Abstract
Background
In non-small cell lung cancer (NSCLC), platelet-derived growth factor receptor (PDGFR) mediates angiogenesis, tissue invasion, and tumor interstitial pressure. Olaratumab (IMC-3G3) is a fully human anti-PDGFRα monoclonal antibody. This Phase II study assessed safety and efficacy of olaratumab + paclitaxel/carboplatin (P/C) versus P/C alone for previously untreated advanced NSCLC.
Materials and Methods
Patients received up to six 21-day cycles of P 200 mg/m2 and C AUC 6 (day 1) ± olaratumab 15 mg/kg (days 1 and 8). Primary endpoint was PFS. Olaratumab was continued in the olaratumab+P/C arm until disease progression.
Results
131 patients were: 67 with olaratumab+P/C and 64 with P/C; 74% had nonsquamous NSCLC. Median PFS was similar between olaratumab+P/C and P/C (4.4 months each) (HR 1.29; 95% CI [0.86–1.93]; p=0.21). Median OS was similar between olaratumab+P/C (11.8 months) and P/C (11.5 months) (HR 1.04; 95% CI [0.68–1.57]; p=0.87). Both arms had similar toxicity profiles. All evaluable cases were PDGFR-negative by immunohistochemistry. Tumor stroma PDGFR expression was evaluable in 23/131 patients, of which 78% were positive.
Conclusions
The addition of olaratumab to P/C did not result in significant prolongation of PFS or OS in advanced NSCLC. Olaratumab studies in other patient populations, including soft tissue sarcoma (NCT02783599), pancreatic cancer (NCT03086369), and pediatric malignancies (NCT02677116) are underway.
Keywords: Olaratumab, NSCLC, PDGFR, paclitaxel/carboplatin
1. Introduction
Lung cancer remains one of the leading causes of cancer mortality internationally. Across all stages of disease, 5-year survival is only 17%[1]. Substantial advances have been made for patients with non-small cell lung cancer (NSCLC) harboring specific oncogenic alterations such as EGFR mutations[2] and ALK rearrangements[3]. Additionally, immune checkpoint inhibitors may offer prolonged disease control for a subset of patients[4,5]. Nevertheless, the necessity for conventional cytotoxic chemotherapy remains. Biologic agents such as bevacizumab (for non-squamous cases) and necitumumab (for squamous cases) have enhanced outcomes of platinum doublet chemotherapy[6–8].
Platelet-derived growth factor receptor (PDGFR) is a transmembrane receptor tyrosine kinase with potential as both a tumor cell and stromal target in NSCLC. Upon binding of circulating PDGF ligand, PDGFRα and β subunits homodimerize or heterodimerize, undergo autophosphorylation, and activate downstream signal transduction molecules including phosphoinositide 3-kinase, Ras, phospholipase C-γ, Src, and signal transducer and activator of transcription[9–11].
PDGFRα has been implicated in cancer development and progression[12]. In various malignancies, co-expression of PDGF and PDGFRα has been reported, consistent with autocrine-mediated growth[11]. In tumor stroma, the PDGF-PDGFRα axis functions in fibroblast activation, aberrant epithelial-stromal interactions, modulation of tumor interstitial pressure, and production and secretion of vascular endothelial growth factor (VEGF) [13–15]. Although gene fusions and activating mutations involving PDGFRα have been implicated in other cancers such as gastrointestinal stromal tumors[16] and gliomas[17], they appear to be relatively uncommon in lung cancer[18].
In lung cancer, expression of PDGF and/or PDGFRα is associated with more aggressive tumor biology and worse prognosis[19]. In rare cases of NSCLC (approximately 1%), PDGFRA amplification results in oncogene addiction, driving tumor cell proliferation and conferring sensitivity to PDGFR inhibitors such as sunitinib[18,20]. Earlier work demonstrates that inhibition of stromal PDFGRα, independent of tumor cell PDFGRα expression, results in lung cancer growth inhibition and enhancement of chemotherapy effects[21].
