Abstract
Objective
Executive functioning (EF) problems may serve as vulnerability or maintenance factors for Binge-eating Disorder (BED). However, it is unclear if EF problems observed in BED are related to overweight status or BED status. The current study extends this literature by examining EF in overweight and normal-weight BED compared to weight-matched controls.
Method
Participants were women with normal-weight BED (n=23), overweight BED (n=32), overweight healthy controls (n=48), and normal-weight healthy controls (n=29). The EF battery utilized tests from the National Institutes of Health (NIH) Toolbox and Delis-Kaplan Executive Function System (D-KEFS).
Results
After controlling for years of education and minority status, overweight individuals performed more poorly than normal-weight individuals on a task of cognitive flexibility requiring generativity (p < 0.01) and speed on psychomotor performance tasks (p = 0.01). Normal-weight and overweight BED performed worse on working memory tasks compared to controls (p = 0.04). Unexpectedly, normal-weight BED individuals out-performed all other groups on an inhibitory control task (p < 0.01). No significant differences were found between the four groups on tasks of planning.
Discussion
Regardless of weight status, BED is associated with working memory problems. Replication of the finding that normal-weight BED is associated with enhanced inhibitory control is needed.
Keywords: Executive functioning, neuropsychology, Binge-eating Disorder, obesity, normal-weight
Introduction
Binge-eating Disorder (BED) is the most prevalent eating disorder, affecting approximately 3.5% of the population and is characterized by recurrent episodes of binge-eating accompanied by a loss of control over eating (Hudson, Hiripi, Pope, & Kessler, 2007). BED is highly comorbid with obesity, as over 40% of individuals with BED are obese (Hudson et al., 2007). Moreover, BED appears to confer greater medical, psychological and psychosocial problems than obesity alone, including depression and serious cardiovascular events (Bulik, Sullivan, & Kendler, 2002; Succurro et al., 2015).
Preliminary evidence suggests that executive functions (EFs), broadly defined as self-regulatory skills enabling one to engage in self-initiated and adaptive goal-directed behaviors, may be compromised in both individuals with BED and obesity (Fitzpatrick, Gilbert, & Serpell, 2013; Voon et al., 2015). EFs are often referred to as “higher level” abilities due to their role in modulating “lower level” abilities, such as feeding behaviors (Gilbert & Burgess, 2008). Evidence across multiple populations suggests that EFs are correlated, but separable, activating both common and specific neural areas, a pattern described as “unity and diversity” (Stuss & Alexander, 2007). It has been proposed that difficulties in EF may contribute to both the development and maintenance of binge-eating and obesity (Boeka & Lokken, 2008; Cohen, 2008); however, a greater understanding of specific EF problems for those with BED, above and beyond weight status, is needed.
Cognitive flexibility
Impaired cognitive flexibility may lead to an over-focus on eating as a coping strategy. In two of the six studies available, overweight individuals with BED exhibit poorer cognitive flexibility than controls (Aloi et al., 2015; Svaldi, Brand, & Tuschen-Caffier, 2010). Other studies showing no significant differences were limited by including either sub-threshold BED group or those without a current diagnosis of BED (Duchesne et al., 2010; Galioto et al., 2012; Lavender et al., 2014; Manasse et al., 2014). To our knowledge, there has been one study that included normal-weight women who engaged in either subjective or objective binge-eating episodes compared to normal-weight women with no eating disordered behavior (Kelly, Bulik, & Mazzeo, 2013). This study found no group differences on a measure of cognitive flexibility, but did find a significant negative correlation between body mass index (BMI) and performance.
Inhibitory control
Inhibitory control deficits may contribute to eating in response to a trigger as well as the drive to continue eating despite being uncomfortably full. Of the seven studies that have examined this construct in BED, three found significant differences, with individuals with BED demonstrating poorer performance on measures of inhibitory control (Manasse et al., 2015; Manasse et al., 2014; Mobbs, Iglesias, Golay, & Van der Linden, 2011). Three studies (Duchesne et al., 2010; Voon et al., 2014; Wu et al., 2013) did not find differences in inhibitory control and one study found that overweight individuals with BED performed better than their weight-matched controls (Mole et al., 2015).
Planning
Difficulties with planning could explain an inability to develop and engage in adaptive behaviors to prevent overeating or binge-eating episodes. Poorer performance on measures of planning have been demonstrated in overweight BED when compared to overweight non-BED individuals in two studies utilizing different assessment measures (Duchesne et al., 2010; Manasse et al., 2015).
Working memory
To date, studies examining working memory deficits in BED samples have been inconclusive, with two studies showing that overweight individuals with BED display poorer working memory capacity than overweight individuals without BED (Duchesne et al., 2010; Manasse et al., 2014) and two studies finding no group differences (Galioto et al., 2012; Muller et al., 2014).
Motor control
Although not defined as a measure of EF, psychomotor performance, the coordination of a cognitive activity and motor performance is thought to be affected by subtle disturbances in the EF network (Reijmer et al., 2013). Of the three studies (Ariza et al., 2012; Cournot et al., 2006; Etou et al., 1989) examining psychomotor processing speed in obesity, two studies showed that obese individuals have slower processing speed compared to normal-weight controls (Cournot et al., 2006; Etou et al., 1989). No studies have examined psychomotor processing speed in individuals with BED.
The primary aim of the current study is to assess the executive functioning constructs associated with weight status and BED by comparing normal-weight BED, overweight BED and healthy, weight-matched controls. While studies have used participant groups with sub-threshold (Kelly et al., 2013; Manasse et al., 2015; Manasse et al., 2014) or history of BED, this study extends the current literature in dissociating the effects of weight status from BED diagnosis. Previous research has shown that obese individuals with and without BED perform more poorly relative to normal-weight healthy individuals on measures of cognitive flexibility, inhibitory control, planning, working memory, and psychomotor processing speed. Due to the related but distinct functions of EFs, we have examined these constructs separately (Galioto et al., 2012). We predicted that BED and overweight status would have a significant effect in these EF domains, however without sufficient past literature we are unable to hypothesize whether the effects of each condition would interact or be additive. The paucity of studies exploring executive functioning in normal-weight BED made it difficult to predict directional hypotheses regarding normal-weight BED. Findings involving the normal-weight BED group are exploratory.
