in the eSBT group.
Interestingly, despite a higher proportion of clinical pregnancy seen in the DBT group (eSBT − 35.6% vs. DBT − 44.6%), similar live birth rates were seen in both the groups. This can be attributed to a significantly higher number of miscarriages seen in the DBT group (eSBT − 4.7% vs. DBT − 10.4%). eSBT ultimately yields similar pregnancy outcome as DBT, but there may be more adverse implications for the pregnancy through the implantation of more than one embryo. Physicians should consider this as well before deciding between eSBT and DBT.
CONCLUSION
We have to maintain a balance between favourable pregnancy success and minimise the most common complication of IVF- Multiple pregnancy. A positive pregnancy test is not a success, but a healthy baby is. eSBT is the answer to avoid all the complications related with multiple pregnancy (MP). With the advances in freezing protocols such as vitrification, it is possible to successfully cryopreserve human blastocysts. This will increase the cumulative pregnancy rate per oocyte harvest and even allows patients to go for a second child in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to acknowledge Dr. Basavaraj Devarashetty and Dr. Lata Nelivigi for providing subjects for the study. We would like to thank Dr. Murali Reddy for his contribution in statistical analysis. We express our gratitude to the entire embryology and nursing staff of Cloudnine Fertility.
REFERENCES
- 1.Esme IK, Bhattacharya S, van der Veen F, Mol BW, Templeton A. Are we overusing IVF? J BMJ. 2014;348:252. doi: 10.1136/bmj.g252. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Assisted Reproductive Medicine. Multiple pregnancy association with infertility therapy. J Fertil Steril. 2006;86:106–10. [Google Scholar]
- 3.Sullivan E. Single embryo transfer reduces the risk of perinatal mortality in IVF. ESHRE Press Release. 2012:0–247. doi: 10.1093/humrep/des315. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AD, Jamieson DJ, Jones HW, Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 5.Allen BD, Adashi EY, Jones HW. On the cost and prevention of iatrogenic multiple pregnancies. Reprod Biomed Online. 2014;29:281–5. doi: 10.1016/j.rbmo.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Kissin DM, Kulkarni AD, Kushnir VA, Jamieson DJ. Number of embryos transferred after in vitro fertilization and good perinatal outcome. J Obstet Gynecol. 2014;123:239–47. doi: 10.1097/AOG.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papankiolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyststage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 8.Criniti A, Thyer A, Chow G, Lin P, Klein N, Soules M. Elective single blastocyst transfer reduces twin rates without compromising pregnancy outcomes. J Fertil Steril. 2005:1613–9. doi: 10.1016/j.fertnstert.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Waters A-M, Dean JH, Sullivan EA. Assisted Reproduction Technology in Australia and New Zealand. Sydney, Australia: AIHW National Perinatal Statistics Unit; 2006. [Google Scholar]
- 10.American Society of Assisted Reproductive Medicine. Elective single-embryo transfer. J Fertil Steril. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Janson R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth: Parthenon Press; pp. 378–88. [Google Scholar]
- 12.Feichtinger W, Szalay S, Kemeter P, Beck A, Janisch H. Twin pregnancy after laparoscopic oocyte recovery, in-vitro fertilization and embryotransfer. J Geburtshilfe Frauenheilkd. 1982;42:197. doi: 10.1055/s-2008-1037262. [DOI] [PubMed] [Google Scholar]
- 13.Kerin J, Quinn P, Kirby C, Seamark R, Warnes G, Jeffrey R, et al. Incidence of multiple pregnancy after in-vitro fertilisation and embryo transfer. J Lancet. 1983;322:537–40. doi: 10.1016/s0140-6736(83)90569-x. [DOI] [PubMed] [Google Scholar]
- 14.Tummers P, De Sutter P, Dhont M. Risk of spontaneous abortion in singleton and twin pregnancies after IVF/ICSI. J Hum Reprod. 2003;18:1720–3. doi: 10.1093/humrep/deg308. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni AD, Jamieson DJ, Jones HW, Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 16.Callahan TL, Hall JE, Ettner SL, Christiansen CL, Greene MF, Crowley WF., Jr The economic impact of multiple-gestation pregnancies and the contribution of assisted-reproduction techniques to their incidence. N Engl J Med. 1994;331:244–9. doi: 10.1056/NEJM199407283310407. [DOI] [PubMed] [Google Scholar]
- 17.The ESHRE Capri Workshop Group. Multiple gestation pregnancy. J Hum Reprod. 2000;15:1856–64. [PubMed] [Google Scholar]