Olaratumab (IMC-3G3; Eli Lilly and Company, Indianapolis, Indiana), a fully human IgG1 anti-PDGFRα monoclonal antibody, selectively binds human PDGFRα with high affinity (approximately 40 pM) and inhibits ligand binding[22]. The antibody blocks PDGF-AA, -BB, and -CC ligands from binding to the receptor, thereby inhibiting ligand-induced receptor autophosphorylation and phosphorylation of downstream signal transduction via Akt and mitogen-activated protein kinase (MAPK). Olaratumab demonstrated anti-tumor activity in in vivo systems on cancer models known to be driven by a PDGF-PDGFRα autocrine loop[23]. In an olaratumab Phase I clinical trial of patients with advanced refractory solid tumors, no dose-limiting toxicities were observed, although two possibly drug-related serious adverse events (SAEs) (increased alkaline phosphatase and tumor hemorrhage; each grade 2) in one patient were noted. Twelve of 19 evaluable patients achieved stable disease[24].
This trial of olaratumab with paclitaxel/carboplatin (P/C) or P/C alone in previously untreated patients with advanced NSCLC was based on biologic rationale and preclinical data.
2. Materials and methods
2.1. Study design and patient enrollment
This was a prospective, randomized, open-label, multicenter Phase II study. The primary endpoint of this trial was progression-free survival (PFS). Secondary endpoints included safety, objective response rate (ORR), overall survival (OS), duration of response, pharmacokinetics (PK), immunogenicity, and pharmacodynamic profile. This study was conducted according to the Declaration of Helsinki and with approval from Institutional Review Boards of all participating study sites. All participants provided written informed consent prior to any study-related procedures. The trial was funded by the study sponsor and designed by the principal investigator (DEG) and the sponsor.
Eligible patients were ≥18 years of age with histologically or cytologically confirmed previously untreated locally advanced or metastatic NSCLC (American Joint Committee on Cancer [AJCC] 6th Edition Stage IIIB with effusion or stage IV, corresponding to AJCC 7th Edition Stage IV M1a or Stage IV M1b, respectively). Squamous and non-squamous histologies were permitted. Additional eligibility criteria included performance status ECOG 0–1; and adequate hematologic, renal, and hepatic function. Exclusions included a history of gross hemoptysis (≥ ½ teaspoon) within 2 months of randomization or evidence of major airway or blood vessel invasion by tumor. Assessment of tumor molecular profile (eg, EGFR, ALK) was not required.
2.2. Study procedures
All patients received paclitaxel 200 mg/m2 over 3 hours on Day 1, followed by carboplatin area under the time-concentration curve (AUC) 6 mg∙hr/L in a 30-minute infusion after the end of paclitaxel infusion. Chemotherapy was administered for up to six 21-day cycles. The carboplatin-paclitaxel chemotherapy backbone was selected because (1) it provided a uniform comparator across histologic subtypes, (2) it had clearly defined and well-established toxicity and efficacy profiles, and (3) at the time of study design and enrollment, first-line pemetrexed-based regimens were not yet used for non-squamous cases. In the olaratumab+P/C arm, olaratumab 15 mg/kg was to be administered at an infusion rate of 25 mg/minute (minimum infusion duration: 30 minutes), on Days 1 and 8 of each 21-day treatment cycle, in combination with P/C administered on Day 1 of each cycle, until disease progression or unacceptable toxicity. At the time of disease progression, patients in the P/C (control) arm could choose to receive olaratumab monotherapy until unacceptable toxicity or disease progression. Because this combination had not been studied previously, an early safety review was performed after six patients received at least four infusions of olaratumab.
Tumor response was based on investigator assessment of target and non-target lesions every 6 weeks measured from the date of randomization. Based on Response Evaluation Criteria in Solid Tumors (RECIST version 1.1)[25,26], tumor measurements were used to determine PFS, ORR, and duration of response. Safety was followed from time of informed consent until 30 days after discontinuation of study treatment or death, whichever happened first. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0).
Pharmacokinetic (PK) sampling measured olaratumab serum concentration levels in all patients enrolled in the olaratumab+P/C arm and patients who received olaratumab on the control arm.
