Abstract
Mutations in the gene encoding comparative gene identification 58 (CGI-58), also known as α β hydrolase domain-containing 5 (ABHD5), cause neutral lipid storage disorder with ichthyosis (NLSDI). This inborn error in metabolism is characterized by ectopic accumulation of triacylglycerols (TAG) within cytoplasmic lipid droplets in multiple cell types. Studies over the past decade have clearly demonstrated that CGI-58 is a potent regulator of TAG hydrolysis in the disease-relevant cell types. However, despite the reproducible genetic link between CGI-58 mutations and TAG storage, the molecular mechanisms by which CGI-58 regulates TAG hydrolysis are still incompletely understood. It is clear that CGI-58 can regulate TAG hydrolysis by activating the major TAG hydrolase adipose triglyceride lipase (ATGL), yet CGI-58 can also regulate lipid metabolism via mechanisms that do not involve ATGL. This review highlights recent progress made in defining the physiologic and biochemical function of CGI-58, and its broader role in energy homeostasis.
Keywords: ABHD5, CGI-58, ATGL, adipocyte, triacylglycerol, lipase
1. The Human Genetic Link Between CGI-58 and Abnormal TAG Storage
Over forty years ago, several patients presented to the clinic with a severe form of dry and scaly skin, which was diagnosed as a new syndrome called Chanarin-Dorfman syndrome; also known as neutral lipid storage disease with ichthyosis (NLSDI) [1–3]. These patients, and many thereafter, have been characterized with accumulation of TAG-rich cytosolic lipid droplets in keratinocytes, circulating leukocytes (known as Jordan’s anomaly), hepatocytes, skeletal myocytes, and several cell types within the central nervous and auditory systems [1–3]. In an initial attempt to delineate the metabolic disturbances in NLSDI patients, several groups isolated skin fibroblasts from affected individuals and found clear defects in TAG hydrolysis and improper recycling of TAG lipolysis products into membrane phospholipids [4–6]. In 2001, a landmark study by Judith Fischer’s group identified the first genetic mutations associated with NLSDI [7]. NLSDI is caused by mutations in the gene encoding comparative gene identification 58 (CGI-58), also known as α β hydrolase domain-containing 5 (ABHD5). Since this original discovery, several additional loss-of-function mutations in CGI-58 have been identified in independent NLSDI subjects [8–11]. This reproducible genetic link between CGI-58 mutations and NLSDI has provided a strong rationale to understand the mechanisms by which CGI-58 regulates TAG metabolism. Given this, many laboratories have attempted to identify molecular mechanisms by which CGI-58 regulates lipid metabolism in diverse cellular contexts. This review discusses the successes and challenges realized in this pursuit, and highlights the fundamentally important role that CGI-58 plays in cellular lipid homeostasis and systemic energy metabolism.
2. The Role of CGI-58 in Adipose Tissue Lipolysis
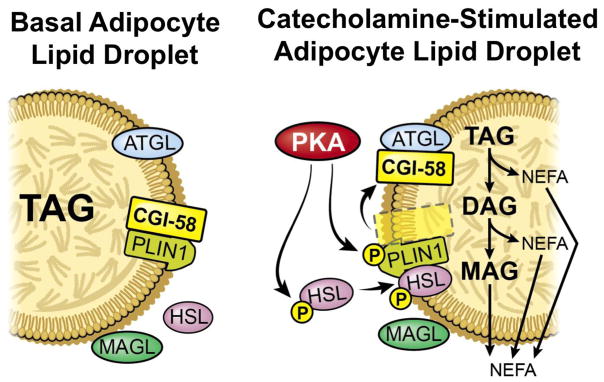
Given that TAG storage is most active in adipose tissue, and that the molecular underpinning of TAG lipolysis is best understood in adipocytes, the vast majority of mechanistic studies with CGI-58 have been conducted in adipocyte cell models. In mature adipocytes, CGI-58 resides primarily on cytosolic lipid droplets due to its direct interaction with the lipid droplet associated protein perilipin 1 (PLIN1) [12–15]. However, during catecholamine-stimulated lipolysis, cAMP-activated protein kinase A (PKA) phosphorylates both PLIN1 [16,17] and CGI-58 itself [18], facilitating the release of CGI-58 from the lipid droplet surface where it can subsequently interact with the major TAG hydrolase adipose triglyceride lipase (ATGL), also known as patatin-like phospholipase domain containing 2 (PNPLA2) [12–15] (Fig. 1). A seminal study by Lass and colleagues demonstrated that direct interaction between CGI-58 and ATGL potently activates ATGL-mediated TAG hydrolysis in adipocytes [19], providing the first clues into how CGI-58 impacts TAG metabolism. This study also showed that wild type recombinant CGI-58 can activate ATGL in an in vitro TAG hydrolysis assay, yet introduction of point mutations that are found in NLSDI patients lack the ability to activate ATGL [19]. Several independent groups have likewise found that CGI-58 co-activates ATGL during catecholamine-stimulated lipolysis in adipocytes [20–25]. Recently, the structural determinants of CGI-58-mediated ATGL co-activation in adipocytes have also been clarified [26,27]. Removal of N-terminal amino acids 10–31 of CGI-58 disrupts both CGI-58’s ability to localize to lipid droplets and its ability to co-activate ATGL [26]. Within the N-terminus, three tryptophan residues (Trp21, Trp25, and Trp29) help form a tether for CGI-58 to stably interact with cytosolic lipid droplets [26]. Using a domain swapping approach from ABHD5 to a structurally similar variant of the α/β hydrolase domain-containing family ABHD4, Sanders and colleagues determined that two conserved amino acids (R299 and G328) were sufficient to confer ATGL co-activation onto ABHD4 [27]. In parallel, mutations of R299 and G328 residues in ABHD5 reduced adipocyte lipolysis without altering CGI-58’s interaction with perilipin [27]. These studies provide important clues into the structural determinants by which CGI-58 regulates lipase activity [26,27]. Another recent study also demonstrated that cytosolic fatty acid binding proteins (FABP) directly interact with CGI-58 within its helix-loop-helix cap region, and FABP-CGI-58 interactions facilitate ATGL-mediated lipolysis [28]. This FABP-CGI-58 interaction during active lipolysis may be important in shuttling fatty acid and acylglycerol lipolysis products away from the lipid droplet [28], which is clearly defective in CGI-58 deficient cells [4–6]. In fact, there is evidence that the fatty acids released from the lipid droplet under FABP-CGI-58 facilitation, are then translocated by FABP to the nucleus to act as peroxisome proliferator-activated receptor (PPAR) ligands [28]. Collectively, these studies provide a molecular mechanism by which CGI-58 directly binds to and co-activates ATGL to promote catecholamine-stimulated lipolysis in adipocytes (Fig. 1). However, it appears that CGI-58’s ability to alter TAG hydrolysis is strictly dependent on ATGL specifically in adipocytes, and its interaction with ATGL does not account for CGI-58’s ability to dictate lipid homeostasis in several other cell types including keratinocytes, hepatocytes, and several cancer cells as discussed in detail below.
Fig. 1.
The role of CGI-58 in adipocyte lipolysis. Under basal conditions, CGI-58 interacts with perilipin 1 (PLIN1) at the lipid droplet surface, where it is tethered away from interactions with the triacylglycerol (TAG) lipase adipose triglyceride lipase (ATGL). Upon catecholamine stimulation, elevations in cellular cyclic AMP activate protein kinase A (PKA), which then phosphorylates the diacylglycerol (DAG) lipase, hormone sensitive lipase (HSL), and also PLIN1 and CGI-58. PKA-mediated phosphorylation of HSL facilitates its translocation from the cytosol to the lipid droplet surface, while phosphorylation of PLIN and CGI-58 causes dissociation of these two proteins and subsequent interaction of CGI-58 and ATGL to drive TAG hydrolysis.