Method
Participants
Participants (N=132) were part of an ongoing research study aimed at investigating the differential effects of BED and weight in adult women aged 18 – 65 years. All participants were assessed at TEDp (Temple Eating Disorders program). Four groups were recruited: overweight women with BED (n = 32), normal-weight women with BED (n = 23), overweight women without BED (n = 48), and normal-weight women without BED (n = 29). Participants were recruited using flyers and local newspaper advertisements. Advertisements for BED and non-BED were identical with the exception of the question “Do you binge-eat?” on advertisements targeting BED participants. Participants received monetary compensation for their participation. No additional incentive or treatment was provided.
Inclusion/exclusion criteria
Individuals were excluded during an initial phone screening if they reported medical conditions including diabetes, seizure disorder, or history of head injury with a loss of consciousness. Individuals in the healthy control groups were excluded if they had any lifetime psychiatric conditions. Individuals with hypertension, high blood pressure, and hypothyroidism were excluded unless their conditions were managed with medication and had been stable for at least 6 months. BED was diagnosed based on DSM-5 (American Psychiatric Association [APA], 2013) criteria. Normal-weight individuals had a BMI between 18.5 and 24.9 kg/m2 and overweight individuals had a BMI ≥25 kg/m2. See Table 1 for details.
Table 1.
Demographics
| Variable | OW-BED (n = 32) | NW-BED (n = 23) | OW-HC (n = 48) | NW-HC (n = 29) |
|---|---|---|---|---|
| Age (M, SD) | 36.34 (2.03) | 23.34 (0.67) | 38.04 (1.78) | 24.52 (1.23) |
| Body Mass Index (M, SD) | 34.20 (0.83) | 22.93 (0.40) | 31.30 (0.56) | 21.56 (0.29) |
| Years of Education (M, SD) | 13.84 (2.37) | 15.39 (2.04) | 15.10 (2.23) | 15.21 (1.59) |
| Race (n, %) | ||||
| White | 31.25 (10) | 16 (70.00) | 16 (33.33) | 22 (76.00) |
| Black | 59.38 (19) | 2 (8.70) | 30 (62.50) | 2 (6.90) |
| Hispanic/Latino | 1 (3.13) | 0 (0) | 1 (2.08) | 1 (3.45) |
| Asian | 1 (3.13) | 5 (21.74) | 1 (2.08) | 4 (13.79) |
| Multiracial | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) |
| Objective Binge Episodes (M, SD) | 3.27 (1.33) | 2.40 (1.24) | 0 (0) | 0 (0) |
| Duration of illness (M, SD) | 14.46 (12.02) | 6.87 (5.84) | 0 (0) | 0 (0) |
| Psychiatric Comorbidity (n, %) | ||||
| Current Mood | 1 (3.13) | 4 (17.40) | 0 (0) | 0 (0) |
| Lifetime Mood | 5 (15.60) | 6 (26.10) | 0 (0) | 0 (0) |
| Current Anxiety | 4 (12.50) | 6 (26.10) | 0 (0) | 0 (0) |
| Lifetime Anxiety | 0 (0) | 2 (8.70) | 0 (0) | 0 (0) |
| Current Substance | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lifetime Substance | 2 (6.30) | 0 (0) | 0 (0) | 0 (0) |
| Medical Comorbidity (n, %) | ||||
| Type II Diabetes | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypertension | 1 (3.13) | 0 (0) | 3 (6.25) | 0 (0) |
| Thyroid Condition | 1 (3.13) | 0 (0) | 2 (4.16) | 1 (3.45) |
| Rate of Medications (n, %) | 4 (12.50) | 3 (13.04) | 5 (10.42) | 4 (13.79) |
| Raven’s Progressive Matrices (M, SD) | 27.26 (26.86) | 54.70 (26.26) | 29.21 (20.46) | 50.55 (25.68) |
Note: OW-BED = Overweight Individuals with Binge Eating Disorder; NW-BED = Normal-weight individuals with Binge Eating Disorder; OW-HC = Overweight Healthy Controls; NW-HC = Normal-weight Healthy Controls; BMI = Body Mass Index; Objective Binge Episodes reported per week; Duration of illness is reported in years.
Procedure
In the first session, participants were screened for eating disorder pathology and psychological disorders with measures described below by Masters-level PhD candidates (KE, AY, and JMA) and diagnoses were confirmed at a weekly best-estimate meeting with a licensed clinical psychologist (EYC) (Klein, Ouimette, Kelly, Ferro, & Riso, 1994; Kosten & Rounsaville, 1992). Weight and height were taken using a scale with a stadiometer to calculate BMI. Neuropsychological measures were administered in accordance with published manuals in the second session.
Measures
Eating Disorders Examination (EDE) Version 16.0 is a standardized semi-structured interview, measuring the severity and frequency of eating disorder psychopathology (Fairburn, Cooper & O’Connor, 2008). The BED module of the EDE reliably assesses objective and subjective binge-eating episode frequency (Grilo, Masheb, Lozano-Blanco, & Barry, 2004; Wilfley, Schwartz, Spurrell, & Fairburn, 1997). The EDE has good internal consistency as well as high test-retest and inter-rater reliability (Rizvi, Peterson, Crow, & Agras, 2000). BED diagnosis was assigned based on DSM-5 (APA, 2013) criteria captured using the EDE. Participants reported when binge-eating became a regular occurrence, which was used to calculate illness duration.
The Structured Clinical Interview for DSM–IV (SCID-I) (First, 2002) is a semi-structured clinical interview that was used to diagnose Axis-I disorders in accordance with the DSM-IV-Text Revision (APA, 2000). The SCID-I has adequate inter-rater reliability (First, 2002).
Delis-Kaplan Executive Function System (D-KEFS) is a comprehensive set of tests that assess higher-level cognitive functions (Delis, Kramer, Kaplan, & Holdnack, 2004). The following subtests were administered: Trail Making Test, Verbal Fluency, Design Fluency, Color-Word Interference Test, and Tower Test. Age-scaled scores were used for the analysis, with scaled scores having a mean of 10 and a standard deviation of 3. See Table 2 for a description of tests and associated EF constructs.
Table 2.