K2-EDTA plasma samples were utilized for analysis of pharmacodynamic markers PDGF-AA and VEGF-A. Pre-treatment circulating protein levels for PDGF-AA and VEGF-A were determined by an analytically validated ELISA at Intertek and were reported as continuous measures. Additionally, optional archival tissue specimens submitted as formalin-fixed paraffin embedded tissues were collected for exploratory biomarker analysis by immunohistochemistry.
2.3. Immunohistochemistry
Immunohistochemistry was performed using a rabbit monoclonal antibody to PDGFRα (clone D13C6, Cell Signaling Technologies, Danvers, MA) diluted to 0.446 µg/mL working concentration in Leica Bond™ Primary Antibody Diluent. In short, a 4-µm section of submitted patient biopsy preserved as a formalin-fixed, paraffin-embedded block was cut by microtome and placed onto a positively charged slide. Immunohistochemistry with the D13C6 antibody was then automated on the Leica Bond™ Autostainer, using Bond Epitope Retrieval Solution 2. The prepared slides were then evaluated by a board-certified pathologist.
A case was adequate for interpretation of tumor if approximately 100 well-preserved tumor cells were identified, and adequate for interpretation of stroma if sufficient well-preserved tumor-associated stroma was present for the pathologist to render a professional interpretation of its staining. Cases were evaluated qualitatively as tumor positive or negative and stroma positive or negative, and only if positive and negative staining controls for each batch performed appropriately. Cases with insufficient tumor were not scored. Cases with insufficient stroma for evaluation were scored for tumor staining, but not stromal staining. Tumor cells were considered positive for PDGFRα if >30% of the tumor showed at least weak membranous staining or ≥5% of the tumor showed moderate-to-strong intensity membranous staining. Tumor cells not meeting these criteria were scored “tumor negative.” Any stromal staining at any intensity comprising >5% of the overall tumor-associated stroma present was scored as “stroma positive”[27]. Any stromal staining not meeting this requirement was scored as “stroma negative.” These criteria were established as part of validation against a set of samples known by other means to express (or not express) PDGFRα.
2.4. Statistical analysis
Assuming a PFS hazard ratio of 0.67 and 80% statistical power, the sample size of 136 patients was sufficient to show a statistically significant improvement in PFS at a one-sided 10% significance level.
Primary and secondary efficacy analyses were based on the modified intent-to-treat (mITT) population. This population was defined as randomized patients who received any quantity of study drug. The safety population, used for all safety analyses, included all patients who received any quantity of study drug.
Olaratumab serum concentration data were analyzed by standard non-compartmental methods.
3. Results
3.1. Baseline patient and disease characteristics
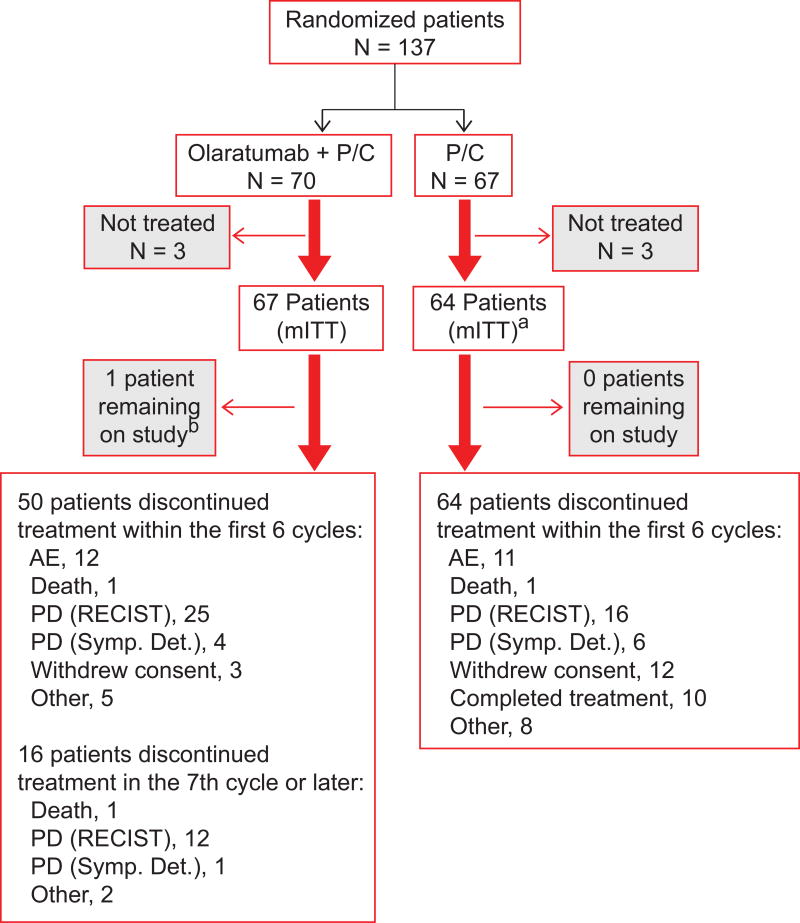