3. CGI-58 Regulates Skin Lipid Homeostasis in an ATGL-Independent Manner
One of the defining features of NLSDI is the presence of ichthyosis, a dermatological condition where the skin appears dry and scaly [1–3]. Interestingly, genetic deficiency of CGI-58 in mice also results in dramatic alterations in lipid homeostasis and defective epidermal barrier function [29]. These two findings in human and mouse models of CGI-58 loss-of-function strongly suggest that CGI-58 plays a major role in skin lipid homeostasis. In contrast, neither loss of function human ATGL mutations [30,31] or genetic deletion of ATGL in mice [32] results in ichthyosis or other skin abnormalities. In fact, unlike CGI-58, ATGL is not abundantly expressed in the skin, creating a condition where CGI-58 most likely regulates skin lipid metabolism via a mechanism that does not rely on ATGL-coactivation. CGI-58 global knockout mice die postnatally due to a severe skin barrier defect, which is characterized by defective TAG hydrolysis and the absence of key barrier structure lipids called Ω-(O)-acylceramides [29]. These lipid abnormalities are rescued by the addition of recombinant CGI-58, but not by addition of ATGL [29], further suggesting an ATGL-independent mechanism. Recent studies in epidermal-specific CGI-58 knockout mice reveal that CGI-58 is essential for Ω-(O)-acylceramide synthesis and the formation of the cornified lipid envelope [33]. In agreement, transgenic overexpression of CGI-58 in differentiated, but not basal, keratinocytes can rescue global CGI-58−/− mice from lethal postnatal barrier dysfunction [33]. In human skin CGI-58 is highly enriched in the stratum granulosum within lamellar bodies, which is a skin microenvironment that is thought to originate from differentiated keratinocytes [34]. Several studies have shown that CGI-58 expression is elevated during keratinocyte differentiation, and knockdown of CGI-58 results in diminished expression of keratinocyte differentiation markers [29,35]. Collectively, data collected in both human and mouse models of CGI-58 deficiency support the concept that CGI-58 is necessary for the hydrolysis of TAGs and synthesis of Ω-(O)-acylceramides in the skin. Given its key roles in keratinocyte lipid metabolism, it is clear that CGI-58 is a gatekeeper of the cornified lipid envelope, which is necessary for skin barrier function. Unfortunately, at this point the molecular mechanisms linking CGI-58 to TAG hydrolysis, Ω-(O)-acylceramide synthesis, and keratinocyte differentiation in the skin are not known. However, there is now unequivocal evidence that CGI-58 regulates skin lipid homeostasis via an ATGL-independent mechanism. The continued search for this mechanism could have broad implications in dermatological diseases such as NLSDI and other related forms of inherited ichthyosis.
4. The Role of CGI-58 in Liver Disease Progression
Much like its role in the skin, CGI-58 is a potent regulator of liver lipid metabolism via mechanisms that do not rely on ATGL co-activation. In addition to ichthyosis, another common finding in people with CGI-58 loss-of-function mutations is severe liver disease including hepatic steatosis, non-alcoholic steatohepatitis (NASH), and cirrhosis [36–40]. In contrast to CGI-58 mutations, human ATGL mutations are not associated with hepatic fat accumulation or liver disease, but instead are associated with skeletal and cardiac muscle lipid accumulation [30,31]. Mice with diminished CGI-58 function in hepatocytes, accomplished by either antisense oligonucleotide (ASO)-mediated knockdown or by hepatocyte-specific CGI-58 genetic deletion, have striking hepatic steatosis which progresses with age into NASH and fibrosis [41–43]. Although ATGL−/− mice also develop mild hepatic steatosis, they never progress into NASH or fibrosis [32,44]. However, it is important to note that global ATGL−/− mice die prematurely due to cardiomyopathy, which makes it difficult to know whether long-term ATGL deficiency would indeed advance towards frank fibrosis. A recent study directly tested whether CGI-58 regulates TAG metabolism via an ATGL-dependent mechanism by knocking down CGI-58 in the liver of wild type or ATGL−/− mice [45]. This work demonstrated that CGI-58 can regulate hepatic steatosis and inflammation in the complete genetic absence of ATGL, indicating that CGI-58 regulates hepatic TAG metabolism and inflammation via ATGL-independent mechanisms [46].
In addition to its role in regulating fatty liver and NASH, CGI-58 has also been linked to mechanisms driving viral hepatitis [46,47]. The hepatitis C virus (HCV) encodes a structural protein known as core, which directly interacts with cytosolic lipid droplets in hepatocytes via a unique lipid-binding domain [48,49]. In HCV infected individuals, core’s avid interaction with cytosolic lipid droplets interferes with normal lipase activity and as a result, TAG hydrolysis is blunted [50]. A recent study demonstrated that HCV-core induced hepatic steatosis requires ATGL activity, yet unexpectedly is associated with increased interaction of ATGL and CGI-58 at the lipid droplet surface [46]. This results strongly suggests that CGI-58 and ATGL can interact at the hepatocyte lipid droplet surface, similar to what is know in adipocytes [19–25], yet unlike in adipocytes, this interaction is not coupled to ATGL activation and TAG hydrolysis [45,46]. Therefore, additional work is required to determine the functional consequence of ATGL-CGI-58 interaction in hepatocytes. In the context of HCV infection, it has recently been shown that CGI-58 is necessary for the assembly of the HCV viral particle by facilitating the hydrolysis of lipid droplet TAG stores for the re-packaging into the nascent lipo-viroparticle [47]. Previous studies in hepatocyte cell lines and ASO-treated mice have demonstrated that CGI-58 is necessary for the packaging of TAG into nascent very low density lipoproteins (VLDL) [41,51,52]. However, hepatocyte-specific genetic deletion of CGI-58 was not associated with altered VLDL-TAG secretion [43]. Therefore, addition work is needed to determine whether CGI-58 is indeed rate-limiting for the delivery of cytosolic lipid cargo into the endoplasmic reticulum and Golgi for VLDL or HCV lipo-viroparticle assembly and secretion. In addition to its role in hepatocytes, it is important to note that CGI-58 is also expressed in non-parenchymal cells in the liver including stellate cells [53] and macrophages [54–57], and regulates TAG hydrolysis in these cells as well [53–57]. However, the cell autonomous roles of CGI-58 in stellate cells and macrophages within the liver microenvironment have not been well characterized. Additional studies are needed to determine whether CGI-58 function in these non-parenchymal cells plays a role in liver disease progression. Collectively, CGI-58 plays a major role in hepatic TAG hydrolysis and the progression of liver disease from simple steatosis to NASH and cirrhosis via mechanisms that do not appear to rely on ATGL co-activation.
5. The Collaborative Role of CGI-58 in Skeletal and Cardiac Muscle Lipid Metabolism and Signaling
Fatty acids are key energy substrates in both skeletal and cardiac myocytes, where both CGI-58 and ATGL act in concert to promote metabolic flux of fatty acids from the lipid droplet to the mitochondria in these cells. Much like its role in adipocytes, CGI-58 appears to primarily regulate muscle lipolysis and downstream oxidative metabolism via direct co-activation of ATGL (Fig. 2). In support of this concept, human mutations in either CGI-58 [2] or ATGL [30,31] result in the accumulation of TAG-rich cytosolic lipid droplets in skeletal muscle, with ATGL mutations causing a much more severe lipid accumulation phenotype. Furthermore, people with loss-of-function mutations in ATGL exhibit symptomatic muscle weakness and cardiomyopathy [30,31,58–60], whereas symptomatic myopathy is less common in people with primary CGI-58 mutations [1–3]. Given the striking cardiac and skeletal muscle lipid accumulation seen in humans with ATGL mutations, affected patients are diagnosed with a variant of NLSDI called neutral lipid storage disease with myopathy (NLSDM) [30,31,58–60]. In agreement with findings in humans, several independent groups have generated genetically modified mouse and cell models that support a clear link between CGI-58 and ATGL in myocyte lipolysis and fatty acid metabolism [30,31,58–60]. A seminal study by Haemmerle and colleagues first showed that global genetic deficiency of ATGL in mice is associated with massive skeletal and cardiac muscle TAG accumulation, which results in premature lethality due to cardiac dysfunction [32]. Young global ATGL−/− mice, lacking ATGL-mediated lipolysis in both cardiac and skeletal muscle, also have impaired exercise performance [61]. This issue is due in part to limited free fatty acid supply to the working muscle as well as lower basal glycogen stores in liver and skeletal muscle [61]. Highlighting the key role ATGL plays in the cardiac TAG metabolism, global ATGL−/− mice have profound cardiac TAG accumulation [32], and cardiomyocyte-specific reintroduction of ATGL in global ATGL−/− mice rescues these mice from premature lethality [62]. In addition to regulating fatty acid fuel availability in myocytes, ATGL-driven lipolysis also liberates lipid agonists for the nuclear hormone receptor peroxisome proliferator-activated receptor α (PPARα) [63,64]. Given that ATGL−/− mice lack ATGL-driven provision of endogenous PPARα agonists, several groups have demonstrated that treatment with exogenous PPARα agonists can effectively rescue the lethal cardiomyopathy in global ATGL−/− mice [63,64].