Demographic Correlations
| Pearson Correlations | Education | Age | Minority Status | Raven’s Matrices | |
|---|---|---|---|---|---|
|
| |||||
| Education | Correlation | 1 | −.03 | −.23** | .40** |
| Sig. (2-tailed) | .72 | .01 | <.01 | ||
|
| |||||
| Age | Correlation | −.03 | 1 | .20* | −.37** |
| Sig. (2-tailed) | .72 | .03 | <.01 | ||
|
| |||||
| Minority Status | Correlation | −.23** | .20* | 1 | −.36** |
| Sig. (2-tailed) | .01 | 0.03 | <.01 | ||
|
| |||||
| Raven’s Matrices | Correlation | .42** | −.37** | −.36** | 1 |
| Sig. (2-tailed) | <.01 | <.01 | <.01 | ||
Note:
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed). Raven’s Matrices are scored with age norms. Minority Status (0=Caucasian, 1 = All other races/ethnicities).
Raven’s Progressive Matrices (RPM) assesses non-verbal abstract reasoning and is regarded as an estimate of fluid intelligence (Raven, 1998).
NIH Toolbox Cognition Battery comprises standardized, computer-administered tests assessing EF (Figley, Asem, Levenbaum, & Courtney, 2016). Age-adjusted standardized scores have a mean of 100 and a standard deviation of 15. See Table 2 for details.
Data Analytic Plan
All analyses were conducted using the IBM Statistical Package for Social Sciences (SPSS) (version 24.0). Skewness and kurtosis were examined for all EF variables to determine normal distribution. Age-scaled scores for all the EF measures were used for analyses, thus age was not included as a covariate. Previous neuropsychological research suggests that statistically controlling for age is unnecessary when using age-adjusted standard scores (Agbayani & Hiscock, 2013; Salthouse, 2013). Minority status was coded as 0=Caucasian and 1=non-Caucasian (African-American, Hispanic/Latina, Asian or multiracial). A Chi-square test assessed minority status differences (Caucasian vs. non-Caucasian) between the groups. If minority composition differed between groups, minority status was included as a covariate. Likewise, years of education were compared between groups via one-way ANOVA and if significant differences existed, years of education was included as a covariate.
Primary analyses compared the four groups (i.e., NW-HC, OW-HC, NW-BED, OW-BED) on each of the five EF constructs (i.e., cognitive flexibility, inhibitory control, planning, psychomotor performance, working memory) using five multivariate analyses of co-variance (MANCOVA). If the multivariate F was significant where α = .05, follow-up ANCOVAs on individual EF tests were examined. Where significant univariate mean effects were found, where α = .05, SPSS-Bonferroni adjusted p-values were reported for post-hoc group comparisons. Two-sided planned contrasts, one for weight class (NW versus OW) and one for diagnosis (BED versus HC) were conducted within each construct with Bonferroni adjusted p-values reported.
Results
Demographics
Table 1 contains demographic information and Table 2 shows correlations between demographic variables. Groups differed significantly on age (F(3, 124) = 9.13, p < 0.01), such that overweight individuals were older than normal-weight individuals. Groups differed significantly on years of education (F(3, 124) = 8.86, p < 0.01), such that overweight individuals had fewer years of education than normal-weight individuals. Groups differed significantly on race (χ2[3] = 38.08, p < 0.01), with a higher proportion of individuals of minority status in the OW groups than the NW groups. Groups differed significantly on their performance on the RPM test (F(3, 124) = 8.59, p = 0.02), such that OW individuals performed more poorly than NW individuals. Performance on the RPM was significantly correlated with years of education (Pearson’s r = .41, p < 0.01). Years of education is recommended as a better measure of IQ in socioeconomically and racially diverse populations where there is a strong positive correlation between years of education and IQ (Lange, Froimowitz, Bigler & Lainhart, 2010; Matarazzo, & Herman, 1984). In addition, there is there is an overlap in measures of fluid intelligence tests and working memory and processing speed tasks, due to the memory maintenance required on fluid intelligence tests (Conway, Cowan, Bunting, Therriault & Minkoff, 2002; Fry & Hale, 1996; Little, Lewandowsky & Craig, 2014). Given this, we chose to control for years of education and minority status in all subsequent analyses.
Clinical Characteristics of BED participants
All BED participants met DSM-5 (APA, 2013) BED criteria for the last three months. Participants with BED reported an average of 2.98 (SD = 1.29) episodes of binge-eating per week, with no significant differences in binge-eating frequencies between NW-BED and OW-BED (t(52) = 1.23, p = 0.23). There was a significant difference in illness duration (t(52) = 2.76, p < 0.01), with NW-BED having a shorter illness duration than OW-BED. Duration of illness and the age of BED participants were significantly correlated (r = .68, p < 0.01), as was duration of illness and BMI in BED (r = .40, p < 0.01). See Table 1.
Psychiatric and medical comorbidities
Of the participants with BED, 55% (30/55) had a history of psychiatric disorders: 29% (16/55) reporting history of mood disorder and 22% (12/55) reporting history of anxiety disorder. There were no significant differences between prevalence of lifetime mood disorders (χ2[1] = .92, p = 0.34), current mood disorders (χ2[1] = 1.80, p = 0.18), lifetime anxiety disorder (χ2[1] = 2.90, p = 0.09), current anxiety disorder (χ2[1] = 1.66, p = 0.20), and lifetime substance abuse (χ2[1] = 1.45, p = 0.22) in BED and non-BED groups (see Table 1). Presence of psychiatric disorders did not significantly correlate with performance on any EF measures (Spearman’s rs = −.08 to .16, ps > 0.05). In our sample, of the participants with BED, 3.6% (2/55) had a comorbid medical condition. Prevalence of hypertension (χ2[3] = 3.32, p = 0.35) and thyroid conditions (χ2[3] = 0.95, p = 0.81) did not significantly differ between groups, nor did medication use (χ2[3] = 1.12, p = 0.18). Presence of comorbid medical conditions did not significantly correlate with performance on any EF measure (Spearman’s rs = −.06 to .11, ps > 0.05), nor did number of medications (Spearman’s rs = −.09 to .13, ps > 0.05). Therefore, only years of education and minority status were controlled for in all subsequent analyses.
Executive functioning outcomes
Scores for the all EF measures fell within an acceptable range of normality, skew and kurtosis <1.5 and > −1.5 (Kline, 2011).