A total of 137 patients were randomized at 22 study sites in the United States and Canada between January 2010 and August 2013. At least one dose of study drug was received by 131 patients. Patient disposition is displayed in Figure 1. Sixty-seven patients received olaratumab+P/C; 64 patients were treated with P/C. Seventeen patients from the control arm opted to receive olaratumab monotherapy. Demographic and disease characteristics were balanced between treatment arms. In total, 37% of patients had prior surgery, 37% had prior radiation therapy, and 12% had prior chemotherapy in the neoadjuvant/adjuvant setting. Prior lung cancer therapy was balanced across arms (Table 1).
Figure 1.
Patient disposition
aIncludes 17 patients who received olaratumab monotherapy.
bAs of June 25, 2015.
P/C, paclitaxel/carboplatin; mITT, modified intent to treat; AE, adverse event; PD, progressive disease; RECIST, Response Evaluation Criteria in Solid Tumors; Symp. Det., symptomatic deterioration.
Table 1.
Baseline demographic and disease characteristics
| Characteristic | Olaratumab plus paclitaxel-carboplatin (Arm A) (N = 67) n (%) |
Paclitaxel-carboplatin (Arm B)(N=64) n(%) |
|---|---|---|
| Gender | ||
| Male | 40 (60) | 34 (53) |
| Female | 27 (40) | 30 (47) |
| Race/Ethnicity | ||
| White | 54 (81) | 56 (88) |
| Black or African American | 10 (15) | 6 (9) |
| Asian | 1 (2) | 1 (2) |
| Multiple | 1 (2) | 0 |
| Other | 1 (2) | 1 (2) |
| Age (y) (mean ± SD) | 64 ± 11 | 64 ± 10 |
| Performance status | ||
| ECOG 0 | 25 (37) | 16 (25) |
| ECOG 1 | 42 (63) | 48 (75) |
| Histology | ||
| Non-squamous | 48 (72) | 48 (75) |
| Squamous | 19 (28) | 16 (25) |
| Stage | ||
| Stage 3B | 3 (5) | 0 |
| Stage 4 | 40 (60) | 41 (64) |
| Missinga | 24 (36) | 22 (34) |
| MXb | 0 | 1 (2) |
| Prior therapy | ||
| Biological | 0 | 1 (2) |
| Chemotherapy | 10 (15) | 5 (8) |
| Radiotherapy | 24 (36) | 24 (38) |
SD = standard deviation, ECOG = Eastern Cooperative Oncology Group.
Missing data were not imputed, except for missing dates concerning major safety and efficacy parameters.
Metastasis cannot be measured.
3.2. Treatment
The median duration of olaratumab therapy was 3.7 months (range: 0.7–31.4 months) for patients in the olaratumab+P/C arm. The median durations of paclitaxel and carboplatin administrations, respectively, were 2.8 months (range: 0.7–5.1 months) for patients in the olaratumab+P/C and 2.8 months (range: 0.7–4.9 months) for patients in the control arm. The median duration of olaratumab treatment for patients in the control arm who elected to receive olaratumab monotherapy at time of disease progression was 1.6 months (range: 0.7–11.7 months). Paclitaxel and carboplatin dose delays/reductions were comparable in the olaratumab+P/C (paclitaxel delay/reduction: 31%/22%; carboplatin delay/reduction: 33%/15%) and control arm (paclitaxel delay/reduction: 33%/23%; carboplatin delay/reduction: 33%/14%). In the olaratumab+P/C arm, 33 patients (49%) had dose delay of olaratumab, 13 patients (19%) had dose reduction of olaratumab, and 25 patients (37%) had dose hold of olaratumab.