Fig. 2.
The role of CGI-58 in skeletal muscle metabolism and transcriptional regulation of mitochondrial function. Non-esterified fatty acids (NEFA) are delivered to skeletal muscle either complexed to albumin or via lipoprotein lipase-driven lipolysis of triglyceride-rich lipoproteins (TGRLP), and taken up into the cell helped by fatty acid transport proteins (FATPs) or the scavenger receptor cluster of differentiation 36 (CD36). Once inside the cell, a portion of newly delivered NEFA are activated into fatty acyl-coenzyme A (FA-CoA) molecules and either used for oxidative fuel in the mitochondria or are esterifed into triacylglycerols (TAG) via the action of diacylglycerol acyltransferase (DGAT) enzymes. At the lipid droplet surface CGI-58 co-activates adipose triglyceride lipase (ATGL) to promote hydrolysis of TAG, and the liberated NEFAs and other acylglycerol lipolysis products are delivered to either the mitochondria for oxidation or the nucleus to activate peroxisome proliferator-activated receptor α (PPARα) signaling to further drive oxidative gene expression.
Similar to ATGL−/− mice, ice lacking CGI-58 in skeletal or cardiac myocytes also exhibit TAG accumulation and diminished PPARα signaling [65,66]. Selective deletion of CGI-58 in cardiac and skeletal myocytes results in muscle TAG accumulation, decreased PPARα-target gene expression, and defective mitochondrial fatty acid oxidation [65,66]. Interestingly, myocyte-specific deletion of CGI-58 preferentially increases TAG storage in type I slow twitch muscle fibers, which are known to rely predominantly on oxidative degradation of fatty acids during endurance type of exercise. In fact, CGI-58 likely plays a key role in oxidative metabolism in human muscle as well. Overexpression of CGI-58 in human myotubes promotes TAG hydrolysis and increases fatty acid oxidation, whereas CGI-58 knockdown reciprocally diminishes mitochondrial fatty acid oxidation [67]. Interestingly, in human myotubes CGI-58 function is closely linked to the expression of peroxisome proliferator-activated receptor δ target genes (PPARδ) [67]. Furthermore, the expression levels of both CGI-58 and ATGL correlate with markers of fatty acid oxidation in human skeletal muscle [68]. During a exercise, CGI-58 and ATGL interactions are more apparent at the lipid droplet surface in both human and rodent skeletal muscle [69,70]. In the context of skeletal muscle, the ability of CGI-58 and ATGL to interact at the lipid droplet surface is likely facilitated via direct interactions of either protein with the muscle-enriched lipid droplet coat protein perilipin 5 (PLIN5) [71–73]. Collectively, a large body of evidence in humans and rodents supports a role for CGI-58 as a key regulator of muscle mitochondrial oxidative metabolism via its ability to co-activate ATGL-driven TAG lipolysis [58–73] (Fig. 2).
6. The Role of CGI-58 in Macrophage Function and Atherosclerosis
It is well known that in the context of cardiovascular disease (CVD), that lipid metabolism and lipid signaling in macrophages plays a central role in orchestrating atherosclerotic plaque formation [74]. Given CGI-58’s ability to regulate TAG hydrolysis to generate endogenous ligands for peroxisome proliferator-activated receptors (PPARs) in other contexts, there has been considerable interest in the role CGI-58 plays in macrophage function in the context of CVD and other cardiometabolic diseases. To study the role of macrophage CGI-58 in the context of atherosclerosis, Goeritzer and colleagues crossed myeloid-specific CGI-58 knockout mice to the hyperlipidemic apolipoprotein E (apoE) null background [57]. Deletion of CGI-58 in macrophages did not significantly affect atherosclerosis progression, but this work did show that macrophages lacking CGI-58 are skewed towards the classical M1 activation state when maintained in culture [57]. An independent study also demonstrated that macrophage-specific deletion of CGI-58 causes macrophages to acquire an M1-like phenotype, which is associated with activation of the NLRP3 inflammasome [54]. In stark contrast to the role that CGI-58 plays in M1 skewing and inflammasome activation, macrophage-selective deficiency of ATGL has a much more dramatic effect [75–78]. ATGL deficient macrophages are polarized towards the alternative M2-like phenotype without showing signs of inflammasome activation [75–78]. Macrophage deletion of ATGL also results in significantly reduced atherosclerosis in low density lipoprotein receptor deficient mice [76]. Given the role that macrophages play in sensing bacterial pathogens, several groups have also evaluated the role of CGI-58 and ATGL in the in vivo response to bacterial endotoxin (lipopolysaccharide; LPS) [79,80]. Interestingly, both global ATGL−/− mice and CGI-58 ASO-treated models have increased levels of circulating pro-inflammatory cytokines when challenged with LPS, yet the tissue source of these cytokines seems quite different [79,80]. CGI-58 ASO-treated mice have reduced LPS-induced cytokine gene expression in the liver [79], whereas ATGL deficient mice have elevated LPS-induced cytokine gene expression in the liver [80]. In CGI-58 ASO-treated mice, it seems that the major source of elevated circulating pro-inflammatory cytokines is white adipose tissue [79]. Collectively, multiple studies clearly indicate cell autonomous roles for both CGI-58 and ATGL in macrophage function in vivo. However, based on the phenotypes of macrophage-specific knockout mouse models, it is most likely that CGI-58 and ATGL impact macrophage function independent from one another.
7. The Role of CGI-58 in the Intestinal Lipid Absorption
Both CGI-58 and ATGL are abundantly expressed in the small and large intestine in rodents and non-human primates, where they help determine enterocyte TAG hydrolysis and fatty acid flux [51,81,82]. It is well known that intestinal enterocytes can transiently store dietary fatty acids after esterification into TAG in cytosolic lipid droplets [83,84]. In fact, a significant portion of absorbed fatty acids are first esterified into cytosolic lipid droplets as TAG, and then subsequently liberated by lipase action to be delivered into the endoplasmic reticulum where they are re-esterified and packaged onto nascent chylomicrons [85]. However, our understanding of the molecular mechanisms regulating intestinal TAG lipolysis and fatty acid re-esterification for packaging into nascent chylomicrons is still in its infancy. Recent studies implicate both CGI-58 and ATGL in regulation of the cytosolic TAG hydrolysis and chylomicron assembly in intestinal enterocytes [81,82]. To study the role of CGI-58 in intestinal lipid absorption, Xie and colleagues selectively deleted CGI-58 in intestinal enterocytes using Cre-LoxP technology [81]. Enterocyte-specific deletion of CGI-58 results in a 4-fold increase in intestinal TAG levels resulting from diminished TAG hydrolysis activity [81]. Moreover, enterocyte-specific CGI-58 knockout mice show significantly reduced postprandial plasma TAG levels, but total intestinal fat absorption is only reduced by <0.5% [81]. These results suggest that CGI-58 is a key determinant of the hydrolysis of cytosolic TAG in enterocytes, yet does not dramatically alter the efficient packaging of TAG into nascent chylomicrons. Using a tissue-specific genetic approach, Obrowsky and colleagues found some similar and some divergent results when deleting ATGL from enterocytes [82]. Enterocyte-specific deletion of ATGL reduced total TAG hydrolase activity and increased intestinal TAG levels [82]. Although this was not associated with alterations in postprandial TAG levels, paradoxically, intestinal cholesterol absorption decreased [82]. These studies clearly demonstrate a role for CGI-58 and ATGL in TAG and cholesterol metabolism in the intestine. However, additional work is needed to determine if CGI-58 and ATGL work through independent pathways in intestinal enterocytes.