Cognitive Flexibility
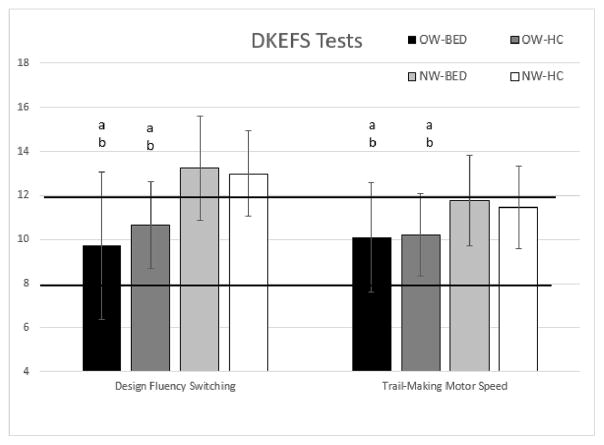
A one-way MANCOVA for cognitive flexibility (i.e., DKEFS Trail-Making Switching, DKEFS Verbal Fluency, DKEFS Design Fluency, NIH Toolbox Dimensional Card Sort), controlling for education and minority status, revealed a significant multivariate group effect, F(12, 320) = 1.89, p = .03; Wilk’s Λ = 0.83, partial η2 = .06. Subsequent ANCOVA’s showed a significant overall group difference for the DKEFS Design Fluency subtest (see Table 4, pFigure 1), but not for the DKEFS Trail-Making Switching, Verbal Fluency, or the NIH Toolbox Dimensional Change Sort. Post-hoc group comparisons with Bonferroni adjusted -values indicated that on the DKEFS Design Fluency subtest, NW-BED performed better than OW-BED (p < 0.01) and OW-HC (p < 0.01). The NW-HC group performed better on the DKEFS Design Fluency subtest than OW-BED (Bonferroni adjusted p < 0.01) and OW-HC (Bonferroni adjusted p = 0.03). OW-BED and OW-HC did not differ significantly on their performance, nor did NW-HC and NW-BED. Separate contrasts revealed that normal-weight individuals performed better than overweight individuals (Bonferroni adjusted p < 0.01) and that there were no differences between BED and non-BED groups (Bonferroni adjusted p = 0.93).
Table 4.
Scaled scores on individual subtests, grouped by executive functioning constructs and accompanying MANCOVA analysis, controlling for minority status and years of education.
| OW-BED M (SD) |
NW-BED M (SD) |
OW-HC M (SD) |
NW-HC M (SD) |
F df = 3, 124 |
Sig. | ηp2 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| (n = 32) | (n = 23) | (n = 48) | (n = 29) | ||||
| Cognitive Flexibility | |||||||
| DKEFS Trail-Making Number-Letter Switching | 9.39 (3.35) | 10.61 (2.37) | 10.35 (2.63) | 10.38 (1.95) | 0.37 | 0.77 | 0.01 |
| DKEFS Verbal Fluency Category Switching | 11.23 (3.52) | 12.30 (3.36) | 12.21 (3.56) | 13.10 (4.00) | 0.50 | 0.69 | 0.01 |
| DKEFS Design Fluency Switching | 9.74 (3.07) | 13.26 (3.45) | 10.65 (2.99) | 13.00 (3.12) | 4.03 | 0.01 | 0.09 |
| NIH Toolbox Dimensional Change Cart Sort | 89.58 (8.56) | 95.24 (7.27) | 89.64 (10.02) | 92.47 (8.11) | 1.38 | 0.25 | 0.03 |
| Inhibitory Control | |||||||
| DKEFS Color Word Interference Inhibition | 9.19 (3.47) | 11.83 (1.99) | 9.65 (3.00) | 10.69 (2.92) | 1.56 | 0.20 | 0.04 |
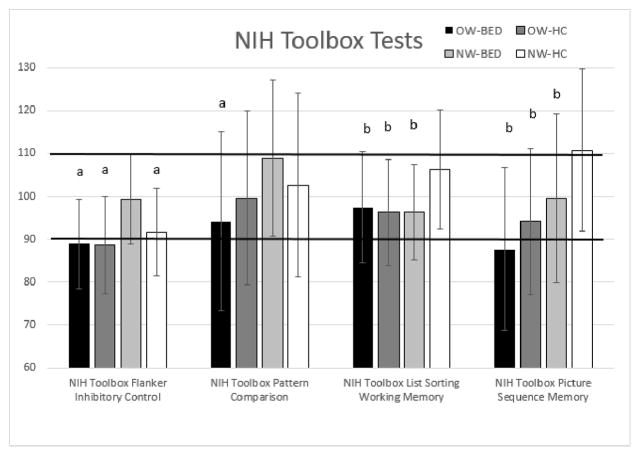
| NIH Toolbox Flanker Inhibitory Control | 88.9 (10.46) | 99.35 (10.47) | 88.67 (11.31) | 91.64 (10.22) | 4.05 | 0.01 | 0.09 |
| Planning | |||||||
| DKEFS Tower Achievement Score | 9.52 (2.90) | 10.52 (2.71) | 9.69 (2.71) | 12.97 (2.12) | 1.11 | 0.35 | 0.03 |
| Rey Complex Figure Copy T-score | 35.50 (34.8) | 37.80 (35.0) | 37.85 (35.67) | 38.45 (34.68) | 0.54 | 0.66 | 0.02 |
| Psychomotor Performance | |||||||
| DKEFS Trail-Making Motor Speed | 10.10 (2.47) | 11.78 (2.04) | 10.21 (2.35) | 11.45 (1.88) | 4.16 | 0.01 | 0.09 |
| NIH Toolbox Pattern Comparison | 94.19 (20.8) | 108.95 (18.28) | 99.63 (20.35) | 102.67 (21.36) | 2.85 | 0.04 | 0.06 |
| Working Memory | |||||||
| NIH Toolbox List Sorting Working Memory | 93.46 (13.02) | 96.24 (11.16) | 96.24 (12.39) | 106.32 (13.98) | 3.14 | 0.02 | 0.08 |
| NIH Toolbox Picture Sequence Memory | 87.73 (19.11) | 99.56 (19.61) | 94.14 (17.02) | 110.79 (18.99) | 3.67 | 0.02 | 0.08 |
Note: F statistics are of overall group comparisons for each construct’s MANCOVA; α = .05. OW-BED = Overweight Individuals with Binge Eating Disorder; NW-BED = Normal-weight individuals with Binge Eating Disorder; OW-HC = Overweight Healthy Controls; NW-HC = Normal-weight Healthy Controls
Figure 1. DKEFS: Design Fluency Switching (Cognitive Flexibility) and Trail-Making Motor Speed (Psychomotor Processing Speed) Age-Adjusted Scaled Scores.