- 18.Wang J, Sauer M. In vitro fertilization (IVF): A review of 3 decades of clinical innovation and technological advancement. J Ther Clin Risk Manag. 2006;2:355–364. doi: 10.2147/tcrm.2006.2.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int J Gynecol Obstet. 2001;75:221–8. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 20.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460–5. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 21.Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single embryo transfer versus double-embryo transfer in in vitro fertilization. New Eng J of Med. 2004;351:2392–402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 22.Veleva Z, Vilska S, Hydén-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H. Elective single embryo transfer in women aged 36–39 years. J Hum Reprod. 2006;21:2098–102. doi: 10.1093/humrep/del137. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra N, Shah D, Pai R, Pai HD, Bankar M. Assisted reproductive technology in India: A 3 year retrospective data analysis. J Hum Reprod. 2013;6:235–40. doi: 10.4103/0974-1208.126286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerris J, De Neoubourg D, Mangelschots K, Van Royen E, Van de Meerssche M, Valkenburg M. Prevention of twin pregnancy after in vitro fertilization or intracytoplasmic sperm injection based on strict embryo criteria: A prospective randomized clinical trial. J Hum Reprod. 1999;14:2581–7. doi: 10.1093/humrep/14.10.2581. [DOI] [PubMed] [Google Scholar]
- 25.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 26.Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in vitro fertilization or intracytoplasmic sperm injection. J Hum Reprod. 1998;13:2869–73. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 27.Diedrich K, Fauser BC, Devroey P, Griesinger G. The role of the endometrium and embryo in human implantation. J Hum Reprod. 2007;13:365–77. doi: 10.1093/humupd/dmm011. [DOI] [PubMed] [Google Scholar]
- 28.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- 29.Coskun S, Hollanders J, Al Hassan S, Al Sufyan H, Al Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: A controlled randomized trial. J Hum Reprod. 2000;15:1947–52. doi: 10.1093/humrep/15.9.1947. [DOI] [PubMed] [Google Scholar]
- 30.Karaki RZ, Samarraie SS, Younis NA, Lahloub TM, Ibrahim MH. Blastocyst culture and transfer: A step toward improved in vitro fertilization outcome. J Fertil Steril. 2002;77:114–8. doi: 10.1016/s0015-0282(01)02939-9. [DOI] [PubMed] [Google Scholar]
- 31.Levron J, Shulman A, Bider D, Seidman D, Levin T, Dor J. A prospective randomized study comparing day 3 with blastocyst-stage embryo transfer. J Fertil Steril. 2002;77:1300–1. doi: 10.1016/s0015-0282(02)03090-x. [DOI] [PubMed] [Google Scholar]
- 32.Utsunomiya T, Naitou T, Nagaki M. A prospective trial of blastocyst culture and transfer. J Hum Reprod. 2002;17:1846–51. doi: 10.1093/humrep/17.7.1846. [DOI] [PubMed] [Google Scholar]
- 33.Rienzi L, Ubaldi F, Iacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. J Hum Reprod. 2002;17:1852–5. doi: 10.1093/humrep/17.7.1852. [DOI] [PubMed] [Google Scholar]
- 34.Van der Auwera I, Debrock S, Spiessens C, Afschrift H, Bakelants E, Meuleman C, et al. A prospective randomized study: Day 2 versus day 5 embryo transfer. J Hum Reprod. 2002;17:1507–12. doi: 10.1093/humrep/17.6.1507. [DOI] [PubMed] [Google Scholar]
- 35.Frattarelli JL, Leondires MP, McKeeby JL, Miller BT, Segars JH. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: A randomized controlled trial. Fertil Steril. 2003;79:228–30. doi: 10.1016/s0015-0282(02)04558-2. [DOI] [PubMed] [Google Scholar]
- 36.Margreiter M, Weghofer A, Kogosowski A, Mahmoud KZ, Feichtinger W. A prospective randomised multicenter study to evaluate the best day for embryo transfer: Does the outcome justify prolonged embryo culture? J Assist Reprod Genet. 2003;20:91–3. doi: 10.1023/A:1021744209193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martikainen H, Tiitinen A, Tomás C, Tapanainen J, Orava M, Tuomivaara L, et al. One versus two embryo transfer after IVF and ICSI: A randomized study. J Hum Reprod. 2001;16:1900–3. doi: 10.1093/humrep/16.9.1900. [DOI] [PubMed] [Google Scholar]
- 38.Gardner D, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft W. Single blastocyst transfer: A prospective randomized trial. J Fertil Steril. 2004;81:551–5. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Mullin CM, Fino ME, Talebian S, Krey LC, Licciardi F, Grifo JA. Comparison of pregnancy outcomes in elective single blastocyst transfer versus double blastocyst transfer stratified by age. J Fertil Steril. 2010;93:1837–43. doi: 10.1016/j.fertnstert.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 40.Aston KI, Peterson CM, Carrell DT. Monozygotic twinning associated with assisted reproductive technologies: A review. J Reprod. 2008;136:377–86. doi: 10.1530/REP-08-0206. [DOI] [PubMed] [Google Scholar]
- 41.Moayeri SE, Behr B, Lathi RB, Westphal LM, Milki AA. Risk of monozygotic twinning with blastocyst transfer decreases over time: An 8-year experience. J Fertil Steril. 2007;87:1028–32. doi: 10.1016/j.fertnstert.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Bulmer MG. The Biology of Twinning in Man. Oxford, UK: Oxford University Press; 1970. [Google Scholar]