8. The Unexpected Role of CGI-58 in Insulin Sensitivity
Given the critical roles that CGI-58 and ATGL play in intracellular fatty acid metabolism and signaling lipid generation, these two proteins are uniquely positioned to impact cellular insulin action. It has been repeatedly reported that accumulation of lipid intermediates in the TAG biosynthetic pathway, including long chain acyl-CoAs and diacylglycerol (DAG) species, are associated with insulin resistance in rodents and humans [86]. Originally proposed by Dr. Roger Unger, the “lipotoxicity” theory of insulin resistance posits that abnormal accumulation of lipid intermediates can antagonize normal insulin action in the liver and skeletal muscle [87]. Based on the lipotoxicity theory, one would predict that CGI-58 or ATGL loss of function would result in lipotoxicity-induced insulin resistance, but this is not the case in rodents or humans. First, neither CGI-58 nor ATGL mutations are associated with insulin resistance or type 2 diabetes in humans [1–3,30,31]. Furthermore, mice lacking either ATGL [32,88,89] or CGI-58 [41,42,79,66] in the liver or skeletal muscle have improvements in systemic insulin sensitivity. Although both ATGL and CGI-58 deficient mice exhibit similar improvements in insulin sensitivity, the mechanisms driving this phenotype are quite different in the two models. In the case of CGI-58, the primary organ driving improvements in insulin sensitivity is the liver [42,79]. ASO-mediated knockdown of CGI-58 promotes a large accumulation of DAG species in the liver [41,42], and such a large accumulation of DAGs would be predicted to drive hepatic insulin resistance [86]. Despite this accumulation of DAG lipids, CGI-58 ASO-treated mice are protected from DAG-induced insulin resistance due to sequestration of DAGs in the lipid droplet and endoplasmic reticulum [42]. This lipid droplet sequestration of DAGs results in prevention of high fat diet-induced accumulation of DAGs at the plasma membrane where they normally act to negatively regulate insulin receptor-driven signaling events [86]. Collectively, the improvements in glucose tolerance seen in CGI-58 ASO treated mice stem almost exclusively from improvements in hepatic insulin action via a mechanism involving DAG sequestration [41,42,79]. In contrast, the improvements in glucose tolerance in global ATGL knockout mice have been primarily linked to improvements in skeletal muscle insulin signaling and stimulation of insulin-stimulated glucose uptake [88,89]. Therefore, the striking improvements in glucose tolerance seen with either ATGL or CGI-58 deficiency in mice derive from skeletal muscle-specific or liver-specific improvements in insulin action, respectively [41,42,79,88,89].
9. CGI-58’s Role in Colorectal Malignancy
It is well known that many types of cancers are characterized by dysregulated cellular metabolism [90]. The vast majority of cancer metabolism studies have focused on specific alterations in glycolytic pathways, but emerging evidence suggests that reorganization of CGI-58-related lipid metabolic networks also plays a role in cancer pathogenesis. Several recent reports have linked epigenetic or genetic regulation of CGI-58 function to malignant transformation in the context of skin, cervical, and colorectal cancer [56,91–94]. A study by Ou and colleagues showed that silencing of CGI-58 in normal fibroblasts is sufficient to induce malignant transformation [93]. Furthermore, enterocyte-specific deletion of CGI-58 promotes in vivo malignant transformation of adenomatous polyps in the colorectal cancer-prone ApcMin/+ mouse model [93]. In this model CGI-58 deletion in enterocytes was associated with increased aerobic glycolysis and induction of epithelial-mesenchymal transition (EMT) [93]. Another report from the same group showed that transgenic overexpression of CGI-58 in macrophages promotes colorectal cancer via a mechanism that involved suppression of spermidine synthase (SRM)-driven spermidine production in the colonic microenvironment [56]. Importantly, the ability of CGI-58 to regulate colorectal tumorigenesis does not appear to depend on co-activation of ATGL, but instead involves regulation of autophagic flux in cancer cell lines [94]. These studies support a role for CGI-58 as a tumor suppressor in the context of colorectal cancer in ApcMin/+ mice, but whether this holds true in human malignancies is still not known. Currently, there are no reported cases of cancer (colorectal or other types) in patients with primary mutations in CGI-58 (i.e. NLSDI mutants), so additional work is needed to clarify the role of CGI-58 in malignant transformation and cancer cell metabolism in humans.
10. Conserved Roles of CGI-58 Homologues in Cellular Lipid Metabolism and Signaling
The storage of energy in the form of TAG is not unique to higher vertebrates [95]. In fact, almost all known organisms have evolved intricate systems to regulate both TAG synthesis and lipolysis to provide fatty acid fuel as well as critical signaling lipids. Much like its role in mammals, CGI-58 homologues also regulate TAG metabolism and signal transduction in several other organisms including Arabidopsis thaliana, Caenorhabditis elegans, and Saccharomyces cerevisiae. [96–107]. In the model plant organism Arabidopsis thaliana, loss of the CGI-58 homologue results in abnormal accumulation of TAG-rich lipid droplets in leaves, which do not typically store TAG [96,97]. Interestingly, plant CGI-58 does not appear to interact with an ATGL-like lipase, but instead directly interacts with an ATP-binding cassette protein known as PXA1 [98]. In plants PXA1 is critical for the uptake of fatty acids into peroxisomes for subsequent β-oxidation [98]. In addition to facilitating PXA1-driven peroxisomal β-oxidation, plant CGI-58 also facilitates PXA1’s ability to generate the critical plant hormones jasmonic acid and indole acetic acid [98], both of which are critical regulators of plant growth and homeostasis [99]. Another recent report demonstrated that plant CGI-58 can also interact with spermidine synthase 1 (SPDS1), and this direct interaction facilitates the conversion of the polyamine putrescine into spermidine [100]. This report is in agreement with similar findings in human colorectal cancer cells [56]. Also, CGI-58-facilitated TAG hydrolysis in plants has recently been shown to be necessary for the physiological process of light-induced stomatal opening [101]. Therefore, in a manner similar to vertebrate CGI-58 isoforms, plant CGI-58 regulates TAG hydrolysis to provide critical fuel for ATP synthesis, but also participates in the generation of hormone-like second messengers that further shape cellular energy metabolism and physiological processes such as stomatal opening (Fig. 3). In the free-living nematode Caenorhabditis elegans CGI-58 likewise regulates TAG hydrolysis during periods of nutrient deprivation [102]. During times of prolonged nutrient deprivation Caenorhabditis elgans can enter the “dauer” stage, which represents a static developmental period that is thought to occur to facilitate survival during time of stress. During the dauer stage, worm CGI-58 interacts with the lipase ATGL-1, but facilitates lipid droplet shrinkage and TAG hydrolysis in a largely ATGL-1-independent fashion [102]. Interestingly, worm CGI-58 promotes TAG hydrolysis and preferentially liberates 20 carbon-containing polyunsaturated fatty acids [102]. Finally, homologues of CGI-58 in the model yeast organism Saccharomyces cerevisiae also regulates cellular TAG and phospholipid homeostasis [103]. This study found that human CGI-58 was closely related to a yeast encoded lysophosphatidic acid acyltransferase (LPAAT) enzyme known as Ict1p [103]. Following up on this observation Ghosh and colleagues showed that introduction of human CGI-58 into Saccharomyces cerevisiae resulted in reduced TAG levels, and increased LPAAT activity [103]. The implications of this paper will be discussed below in detail, as this reported LPAAT activity has since been called into question. Collectively, CGI-58 appears to have a conserved role in TAG hydrolysis as well as in the generation of key hormone-like signaling molecules that impact energy homeostasis.
Fig. 3.
The role of CGI-58 in plant lipid metabolism and signaling. In the model plant organism Arabidopsis thaliana CGI-58 directly interacts with the peroxisomal ATP-binding cassette transporter PXA1. These proteins collaborate to promote transport of non-esterified fatty acids (NEFA) to provide energy and also stimulate the transport of key intermediate metabolites that are critical for plant hormone production. First, the interaction between CGI-58 and PXA1 facilitates the transport and β-oxidation of NEFA in peroxisomes. In parallel, CGI-58 facilitates PXA1-dependent transport of the plant hormone lipid intermediates 12-oxo phytodienoic acid (OPDA) and indole butyric acid (IBA) into the peroxisome where they are converted, respectively, to jasmonic acid or indole acetic acid, which are key regulatory hormones in plants promoting pathogen resistance and growth.