Note: a: Performance of NW-BED is significantly better than denoted groups, p<.05; b: Performance of NW-HC is significantly better than denoted groups, p<.05. The normative range falls within the bolded horizontal lines. Age-adjusted raw means and standard deviations are presented. OW-BED = Overweight Individuals with Binge Eating Disorder; NW-BED = Normal-weight individuals with Binge Eating Disorder; OW-HC = Overweight Healthy Controls; NW-HC = Normal-weight Healthy Controls.
Inhibitory Control
A one–way MANCOVA for inhibitory control (i.e., DKEFS Color Word Interference, NIH Flanker Inhibitory Control), controlling for education and minority status, showed a significant multivariate effect of group, F(6, 246) = 2.34, p = .03; Wilk’s Λ = 0.90, partial η2 = .05. Follow-up ANCOVAS revealed group differences for the NIH Toolbox Flanker Inhibitory Control test (see Table 4, pFigure 2). Post-hoc comparisons with Bonferroni adjusted -values revealed that NW-BED performed better than the other three groups (ps < 0.01). On the Flanker Test, normal-weight individuals performed better compared to overweight individuals (Bonferroni adjusted p = 0.03) and BED performed better than non-BED (Bonferroni adjusted p = 0.02). There were no significant group differences for the DKEFS Color Word Interference subtest (see Table 4).
Figure 2. NIH Toolbox: Flanker Inhibitory Control, Pattern Comparison, List Sorting Working Memory, Picture Sequence Memory Age-Adjusted Scaled Scores.
Note: a: Performance of NW-BED is significantly better than denoted groups, p<.05; b: Performance of NW-HC is significantly better than denoted groups, p<.05. The normative range falls within the bolded horizontal lines. Error bars represent standard deviation. Age-adjusted raw means and standard deviations are presented. OW-BED = Overweight Individuals with Binge Eating Disorder; NW-BED = Normal-weight individuals with Binge Eating Disorder; OW-HC = Overweight Healthy Controls; NW-HC = Normal-weight Healthy Controls
Planning
The one-way MANCOVA for planning, controlling for education and minority status, using the DKEFS Tower test and RCFT Copy scores as dependent variables revealed no significant multivariate effect of group, F(6, 246) = 1.06, p = .39; Wilk’s Λ = 0.95, partial η2 =.03.
Working Memory
A one-way MANCOVA for working memory (i.e., NIH Toolbox List Sorting Working Memory, NIH Toolbox Picture Sequence Memory), controlling for education and minority status, showed a significant multivariate effect of group, F(6, 246) = 2.98, p < .01; Wilk’s Λ = 0.87, partial η2 = .07. Follow-up ANCOVAS with Bonferroni adjusted p-values revealed group differences for both tasks (see Table 4). Post-hoc comparisons revealed that NW-HC performed better than the remaining groups on both NIH Toolbox List Sorting Working Memory and Picture Sequence Memory task (Bonferroni adjusted ps < 0.01) (see Figure 2). Planned contrasts suggest that HCs performed better than BED for both subtests (Bonferroni adjusted p = 0.04; p = 0.03, respectively).
Psychomotor Performance
A one-way MANCOVA for psychomotor performance (i.e., DKEFS Trail-making Motor Speed task, NIH Toolbox Pattern Comparison), controlling for education and minority status, showed a significant multivariate effect of group, F(6, 246) = 2.67, p = .02; Wilk’s Λ = 0.88, partial η2 = .06. Follow-up ANCOVAS revealed group differences for both tasks (see Table 4). Post-hoc comparisons with Bonferroni adjusted p-values for DKEFS Motor Speed (see Figure 1) revealed that NW-BED performed better than OW-BED (p < 0.01) and OW-HC (p < 0.01). NW-HC also performed better than OW-BED (p = 0.03) and OW-HC (p = 0.02). On the NIH Toolbox Pattern Comparison, NW-BED performed better than OW-BED (see Figure 2, Bonferroni adjusted p < 0.01) and there was a trend for better performance for NW-HC compared to OW-HC (Bonferroni adjusted p = 0.07) and in NW-BED compared to OW-HC (Bonferroni adjusted p = 0.07). Planned contrasts suggest that NW individuals performed better than OW individuals (Bonferroni adjusted p = 0.01). There was no difference between BED and non-BED groups (Bonferroni adjusted p = 0.69). On the D-KEFS Motor Speed task, planned contrasts suggest that normal-weight individuals performed better compared to overweight individuals (Bonferroni adjusted p < 0.01). There were no differences between the BED and non-BED groups (Bonferroni adjusted p = 0.89).
Discussion
The aim of the current study was three-fold: to replicate previous findings demonstrating unique associations between BMI, BED status and EF, to examine how EF may differ with membership to one or both BED and BMI groups, and to explore the relatively unexamined NW-BED group. We confirmed previous findings showing that NW individuals performed significantly better than OW individuals on tasks requiring speed (DKEFS Trail-making Motor Speed, NIH Pattern Comparison) or cognitive flexibility (DKEFS Design Fluency). Relative to the non-BED groups, participants in the BED groups showed poorer performance on tasks of working memory (NIH Toolbox List Sorting Working Memory, Picture Sequence Memory), though OW-HCs also performed significantly poorer than NW-HCs. NW-BED individuals demonstrated significantly higher inhibitory control as measured by the Flanker Inhibition task than all other groups. Medical conditions and use of medication was not associated with EF findings. The co-occurrence of other psychological conditions was not associated with EF findings and the rate of psychiatric disorders in our sample is consistent with previous studies (Kessler et al., 2013; Hudson et al., 2007).
Our findings support a link between poorer psychomotor performance and overweight status (Cournot et al., 2006; Etou et al., 1989). Planned contrasts revealed that weight group was significantly related to performance on three tasks where psychomotor speed and reaction time were central (DKEFS Trail-making Motor Speed, DKEFS Design Fluency, NIH Pattern Comparison tasks). Thus, our findings suggest that EF of participants in the NW range, even with a diagnosis of BED is generally characterized by better psychomotor performance than OW, as captured by multiple EF tasks. Higher BMI has been associated with a reduction in white matter integrity (Bolzenius et al., 2015; Figley et al., 2016; Verstynen et al., 2012) and poorer performance on tasks of speed (Bolzenius et al., 2015), suggesting a possible explanation for poorer performance of the OW group on psychomotor tasks.