The most common reasons for discontinuation from study therapy in both groups were progressive disease per RECIST (41%), followed by adverse events (AEs) (18%) and withdrawal of consent and “other” reasons (12% each). Additional anticancer treatments after therapy were received by 37 of 67 patients (55%) in the olaratumab+P/C arm, and by 30 of 64 patients (47%) in the control arm. These therapies included additional chemotherapy (42% in the olaratumab+P/C arm; 30% in the control arm) and radiation therapy (21% in the olaratumab+P/C arm; 9% in the control arm).
3.3. Efficacy outcomes
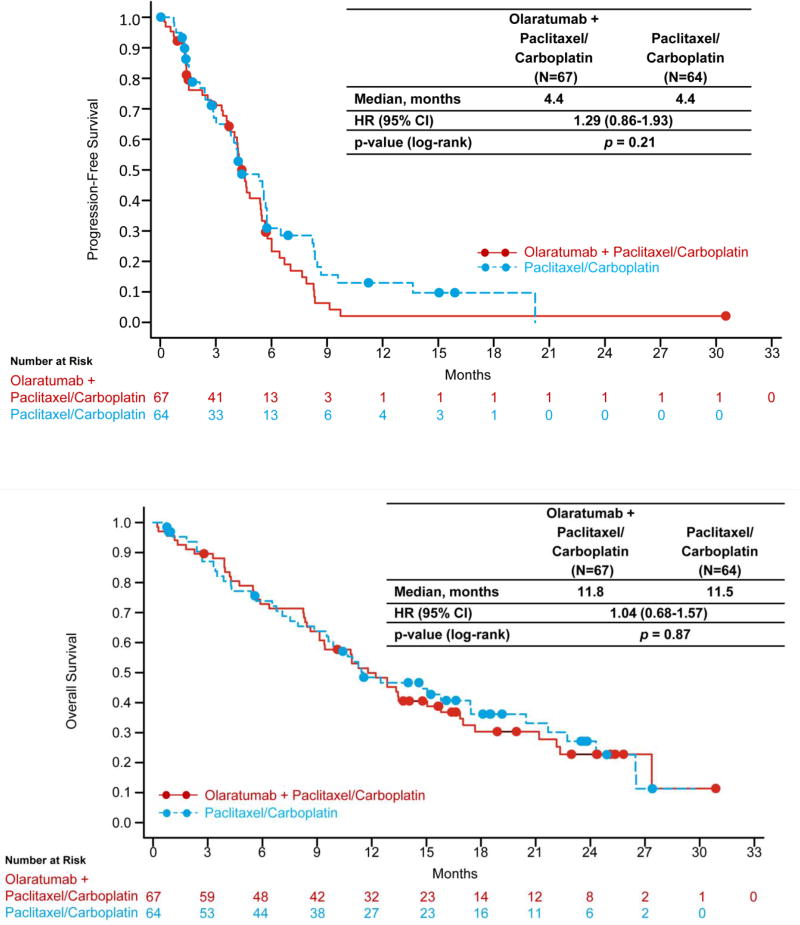
Fifty-four patients (81%) in the olaratumab+P/C arm and 44 patients (69%) in the control arm had PFS events. Median PFS was 4.4 months (95% CI, 3.8–5.4 months: olaratumab+P/C) and 4.4 months (95% CI, 3.5–5.7 months: control arm) (HR 1.29; 95% CI, 0.86–1.93; P=0.21) (Figure 2a). Among patients with squamous cell cancers, median PFS was 4.0 months (95% CI, 1.5–5.5 months: olaratumab+P/C); 5.3 months (95% CI: 2.7–8.2 months: control arm) (HR 1.54; 95% CI, 0.70–3.37). Among patients with non-squamous cancers, median PFS was 4.6 months (95% CI, 4.1–5.7: olaratumab+P/C) and 4.4 months (95% CI, 2.9–5.7 months: control arm) (HR 1.21; 95% CI, 0.75–1.94).