11. Conclusions and Perspectives: The Continued Search for ATGL-Independent Functions of CGI-58
Even though it is apparent that CGI-58 plays a fundamentally important role in TAG lipolysis and the generation of key signaling molecules in every organism studied, we still do not fully understand how this enigmatic protein achieves this regulatory role. CGI-58 is a member of the alpha beta hydrolase domain-containing (ABHD) family of enzymes, many of which possess a classic lipase catalytic triad [104]. However CGI-58 lacks the predicted nucleophile serine within the lipase active site, and by all accounts has no intrinsic lipase activity towards TAG or many other glycerolipid and glycerophospholipid substrates [104]. There is now unequivocal evidence in adipocytes [19–25], myocytes [65–69], and hepatic stellate cells [53] that CGI-58 can stimulate TAG hydrolysis and alter PPAR signaling by co-activating ATGL (Fig. 1 & 2). However, CGI-58 can regulate TAG hydrolysis and cellular signaling in many other cell types including hepatocytes [45], keratinocytes [33], and several cancer cell lines [94] via an ATGL-independent mechanism. Given these ATGL-dependent and ATGL-independent roles for CGI-58, human mutations of either CGI-58 or ATGL are associated with some shared yet several divergent phenotypes [11]. In people with primary mutations in either CGI-58 or ATGL, the main shared phenotype is ectopic TAG storage in circulating leukocytes known as Jordans’ anomaly [11], In contrast to this shared phenotype, primary ATGL mutations are always associated with cardiomyopathy, yet much less frequently associated with ichthyosis, hepatomegaly, hepatic steatosis when compared to primary CGI-58 mutations [11]. Also, primary CGI-58 mutations are always associated with ichthysosis, but much less frequently associated with skeletal or cardiac myopathy [11]. Collectively, the findings in animal models and humans strongly suggest both ATGL-dependent and independent roles for CGI-58.
In the search for the elusive ATGL-independent function of CGI-58, many avenues have been explored with limited success. As mentioned above, mammalian CGI-58 has previously been reported to possess lysophosphatidic acid acyltransferase (LPAAT) activity [97,105], suggesting a potential role in the generation of the critical signaling lipid phosphatidic acid. However, subsequent investigation has revealed that the LPAAT activity originally associated with recombinant CGI-58 [97,105] was due to a bacterial contaminant LPAAT acquired during the affinity purification process [106]. This was demonstrated by the fact that mutations of the predicted acyltransferase active site of CGI-58 does not reduce LPAAT activity [106]. Moreover, affinity purification of recombinant CGI-58 from a bacterial strain that lacks the sole LPAAT found in the Escherichia coli genome (plsC) yielded no detectable LPAAT activity [106]. It is important to note that other proteins have been mistakenly identified as LPAAT enzymes due to similar problems with affinity co-purification of bacterial LPAATs [107]. Another recent report also suggested that CGI-58 instead possesses intrinsic lysophosphatidylglycerol acyltransferase (LPGAT) activity to convert lysophosphatidylglycerols (LPG) to phosphatidylglycerol (PG) [108]. However, CGI-58 knockdown in mouse liver is associated with marked accumulation of PG lipids, which is inconsistent with a LPGAT activity for CGI-58 [41]. Moreover, an independent study recently found that both plant and mouse versions of CGI-58 do not have detectable LPGAT activity [109]. Therefore, additional work is needed to clarify whether CGI-58 indeed possesses LPGAT activity, and whether this is linked to ATGL-independent phenotypes driven by CGI-58 loss of function. In the continued search for an acyltransferase activity for CGI-58, it will be important to consider the challenges of purifying recombinant CGI-58 from bacterial expression systems [106].
Given the product of TAG hydrolysis is diacylglycerol (DAG), and CGI-58 knockdown alters hepatic DAG storage [42], it is tempting to speculate that CGI-58 may be a key regulator of cellular DAG metabolism and DAG-mediated signal transduction. CGI-58 knockdown in mice leads to increased hepatic DAG levels [41,42,45], and prevents the inflammatory cytokine-driven generation of several signaling lipids that can be derived from DAG [79]. Two recent reports also support the possibility that CGI-58 could play a direct role in the regulation of DAG metabolism or subcellular localization [42,110]. First, CGI-58 knockdown causes hepatic DAG accumulation in lipid droplets/endoplasmic reticulum, while preventing accumulation of DAG at the plasma membrane [42]. Second, CGI-58 co-activation broadens the selectivity of ATGL for the sn-2 position of TAG to include the sn-1 position, resulting in the generation of both sn-1,3 and sn-2,3 DAG [110]. It is important to note that ATGL deficiency decreases hepatic DAG levels, whereas CGI-58 knockdown increases hepatic DAG both in the presence or absence of ATGL [45]. Although the mechanism by which CGI-58 knockdown causes DAG accumulation within the lipid droplet/endoplasmic reticulum compartment is unknown, the reciprocal reduction in hepatic DAG by ATGL deficiency likely directly stems from defective TAG lipolysis to DAG in hepatocytes. In support of this concept, adipocyte-specific deletion of ATGL likewise reduces total adipose DAG levels [111]. Given that ATGL and CGI-58 reciprocally regulate hepatic DAG levels, and the fact that CGI-58 knockdown increases hepatic DAG in both the presence and absence of ATGL [45], it is tempting to speculate that CGI-58 plays an ATGL-independent role in DAG shuttling or metabolism. An early study by Subramanian and colleagues [12] showed that during active adipocyte lipolysis that CGI-58 moves away from the lipid droplet surface towards the cytoplasm. Based on this trafficking pattern [12], and alterations in cellular DAG levels with CGI-58 deficiency [41,42,45,110], it remains possible that CGI-58 may be important in shuttling the lipolytic product DAG away from the droplet and towards the generation of signaling lipids [5]. In fact, some of the earliest studies in NSLDI fibroblasts showed cleared defective shuttling of acylglycerols from TAG pools [5]. However, additional studies are required to better understand if CGI-58 has the ability to bind and shuttle specific stereoisomers of DAG away from the lipid droplet.
Another potential ATGL-independent role of CGI-58 in TAG hydrolysis could be its recently reported role in regulating cellular autophagy. In fact, autophagy of lipid droplets, also called “lipophagy”, has recently been described as a key means to regulate hepatic TAG levels during times of excessive starvation [112]. CGI-58 has been shown to directly interact with the autophagy protein beclin 1 in colon cancer cells [94]. This work showed that CGI-58 competes for a caspase binding site on beclin 1, thereby blunting caspase-mediated cleavage of beclin 1, and also showed that CGI-58 and beclin 1 expression levels were correlated in human colorectal cancer tissue [94]. Another recent study showed that CGI-58 regulates the autophagy of mitochondria, a process known as “mitophagy” [108]. This study showed CGI-58 overexpression stimulated the activity of 5’-AMP-activated protein kinase (AMPK) and diminished the signaling of the mammalian target of rapamycin complex 1 (mTORC1), both of which are major regulatory nodes in autophagy activation [108]. Although additional work is needed, these studies provide support for the idea that CGI-58 may regulate cellular TAG storage via regulation of lipophagy and mitophagy. As the search for the elusive ATGL-independent function of CGI-58 continues it will be important to consider regulatory roles in cellular autophagy programs. Moreover, recent identification of endogenous and synthetic ligands of CGI-58 that facilitate the release of CGI-58 from perilipins on the lipid droplet surface without altering PKA activation [25] provides an extremely useful tool to further understand the ATGL-independent roles of CGI-58. Collectively, CGI-58 is a conserved regulator of intracellular TAG hydrolysis and the generation of signaling lipids. In some cellular contexts (adipocytes and myocytes), CGI-58 accomplishes this regulatory role via direct co-activation of ATGL-driven TAG hydrolysis. Whereas, in many other cell types CGI-58 potently regulates TAG hydrolysis and lipid signaling via an elusive second ATGL-independent mechanism. The identification of the ATGL-independent function(s) of CGI-58 will have broad implications both in the field of energy metabolism as well as signal transduction.
Highlights.
Mutations in α/β-hydrolase domain (ABHD5) cause neutral lipid storage disorder.
ABHD5/CGI-58 regulates adipose lipolysis via co-activation of ATGL.
ABHD5/CGI-58 regulates skin barrier function in an ATGL-independent manner.
ABHD5/CGI-58 is a key regulator of insulin action and inflammatory responses.
The role of ABDH5/CGI-58 in neutral lipid metabolism is conserved across species.
Acknowledgments
We apologize for not being able to include discussion on all studies surrounding ABHD5/CGI-58 due to space constraints. The authors sincerely thank David Schumick (B.S., C.M.I.) for provided illustrations included in this work. The authors are supported by grants from the National Heart Lung and Blood Institute (R01 HL122283), National Institutes of Digestive and Kidney Diseases (R01 DK060864), the National Institute of Alcohol Abuse and Alcoholism (P50 AA024333), the National Center for Advancing Translational Sciences (4UL1TR000439), the Case Comprehensive Cancer Center (P30 CA043703), and a Cleveland Clinic Research Center of Excellence grant.