Moreover, NW groups performed better than OW groups on DKEFS Design Fluency, a task of cognitive flexibility, which is distinguished from the other cognitive flexibility tasks in this study by a component of visuospatial generativity. There was no difference in performance on the DKEFS Verbal Fluency task, which has no visuospatial component. Though the Dimensional Card Sort task and DKEFS Trail-Making task examine visuospatial switching, these two tasks clearly define the target for the participant, while the Design Fluency task requires the participant to create a novel design. On the Design Fluency task, weight was a significant factor in performance, with NW individuals generating an average of four more designs in the same time frame as OW individuals. This may be confounded partially by the aforementioned psychomotor abilities required by the task. However, this task also has attention and set-shifting demands (Suchy, Kraybill, & Larson, 2010); as designs cannot be repeated, an individual must “update” what designs are possible to generate with each new design drawn. Similar tasks, such as the Wisconsin Card Sort Task (WCST), which target set-shifting, have shown that OW groups perform poorer when compared to NW groups (Fitzpatrick et al., 2013; Kelly et al., 2013). The current study extends the literature in using the DKEFS Design Fluency task in BED and OW groups. Further research is needed to clarify whether difficulties with set-shifting are driving group differences and to control for the potential confound of motor speed.
In this study, individuals with BED performed more poorly on tasks assessing working memory (i.e., NIH Toolbox List Sorting Working Memory, Picture Sequence Memory) relative to those without BED. The performances of both BED groups were significantly lower than NW-HCs; additionally, OW-HCs also performed significantly poorer than NW-HCs. The means for all groups were within the normative range for the NIH Toolbox List Sorting Working Memory task. For the Picture Sequence Memory task, the OW-BED group mean was below the normative range, the NW-BED and OW-HC within the normative range and the NW-HC group mean just above the normative range. Working memory performance is dependent on one’s ability to retain and manipulate target information. Past studies have found poorer working memory in OW and obese groups with and without BED (Catoira et al., 2016; Gonzales et al., 2010; Solis-Ortiz, Gutierrez-Munoz, Morado-Crespo, Trejo-Bahena, & Kala, 2016); additionally, we observed such differences in a NW-BED group. Replication is needed, as this may be attributed to the performance of the NW-HCs, who performed in the higher end of the normative sample for both working memory tasks. However, observing an association between poorer working memory and BED independent of weight status is intriguing. This may have important clinical applications, as compromised working memory may lead to the maintenance of binge-eating by allowing distractors to overwhelm self-regulation goals. Our finding supports results which suggest a link between poor diet quality and memory deficits on tasks thought to recruit the hippocampus, even among normal weight participants (Francis & Stevenson, 2011). Rodent models show that the consumption of a high-energy diet can have significant adverse effects on hippocampus-dependent memory processes, and these deficits can be observed even prior to weight gain (Beilharz, Maniam, & Morris, 2015). The foods consumed during a binge-eating episode, typically characterized by high fat and sugar, may contribute to the difficulties in working memory observed in this study.
An additional aim of this study was to describe the EF of the previously unexamined NW-BED group in an exploratory way. While the NW group relative to the OW group exhibited enhanced psychomotor performance and cognitive flexibility, BED status in the NW group had a negative impact on working memory performance. In contrast, the NW-BED group performed significantly better on the Flanker Inhibitory Control task relative to all other groups, even when age and years of education were controlled for, though performance for all groups was within the normative range. The mean performances of the OW-HC and OW-BED groups was below average and the NW-HC group performed in the in the lower end of the normative range, supporting the importance of further replication of these findings. There were no significant group differences on the verbal inhibition task (D-KEFS Color-Word Interference). The performance of NW-BED on the Flanker Inhibitory Control task, which involves a large speed component, may be partially accounted for by the overall speed of the NW-BED group, as this was similar to NW-HC. In contrast to a past study that showed that obese individuals with BED did better on motor inhibitory tasks than obese HCs (Mole et al., 2015), we did not observe better performance in our OW-BED group, only in our NW-BED group. It is possible that the relatively higher inhibitory control observed in NW-BED relative to all other groups serves as a protective factor, preventing weight gain. As there have been no studies examining the trajectory of NW-BED individuals, we do not know whether this group will maintain their performance, or whether they will become behaviorally similar to the OW-BED group as they age. Our study extends the current literature in examining NW-BED, however replication and longitudinal studies are needed to explore questions of causality and prognosis.
We found no differences in neurocognitive performance on tasks of planning. A previous study found poorer performance in overweight individuals with loss-of-control eating in planning on the D-KEFS Tower Test compared to overweight individuals without loss-of-control eating. This study did not use a full-threshold BED sample (Manasse et al., 2014), making it difficult to fully compare findings. Moreover, there was a large amount of variability within groups on the RCFT-Copy, which may have masked differences between groups. Future studies may also examine planning in these groups using larger samples and different tasks, including disorder-specific tasks.
Observed differences in performance across EF domains support the notion that EF rely on overlapping but distinct neural circuits (Collette, Hogge, Salmon, & Van der Linden, 2006; Friedman & Miyake, 2017). Factor analyses suggest distinct EF constructs, while also showing moderate correlations between tasks of shifting, updating and inhibition, which may reflect common subprocesses involved in each task (e.g., attention, working memory, and inhibitory control) (Miyake et al., 2000). Different tasks used to assess the same EF may differ in their demands on such subprocesses leading to different outcomes. The results of our study suggest that though some subtests yielded similar results within a construct (e.g., psychomotor processing speed, working memory, planning), tasks examining cognitive flexibility and inhibitory control may be tapping into different subprocesses (e.g., novel versus planned, visual versus verbal).
The current study is novel in utilizing the NIH Toolbox Cognition module in a sample with eating disorders. Future studies could incorporate a comprehensive assessment of IQ in order to examine differences among these groups. Additionally, examining performance on the WCST (Berg, 1948) and Delayed Discounting Task (Richards, Zhang, Mitchell, & de Wit, 1999) in a NW-BED sample is needed. Another limitation of the study was the cross-sectional nature of the design; as such, we cannot address the question of directionality.