Abbreviations
- ABHD5
αβ hydrolase domain 5
- ATGL
adipose triglyceride lipase
- CGI-58
comparative gene identification-58
- DAG
diacylglycerol
- FABP
fatty acid binding protein
- G0S2
G0/G1 switch gene 2
- HCV
hepatitis C virus
- LPA
lysophosphatidic acid (LPA)
- LPAAT
lysophosphatidic acid acyltransferase
- LPGAT
lysophosphatidylglycerol acyltransferase
- MAG
monoacylglycerol
- NLSDI
neutral lipid storage disease with ichthyosis
- NLSDM
neutral lipid storage disease with myopathy
- PA
phosphatidic acid
- PLIN1
perilipin 1
- PNPLA2
patatin-like phospholipase domain containing 2
- PPARα
peroxisome proliferator-activated receptor alpha
- TAG
triacylglycerol
- VLDL
very low density lipoprotein
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975;1:553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavin G, Wills EJ, Richmond JE, Chanarin I, Andrews T, Stewart G. Morphological features in a neutral lipid storage disease. J Clin Pathol. 1975;28:701–710. doi: 10.1136/jcp.28.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozenszajn L, Klajman A, Yaffe D, Efrait P. Jordans’ anomaly in white blood cells. Report of case. Blood. 1966;28:258–265. [PubMed] [Google Scholar]
- 4.Williams ML, Coleman RA, Placezk D, Grunfeld C. Neutral lipid storage disease: a possible function defect in phospholipid-linked triacylglycerol metabolism. Biochim Biophys Acta. 1991;1096:162–169. doi: 10.1016/0925-4439(91)90055-e. [DOI] [PubMed] [Google Scholar]
- 5.Igal RA, Coleman RA. Acylglycerol recycling from triacylglycerol to phospholipid, not lipase activity, is defective in neutral lipid storage disease fibroblasts. J Biol Chem. 1996;271:16644–16651. doi: 10.1074/jbc.271.28.16644. [DOI] [PubMed] [Google Scholar]
- 6.Hilaire N, Negre-Salvayre A, Salvayre R. Cytoplasmic triacylglycerols and cholesteryl esters are degraded in two separate catabolic pools in cultured human fibroblasts. FEBS Lett. 1993;328:230–234. doi: 10.1016/0014-5793(93)80933-l. [DOI] [PubMed] [Google Scholar]
- 7.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud'homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H. Trucation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. J Invest Dermatol. 2003;121:1029–1034. doi: 10.1046/j.1523-1747.2003.12520.x. [DOI] [PubMed] [Google Scholar]
- 9.Caux F, Selma ZB, Laroche L, Prud’homme JF, Fischer J. CGI-58/ABHD5 gene is mutated in Dorfman-Chanarin syndrome. Am J Med Genet A. 2004;129:214. doi: 10.1002/ajmg.a.30228. [DOI] [PubMed] [Google Scholar]
- 10.Nur BG, Gencpinar P, Yuzbasioglu A, Emre SD, Mihci E. Chanarin-Dorfman syndrome: genotype-phenotype correlation. Eur J Med Genet. 2015;58:238–242. doi: 10.1016/j.ejmg.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–1089. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 17.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 18.Sahu-Osen A, Montero-Moran G, Schittmayer M, Fritz K, Dihn A, Chang YF, McMahon D, Baeszoermenyl A, Cornaciu I, Russell D, Oberer M, Carman GM, Birner-Gruenberger R, Brasaemle DL. CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: control of subcellular localization. J Lipid Res. 2015;56:109–121. doi: 10.1194/jlr.M055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lass A, Zimmerman R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose trigylceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of adipose trigylceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Yang X, Liu J. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle. 2010;9:2719–2725. doi: 10.4161/cc.9.14.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;288:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Heckmann BL, Zhang X, Smas CM, Liu J. Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol Endocrinol. 2013;27:116–126. doi: 10.1210/me.2012-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders MA, Madoux F, Mladenovic L, Zhang H, Ye X, Angrish M, Mottillo EP, Caruso JA, Halvorsen G, Roush WR, Chase P, Hodder P, Granneman JG. Endogenous and synthetic ABHD5 ligands regulate perilipin interactions and lipolysis in fat and muscle. Cell Metab. 2015;22:851–860. doi: 10.1016/j.cmet.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeszoermenyl A, Nagy HM, Arthanari H, Pillip CJ, Lindermuth H, Luna RE, Wagner G, Zechner R, Zangger K, Oberer M. Structure of a CGI-58 motif provides the molecular basis of lipid droplet anchoring. J Biol Chem. 2015;290:26361–26372. doi: 10.1074/jbc.M115.682203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders MA, Zhang H, Mladenovic L, Tseng YY, Granneman JG. Molecular basis of ABHD5 lipolysis activation. Sci Rep. 2017;7:42589. doi: 10.1038/srep42589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer P, Boeszoermenyl A, Jaeger D, Feiler U, Arthanari H, Mayer N, Zehender F, Rechberger G, Oberer M, Zimmerman R, Lass A, Haemmerle G, Breinbauer R, Zechner R, Preiss-Landl K. Fatty acid-binding proteins interact with comparative gene identification-58 linking lipolysis with lipid ligand shuttling. J Biol Chem. 2015;290:18438–18453. doi: 10.1074/jbc.M114.628958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2009;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 31.Coassin S, Schweiger M, Kloss-Brandstatter A, Lamina C, Haun M, Erhart G, Paulweber B, Rahman Y, Olpin S, Wolinski H, Cornaciu I, Zechner R, Zimmerman R, Kronenberg F. Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genet. 2010;6:e1001239. doi: 10.1371/journal.pgen.1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 33.Grond S, Radner FP, Eichmann TO, Kolb D, Grabner GF, Wolinski H, Gruber R, Hofer P, Heier C, Schauer S, Rulicke T, Hoefler G, Schmuth M, Elias PM, Lass A, Zechner R, Haemmerle G. Skin barrier development depends on CGI-58 protein expression during late-stage keratinocyte differentiation. J Invest Dermatol. 2017;137:403–413. doi: 10.1016/j.jid.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H. Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. 2003;121:1029–1034. doi: 10.1046/j.1523-1747.2003.12520.x. [DOI] [PubMed] [Google Scholar]
- 35.Akiyama M, Sakai K, Takayama C, Yanagi T, Yamanaka Y, McMillan JR, Shimizu H. CGI-58 is an alpha/beta-hydrolase within lipid transporting lamellar granules of differentiated keratinocytes. Am J Pathol. 2008;173:1349–1360. doi: 10.2353/ajpath.2008.080005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igal RA, Rhoads JM, Coleman RA. Neutral lipid storage disease with fatty liver and cholestasis. J Pediatr Gastroenterol Nutr. 1997;25:541–547. doi: 10.1097/00005176-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan R, Hadzic N, Fischer J, Knisely AS. Steatohepatitis and unsuspected micronodular cirrhosis in Dorfman-Chanarin syndrome with documented ABHD5 mutation. J Pediatr. 2004;144:662–665. doi: 10.1016/j.jpeds.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Ronchetti A, Prati D, Pezzotta MG, Tavian D, Columbo R, Callea F, Colli A. Severe steatohepatitis in a patient with a rare neutral lipid storage disorder due to ABHD5 mutation. J Hepatol. 2008;49:474–477. doi: 10.1016/j.jhep.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Cakir M, Bruno C, Cansu A, Cobanoglu U, Erduran E. Liver cirrhosis in an infant with Chanarin-Dorfman syndrome caused by a novel splice-site mutation in ABHD5. Acta Paediatr. 2010;99:1592–1594. doi: 10.1111/j.1651-2227.2010.01869.x. [DOI] [PubMed] [Google Scholar]