This study replicated findings in the existing literature showing that OW status is associated with reduced psychomotor performance and cognitive flexibility. Working memory performance was poorer among overweight participants and both NW and OW participants with BED compared to NW healthy controls. Future research may seek to further explore inhibitory control among NW-BED. Understanding how these specific EFs may relate to or result from binge-eating remains an important area of future study.
Table 3.
Neuropsychological Tests administered, grouped by construct.
| Construct | Test | Description of Test | Scoring Criteria |
|---|---|---|---|
| Cognitive Flexibility | D-KEFS Trail-Making Number-Letter Switching | Participants switch back and forth between connecting numbers and letters in sequential order. | Time to completion |
| D-KEFS Verbal Fluency Category Switching | Participants are asked to generate words, switching between two difference semantic categories (fruits and pieces of furniture). | Number of responses in 60 seconds | |
| D-KEFS Design Fluency Switching | Participants are asked to connect dots, switching between empty and filled dots to generate novel designs. | Number of designs in 60 seconds | |
| NIH Toolbox Dimensional Change Cart Sort | Participants match test pictures to target pictures, first on one dimension (e.g. shape) and then on the other dimension (e.g. color). | Accuracy and reaction time | |
| Inhibitory Control | D-KEFS Color Word Interference Inhibition | Participants must inhibit proponent response (reading) in order to say color ink as quickly as possible. | Time to completion |
| NIH Toolbox Flanker Inhibitory Control | Participants focus on middle arrow while inhibiting attention to surrounding arrows flanking it and respond by selecting direction of middle arrow. | Accuracy and reaction time | |
| Planning | D-KEFS Tower | Participants must move circles on pegs to obtain a target order while following specific rules. | Number of moves to complete trial |
| Rey-Ostereith Complex Figure Test - Copy | The subject must copy a complex geometric figure using organizational strategies | Scored on accuracy and location | |
| Psychomotor Performance | D-KEFS Trail-Making Motor Speed | Participant trace over a dotted line as quickly as possible | Time to completion |
| NIH Toolbox Pattern Comparison | Participants discern whether two side-by-side pictures are the same or not. The items are designed to be simple to measure processing speed. | Number of responses in 90 seconds | |
| Working Memory | NIH Toolbox List Sorting Working Memory | Recall and sequencing of different visually and orally presented stimuli. Pictures are displayed with accompanying audio recording and written text, and the participant is asked to say the items back in size order | Number of items recalled and sequenced correctly |
| NIH Toolbox Picture Sequence Memory | Participants recall increasingly lengthy series of illustrated objects. Sequence length varies increases in difficulty. | Number of items recalled and sequenced correctly |
Acknowledgments
Funding/support: This research was supported by a R21 (R21MH093932-01A1) grant funded by the National Institute of Mental Health to Chen (PI).
Footnotes
Potential conflicts of interest: Eunice Y. Chen, Ph.D. discloses annual royalties from Guilford Press. All other authors declare no potential conflict of interest with the current work.
Role of the sponsors: The supporters had no role in the design, analysis, interpretation, or publication of this study.
References
- Agbayani KA, Hiscock M. Age-related change in Wechsler IQ norms after adjustment for the Flynn effect: Estimates from three computational models. Journal of Clinical and Experimental Neuropsychology. 2013;35:642–654. doi: 10.1080/13803395.2013.806650. doi.org/10.1080/13803395.2013.806650. [DOI] [PubMed] [Google Scholar]
- Aloi M, Rania M, Caroleo M, Bruni A, Palmieri A, Cauteruccio MA, … Segura-García C. Decision making, central coherence and set-shifting: A comparison between binge eating disorder, anorexia nervosa and healthy controls. BMC psychiatry. 2015;15:1–10. doi: 10.1186/s12888-015-0395-z. doi.org/10.1186/s12888-015-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 2000. doi.org/10.1176/appi.books.9780890423349. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5) Arlington, VA: American Psychiatric Association; 2013. doi.org/10.1176/appi.books.9780890425596. [Google Scholar]
- Ariza M, Garolera M, Jurado MA, Garcia-Garcia I, Hernan I, Sanchez-Garre C, … Narberhaus A. Dopamine genes (DRD2/ANKK1-TaqA1 and DRD4-7R) and executive function: Their interaction with obesity. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0041482. doi.org/10.1371/journal.pone.0041482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: The role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7:6719–6738. doi: 10.3390/nu7085307. doi.org/10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. The Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Archives of Clinical Neuropsychology. 2008;23:467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, … Paul RH. Brain structure and cognitive correlates of body mass index in healthy older adults. Behavioural Brain Research. 2015;278:342–347. doi: 10.1016/j.bbr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Medical and psychiatric morbidity in obese women with and without binge eating. International Journal of Eating Disorders. 2002;32:72–78. doi: 10.1002/eat.10072. [DOI] [PubMed] [Google Scholar]
- Catoira NP, Tapajoz F, Allegri RF, Lajfer J, Camara MJR, Iturry ML, Castano GO. Obesity, metabolic profile, and inhibition failure: Young women under scrutiny. Physiology & Behavior. 2016;157:87–93. doi: 10.1016/j.physbeh.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Cohen DA. Neurophysiological pathways to obesity: Below awareness and beyond individual control. Diabetes. 2008;57:1768–1773. doi: 10.2337/db08-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139(1):209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. doi.org/10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan executive function system: An update. Journal of the International Neuropsychological Society. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Duchesne M, Mattos P, Appolinario JC, de Freitas SR, Coutinho G, Santos C, Coutinho W. Assessment of executive functions in obese individuals with binge eating disorder. Revista Brasileira De Psiquiatria. 2010;32:381–388. doi: 10.1590/s1516-44462010005000022. [DOI] [PubMed] [Google Scholar]
- Etou H, Sakata T, Fujimoto K, Kurata K, Terada K, Fukagawa K, … Miller RE. Characteristics of psychomotor performance and time cognition in moderately obese patients. Physiology & Behavior. 1989;45:985–988. doi: 10.1016/0031-9384(89)90225-4. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, O’Connor M. Eating Disorder Examination (Edition 16.0D) In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York: Guilford Press; 2008. pp. 309–314. [Google Scholar]
- Figley CR, Asem JSA, Levenbaum EL, Courtney SM. Effects of body mass index and body fat percent on default mode, executive control, and salience network structure and function. Frontiers in Neuroscience. 2016;10:1–23. doi: 10.3389/fnins.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fitzpatrick S, Gilbert S, Serpell L. Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychology Review. 2013;23:138–156. doi: 10.1007/s11065-013-9224-7. [DOI] [PubMed] [Google Scholar]