- 40.Cakmak E, Alagozlu H, Yonem O, Ataseven H, Citti S, Ozer H. Steatohepatitis and liver cirrhosis in Chanarin-Dorfman syndrome with a new ABHD5 mutation. Clin Res Hepatol Gastroenterol. 2012;36:e34–e37. doi: 10.1016/j.clinre.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, Liu X, Graham MJ, Lee R, Crooke R, Shulman GI, Xue B, Shi H, Yu L. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantley JL, Yoshimura T, Camporez JP, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ, Kahn M, Guigni BA, Serr J, Hankin J, Murphy RC, Cline GW, Bhanot S, Manchem VP, Brown JM, Samuel VT, Shulman GI. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci USA. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo F, Ma Y, Kadegowda AK, Betters JL, Xie P, Liu G, Liu X, Miao H, Ou J, Su X, Zheng Z, Xue B, Shi H, Yu L. Deficiency of liver comparitive gene identification-58 causes steatohepatitis and fibrosis in mice. J Lipid Res. 2013;54:2109–2120. doi: 10.1194/jlr.M035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber R, Hofer P, Taschler U, Voshol PJ, Rechberger GN, Kotzbeck P, Jaeger D, Preiss-Landl K, Lord CC, Brown JM, Haemmerle G, Zimmermann R, Vidal-Puig A, Zechner R. Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity. Proc Natl Acad Sci USA. 2015;112:13850–13855. doi: 10.1073/pnas.1516004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord CC, Ferguson D, Thomas G, Brown AL, Schugar RC, Burrows A, Gromovsky AD, Betters J, Neumann C, Sacks J, Marshall S, Watts R, Schweiger M, Lee RG, Crooke RM, Graham MJ, Lathia JD, Sakaguchi TF, Lehner R, Haemmerle G, Zechner R, Brown JM. Regulation of hepatic triacylglycerol metabolism by CGI-58 does not require ATGL co-activation. Cell Rep. 2016;16:939–949. doi: 10.1016/j.celrep.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camus G, Schweiger M, Herker E, Harris C, Kondratowicz AS, Tsou CL, Farese RV, Jr, Herath K, Previs SF, Roddy TP, Pinto S, Zechner R, Ott M. The hepatitis C virus core protein inhibits adipose triglyceride lipase (ATGL)-mediated lipid mobilization and enhances the ATGL interaction with comparative gene identification 58 (CGI-58) and lipid droplets. J Biol Chem. 2014;289:35770–35780. doi: 10.1074/jbc.M114.587816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieyres G, Welsch K, Gerold G, Gentzsch J, Kahl S, Vondran FW, Kaderali L, Pietschmann T. ABHD5/CGI-58, the Chanarin-Dorfman syndrome protein, mobilises lipid stores for hepatitis C virus production. PLoS Pathog. 2016;12:e1005568. doi: 10.1371/journal.ppat.1005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, Brechot C. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hope RG, McLauchlan J. Sequence motifs required for lipid droplet association and protein stability are unique to the hepatitis C virus core protein. J Gen Virol. 2000:1913–1925. doi: 10.1099/0022-1317-81-8-1913. [DOI] [PubMed] [Google Scholar]
- 50.Harris C, Herker E, Farese RV, Jr, Ott M. Hepatitis C virus core protein decreases lipid droplet turnover: a mechanism for core-induced steatosis. J Biol Chem. 2011:42615–42625. doi: 10.1074/jbc.M111.285148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JM, Chung S, Das A, Shelness GS, Rudel LL, Yu L. CGI-58 facilitates the mobilization of cytoplasmic triglyceride for lipoprotein secretion in hepatoma cells. J Lipid Res. 2004;48:42062–42071. doi: 10.1194/jlr.M700279-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Caviglia JM, Sparks JD, Toraskar N, Brinker AM, Yin TC, Dixon JL, Brasaemle DL. ABHD5/CGI-58 facilitates the assemble and secretion of apolipoprotein B lipoproteins by McA-RH7777 rat hepatoma cells. Biochim Biophys Acta. 2009;179:198–205. doi: 10.1016/j.bbalip.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eichmann TO, Grumet L, Taschler U, Hartler J, Heier C, Woblistin A, Pajed L, Kollroser M, Rechberger G, Thallinger GG, Zechner R, Haemmerle G, Zimmermann R, Lass A. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J Lipid Res. 2015;56:1972–1984. doi: 10.1194/jlr.M062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miao H, Ou J, Ma Y, Guo F, Yang Z, Wiggins M, Liu C, Song W, Han X, Wang M, Cao Q, Chung BH, Yang D, Liang H, Xue B, Shi H, Gan L, Yu L. Macrophage CGI-58 deficiency activates ROS-inflammasome pathway to promote insulin resistance in mice. Cell Rep. 2014;7:223–235. doi: 10.1016/j.celrep.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie P, Zheng X, Xiao J, Sun B, Yang D. Transgenic CGI-58 expression in macrophages alleviates the atherosclerotic lesion development in ApoE knockout mice. Biochim Biophys Acta. 2014;1841:1683–1690. doi: 10.1016/j.bbalip.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Miao H, Ou J, Peng Y, Zhang X, Chen Y, Hao L, Xie G, Wang Z, Pang X, Ruan Z, Li J, Yu L, Xue B, Shi H, Shi C, Liang H. Macrophage ABHD5 promotes colorectal cancer growth by suppressing spermidine production by SRM. Nat Commun. 2016;7:11716. doi: 10.1038/ncomms11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goeritzer M, Schlager S, Radovic B, Madreiter CT, Rainer S, Thomas G, Lord CC, Sacks J, Brown AL, Obrowsky S, Sachdev V, Kolb D, Chandak PG, Graier WF, Sattler W, Brown JM, Kratky D. Deletion of CGI-58 or adipose triglyceride lipase differently affects macrophage function and atherosclerosis. J Lipid Res. 2014;55:2562–2575. doi: 10.1194/jlr.M052613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G. Trigylceride deposit cariomyovasculopathy. N Engl J Med. 2008;359:2396–2398. doi: 10.1056/NEJMc0805305. [DOI] [PubMed] [Google Scholar]
- 59.Reilich P, Horvath R, Krause S, Schramm N, Turnbull DM, Trenell M, Hollingsworth KG, Gorman GS, Hans VH, Reimann J, MacMillan A, Turner L, Schollen A, Witte G, Czemin B, Holinski-Feder E, Walter MC, Schoser B, Lochmuller H. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol. 2011;258:1987–1997. doi: 10.1007/s00415-011-6055-4. [DOI] [PubMed] [Google Scholar]
- 60.Janseen MC, van Engelen B, Kapusta L, Lammens M, van Dijk M, Fishcer J, van der Graaf M, Wevers RA, Fahrleitner M, Zimmermann R, Morava E. Symptomatic lipid storage in carriers for the PNPLA2 gene. Eur J Hum Genet. 2013;21:807–815. doi: 10.1038/ejhg.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:E505–E513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 62.Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose trigylceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res. 2010;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolkart G, Schrammel A, Dorffel K, Haemmerle G, Zechner R, Mayer B. Cardiac dysfunction in adipose triglyceride lipase deficiency: treatment with a PPARα agonist. Br J Pharmacol. 2012;165:380–389. doi: 10.1111/j.1476-5381.2011.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zierler KA, Jaeger D, Pollak NM, Eder S, Rechberger GN, Radner FP, Woelkart G, Kolb D, Schmidt A, Kumari M, Preiss-Landl K, Pieske B, Mayer B, Zimmermann R, Lass A, Zechner R, Haemmerle G. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J Biol Chem. 2013;288:9892–9904. doi: 10.1074/jbc.M112.420620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie P, Kadegowda AK, Ma Y, Guo F, Han X, Wang M, Groban L, Xue B, Shi H, Li H, Yu L. Muscle-specific deletion of comparative gene identification-58 (CGI-58) causes muscle steatosis but improves insulin senstivity in male mice. Endocrinology. 2015;156:1648–1658. doi: 10.1210/en.2014-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baldin PM, Loubiere C, Coonen M, Louche K, Tavernier G, Bourlier V, Mairal A, Rustan AC, Smith SR, Langin D, Moro C. Regulation of skeletal muscle lipolysis and oxidative metabolism by the co-lipase CGI-58. J Lipid Res. 2012;53:839–848. doi: 10.1194/jlr.M019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao-Borengasser A, Varma V, Coker RH, Ranganathan G, Phanavanh B, Rasouli N, Kern PA. Adipose triglyceride lipase expression in human adipose tissue and muscle. Role in insulin resistance and response to training and pioglitazone. Metabolism. 2011;60:1012–1020. doi: 10.1016/j.metabol.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am J Physiol Regul Integr Comp Physiol. 2013;304:R644–R650. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mason RR, Meex RC, Russell AP, Canny BJ, Watt MJ. Cellular localization and associations of the major lipolytic proteins in human skeletal muscle at rest and during exercise. PLoS One. 2014;9:e103062. doi: 10.1371/journal.pone.0103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Granneman JG, Moore HH, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet associated protein. J Biol Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandak PG, Radovic B, Aflaki E, Kolb D, Buchebner M, Frohlich E, Magnes C, Sinner F, Haemmerle G, Zechner R, Tabas I, Levak-Frank S, Kratky D. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J Biol Chem. 2010;285:20192–20201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lammers B, Chandak PG, Aflaki E, Van Puijvelde GH, Radovic B, Hildebrand RB, Meurs I, Out R, Kuiper J, Van Berkel TJ, Kolb D, Haemmerle G, Zechner R, Levak-Frank S, Van Eck M, Kratky D. Macrophage adipose triglyceride lipase deficiency atteunuate atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:67–73. doi: 10.1161/ATVBAHA.110.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aflaki E, Radovic B, Chandak PG, Kolb D, Eisenberg T, Ring J, Fertschi I, Uellen A, Wolinski H, Kohlwein SD, Zechner R, Levak-Frank S, Sattler W, Graier WF, Malli R, Madeo F, Kratky D. Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages. J Biol Chem. 2011;286:7418–7428. doi: 10.1074/jbc.M110.175703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aflaki E, Belenga NA, Luschnig-Schratl P, Wolinski H, Povoden S, Chandak PG, Bogner-Strauss JG, Eder S, Konya V, Kohlwein SD, Heinemann A, Kratky D. Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis. Cell Mol Life Sci. 2013;68:2621. doi: 10.1007/s00018-011-0688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lord C, Betters JL, Ivanova PT, Milne SB, Myers DS, Madenspacher J, Thomas G, Chung S, Liu M, Davis MA, Lee RG, Crooke RM, Graham MJ, Parks JS, Brasaemle DL, Fessler MB, Brown HA, Brown JM. CGI-58/ABHD5-derived signaling lipids regulate systemic inflammation and insulin action. Diabetes. 2012;61:355–363. doi: 10.2337/db11-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jha P, Claudel T, Baghdasaryan A, Mueller M, Halibasic E, Das SK, Lass A, Zimmermann R, Zechner R, Hoefler G, Trauner M. Role of adipose trigylceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59:858–869. doi: 10.1002/hep.26732. [DOI] [PubMed] [Google Scholar]
- 81.Xie P, Guo F, Ma Y, Zhu H, Wang F, Xue B, Shi H, Yang J, Yu L. Intestinal Cgi-58 deficiency reduces postprandial lipid absorption. PLoS One. 2014;9:e91652. doi: 10.1371/journal.pone.0091652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obrowsky S, Pandak PG, Patankar JV, Povoden S, Schlager S, Kershaw EE, Bogner-Strauss JG, Hoefler G, Levak-Frank S, Kratky D. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J Lipid Res. 2013;54:425–435. doi: 10.1194/jlr.M031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young SG, Cham CM, Pitas RE, Burri BJ, Connoly A, Flynn L, Pappu AS, Wong JS, Hamilton RL, Farese RV., Jr A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96:2932–2946. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu J, Lee B, Buhman KK, Cheng JX. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie. 2014;96:48–55. doi: 10.1016/j.biochi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Samuel VT, Shulman GI. The pathogenesis on insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 88.Kienesberger PC, Lee D, Pulinikunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G, Flier JS, Zechner R, Kim YB, Kershaw EE. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoy AJ, Bruce CR, Turpin SM, Morris AJ, Febbraio MA, Watt MJ. Adipose triacylglycerol lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152:48–58. doi: 10.1210/en.2010-0661. [DOI] [PubMed] [Google Scholar]
- 90.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Chen WS, Chen PL, Li J, Lind AC, Lu D. Lipid synthesis and processing proteins ABHD5, PGRMC1, and squalene synthase can serve as novel immunohistochemical markers for sebaceous neoplasms and differentiate sebaceous carcinoma and sebaceoma and basal cell carcinoma with clear cell features. J Cutan Pathol. 2013;40:631–638. doi: 10.1111/cup.12147. [DOI] [PubMed] [Google Scholar]
- 92.Senchenko VN, Kisseljova NP, Ivanova TA, Dmitriev AA, Krasnov GS, Kudryavtseva AV, Panasenko GV, Tsirin EB, Lerman MI, Kisseljov FL, Kashuba VI, Zabarovsky ER. Novel tumor suppressor candidates on chromosome 3 revealed by NotI-microarrays in cervical cancer. Epigenetics. 2013;8:409–420. doi: 10.4161/epi.24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ou J, Miao H, Ma Y, Guo F, Deng J, Wei X, Zhou J, Xie G, Shi H, Xue B, Liang H, Yu L. Loss of abhd5 promotes colorectal tumor development and progression by inducing aerobic glycolysis and epithelial-mesenchymal transition. Cell Rep. 2014;9:1798–1811. doi: 10.1016/j.celrep.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng Y, Miao H, Wu S, Yang W, Zhang Y, Xie G, Xie X, Li J, Shi C, Ye L, Sun W, Wang L, Liang H, Ou J. ABHD5 interacts with BECN1 to regulate autophagy and tumorigenesis of colon cancer independent of PNPLA2. Autophagy. 2016;12:2167–2182. doi: 10.1080/15548627.2016.1217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L, Ding Y, Chen Y, Zhang S, Huo C, Wang Y, Yu J, Zhang P, Na H, Zhang H, Ma Y, Liu P. The proteomics of lipid droplets: Structure, dynamics, and functions of the organelle conserved from bacteria to humans. J Lipid Res. 2012;53:1245–1253. doi: 10.1194/jlr.R024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.James CN, Horn PJ, Case CR, Gidda SK, Zhang D, Mullen RT, Dyer JM, Anderson RG, Chapman KD. Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci USA. 2010;107:17822–17838. doi: 10.1073/pnas.0911359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh AK, Chauhan N, Rajakumari S, Daum G, Rajasekharan R. At4g24160, a soluble acyl-coenzyme A-dependent lysophosphatidic acid acyltransferase. Plant Physiol. 2009;151:869–881. doi: 10.1104/pp.109.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park S, Gidda SK, James CN, Horn PJ, Khuu N, Seay DC, Keereetaweep J, Chapman KD, Mullen RT, Dyer JM. The α/β hydrolase CGI-58 and peroxisomal transport protein PXA1 co-regulate lipid homeostasis and signaling in Arabidopsis. Plant Cell. 2013;25:1726–1739. doi: 10.1105/tpc.113.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shigenaga AM, Argueso CT. No hormone to rule them all: interactions of plant hormones during the responses of plants to pathogens. Semin Cell Dev Biol. 2016;56:174–189. doi: 10.1016/j.semcdb.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Park S, Keereetaweep J, James CN, Gidda SK, Chapman KD, Mullen RT, Dyer JM. CGI-58, a key regulator of lipid homeostasis and signaling in plants, also regulates polyamine metabolism. Plant Signal Behav. 2014;9:e27723. doi: 10.4161/psb.27723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McLauchlan DH, Lan J, Geilfus CM, Dodd AN, Larson T, Baker A, Horak H, Kollist H, He Z, Graham I, Mickelbart MV, Heatherington AM. The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr Biol. 2016;26:707–712. doi: 10.1016/j.cub.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie M, Roy R. The causative gene in Chanarin Dorfman Syndrome regulates lipdi droplet homeostasis in C. elegans. PLoS Genet. 2015;11:e1005284. doi: 10.1371/journal.pgen.1005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lord CC, Thomas G, Brown JM. Mammalian alpha beta hydrolase domain (ABHD) proteins: lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim Biophys Acta. 2013;183:792–802. doi: 10.1016/j.bbalip.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2009;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McMahon D, Dinh A, Kurz D, Shah D, Han GS, Carman GM, Brasaemle DL. Comparative gene identification 58/α/β hydrolase domain 5 lacks lysophosphatidic acid acyltransferase activity. J Lipid Res. 2014;55:1750–1761. doi: 10.1194/jlr.M051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–678. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- 108.Zhang J, Xu D, Nie J, Han R, Zhai Y, Shi Y. Comparative gene identifcation-58 (CGI-58) promotes autophagy as a putative lysophosphatidylglycerol acyltransferase. J Biol Chem. 2015;289:241. doi: 10.1074/jbc.M114.573857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khatib A, Arhab Y, Bentebibel A, Abousalham A, Noriel A. Reassessing the potential activities of plant CGI-58 protein. PLoS One. 2016;11:e0145806. doi: 10.1371/journal.pone.0145806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eichmann TO, Kumari M, Haas JT, Farese RV, Jr, Zimmermann R, Lass A, Zechner R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-senstive lipase, and diacyl-O-acyltransferases. J Biol Chem. 2012;287:41446–41457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]