- Francis H, Stevenson R. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behavioral Neuroscience. 2011;125:943–955. doi: 10.1037/a0025998. doi.org/10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. doi.org/10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, … Gunstad J. Cognitive function in morbidly obese individuals with and without binge eating disorder. Comprehensive Psychiatry. 2012;53:490–495. doi: 10.1016/j.comppsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Burgess PW. Executive function. Current Biology. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Gonzales MM, Tarumi T, Miles SC, Tanaka H, Shah F, Haley AP. Insulin sensitivity as a mediator of the relationship between BMI and working memory-related brain activation. Obesity. 2010;18:2131–2137. doi: 10.1038/oby.2010.183. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. International Journal of Eating Disorders. 2003;35:80–85. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- Houben K, Dassen FCM, Jansen A. Taking control: Working memory training in overweight individuals increases self-regulation of food intake. Appetite. 2016;105:567–574. doi: 10.1016/j.appet.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NR, Bulik CM, Mazzeo SE. Executive functioning and behavioral impulsivity of young women who binge eat. International Journal of Eating Disorders. 2013;46:127–139. doi: 10.1002/eat.22096. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, … Xavier M. The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biological Psychiatry. 2013;73:904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: A Meta-Analysis. Human brain mapping. 2012;33(1):130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Ouimette PC, Kelly HS, Ferro T, Riso LP. Test-retest reliability of team consensus best-estimate diagnoses of Axis I and II disorders in a family study. American Journal of Psychiatry. 1994;151:1043–1047. doi: 10.1176/ajp.151.7.1043. doi.org/10.1176/ajp.151.7.1043. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3. Guilford Press; New York: 2011. [Google Scholar]
- Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. American Journal of Psychiatry. 1992;149:1225–1227. doi: 10.1176/ajp.149.9.1225. doi.org/10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE Brain Development Cooperative Group. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Developmental neuropsychology. 2010;35(3):296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, … Gunstad J. Association between binge eating disorder and changes in cognitive functioning following bariatric surgery. Journal of Psychiatric Research. 2014;59:148–154. doi: 10.1016/j.jpsychires.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, Fitzpatrick KK. Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. International Journal of Eating Disorders. 2015;48:677–683. doi: 10.1002/eat.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Juarascio AS, Forman EM, Berner LA, Butryn ML, Ruocco AC. Executive functioning in overweight Individuals with and without loss-of-control eating. European Eating Disorders Review. 2014;22:373–377. doi: 10.1002/erv.2304\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarazzo JD, Herman DO. Relationship of education and IQ in the WAIS—R standardization sample. Journal of Consulting and Clinical Psychology. 1984;52(4):631. doi: 10.1037/0022-006X.52.4.631. [DOI] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57:263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, … Voon V. Impulsivity in disorders of food and drug misuse. Psychological Medicine. 2015;45:771–782. doi: 10.1017/S0033291714001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Brandl C, Kiunke W, Georgiadou E, Horbach T, Kohler H, de Zwaan M. Food-independent tendency to disadvantageous decisions in obese individuals with regular binge eating. Comprehensive Psychiatry. 2014;55:64–70. doi: 10.1016/j.comppsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s progressive matrices and vocabulary scales. Oxford Psychologists Press; Oxford, UK: 1998. [Google Scholar]
- Reijmer YD, Leemans A, Brundel M, Kappelle LJ, Biessels GJ on behalf of the Utrecht Vascular Cognitive Impairment (VCI) Study Group. Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes. 2013;62:2112–2115. doi: 10.2337/db12-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SL, Peterson CB, Crow SJ, Agras WS. Test-retest reliability of the Eating Disorder Examination. International Journal of Eating Disorders. 2000;28:311–316. doi: 10.1002/1098-108x(200011)28:3<311::aid-eat8>3.0.co;2-k. doi.org/10.1002/1098-108x(200011)28:3%3C311::aid-eat8%3E3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Within-cohort age-related differences in cognitive functioning. Psychological Science. 2013;24:123–130. doi: 10.1177/0956797612450893. doi.org/10.1177/0956797612450893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Ortiz S, Gutierrez-Munoz M, Morado-Crespo L, Trejo-Bahena SA, Kala L. Executive functions correlated with body mass index in overweight middle-aged women. Psychology. 2016;7:410–417. doi: 10.4236/psych.2016.73043. [DOI] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. doi.org/10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, … Arturi F. Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile. Medicine. 2015;94:1–7. doi: 10.1097/MD.0000000000002098. doi.org/10.1097/md.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchy Y, Kraybill ML, Larson JCG. Understanding design fluency: Motor and executive contributions. Journal of the International Neuropsychological Society. 2010;16:26–37. doi: 10.1017/S1355617709990804. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Brand M, Tuschen-Caffier B. Decision-making impairments in women with binge eating disorder. Appetite. 2010;54:84–92. doi: 10.1016/j.appet.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosomatic Medicine. 2012;74:682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Ruck C, Irvine MA, Worbe Y, Enander J, … Bullmore ET. Disorders of compulsivity: A common bias towards learning habits. Molecular Psychiatry. 2015;20:345–352. doi: 10.1038/mp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Irvine MA, Derbyshire K, Worbe Y, Lange I, Abbott S, … Robbins TW. Measuring “waiting” impulsivity in substance addictions and binge eating disorder in a novel analogue of rodent serial reaction time task. Biological Psychiatry. 2014;75:148–155. doi: 10.1016/j.biopsych.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Schwartz MB, Spurrell EB, Fairburn CG. Assessing the specific psychopathology of binge eating disorder patients: Interview or self-report? Behaviour Research and Therapy. 1997;35:1151–1159. doi.org/10.1016/s0005-7967(97)80010-1. [PubMed] [Google Scholar]
- Wu MD, Giel KE, Skunde M, Schag K, Rudofsky G, de Zwaan M, … Friederich HC. Inhibitory control and decision making under risk in bulimia nervosa and binge-eating disorder. International Journal of Eating Disorders. 2013;46:721–728. doi: 10.1002/eat.22143. [DOI] [PubMed] [Google Scholar]