Figure 2.
Efficacy
Median OS was 11.8 months (95% CI, 9.1–15.8: olaratumab+P/C) and 11.5 months (95% CI, 9.5–17.5 months: control arm) (HR 1.04; 95% CI, 0.68–1.57; P=0.87) (Figure 2b). One- and 2-year survival rates were 49.9% (95% CI, 37.3–61.2) and 22.7% (95% CI, 12.4–35: olaratumab+P/C), and 48.4% (95% CI, 35.3–60.3) and 27.1% (95% CI, 15.2–40.6: control arm), respectively. Median OS was similar among patients with squamous tumors (12.1 months [95% CI, 4.3–17.0 months: olaratumab+P/C], 10.9 months [95% CI, 6.5–29.7 months: control], HR 1.34 95% CI, 0.59–3.08) and non-squamous tumors (11.8 months [95% CI, 8.6–16.9 months: olaratumab+P/C], 11.5 months [95% CI, 7.1–20.5 months: control], HR 0.95 [95% CI, 0.59–1.53]), respectively.
No patient had a complete response. A partial response (PR) was observed with 28 patients (42%) (olaratumab+P/C) and 22 patients (34%) (control) (P=0.39). Stable disease (SD) was observed in 17 patients (25%) (olaratumab+P/C) and 18 patients (28%) (control). For 7 patients (10%) (olaratumab+P/C) and 10 patients (16%) (control), radiographic response was not evaluable.
3.4. Safety
Treatment-emergent AEs (TEAEs) are summarized in Table 2. The most frequently reported TEAEs for olaratumab+P/C regardless of causality were fatigue (64%), neuropathy (66%), alopecia (55%), and nausea and neutropenia (52% each). The most frequently reported TEAEs in the control arm regardless of causality were neuropathy (63%), alopecia (59%), fatigue (52%) and nausea (56%). The following TEAEs were reported more frequently (≥10% difference) among olaratumab+P/C vs control patients: neutropenia, thrombocytopenia, decreased appetite, arthralgia, diarrhea, mucositis, infusion-related reactions (IRRs), fatigue, and peripheral edema. TEAEs that were observed at a higher incidence (≥10% difference) (control arm vs olaratumab+P/C) included pain in extremity and decreased weight.
Table 2.
Treatment-emergent adverse events (≥20% of patients)
| Olaratumab plus Paclitaxel-carboplatin (Arm A) (N = 67) |
Paclitaxel-carboplatin (Arm B) (N = 64) |
|||
|---|---|---|---|---|
|
| ||||
| Event | All grades n (%) |
Grade ≥3 n (%) |
All grades n (%) |
Grade ≥3 n (%) |
| Patients with any adverse event | 67 (100) | 54 (81) | 64 (100) | 40 (63) |
| Fatigue | 43 (64) | 7 (10) | 33 (52) | 2 (3) |
| Alopecia | 37 (55) | 0 | 38 (59) | 0 |
| Nausea | 35 (52) | 3 (5) | 36 (56) | 2 (3) |
| Neutropenia* | 35 (52) | 25 (37) | 21 (33) | 14 (22) |
| Decreased appetite | 31 (46) | 1 (2) | 19 (30) | 0 |
| Thrombocytopenia | 29 (43) | 9 (13) | 15 (23) | 3 (5) |
| Diarrhea | 29 (43) | 2 (3) | 19 (30) | 0 |
| Vomiting | 28 (42) | 0 | 22 (34) | 0 |
| Arthralgia | 25 (37) | 0 | 14 (22) | 3 (5) |
| Constipation | 24 (36) | 1 (2) | 27 (42) | 0 |
| Anemia | 23 (34) | 4 (6) | 27 (42) | 6 (9) |
| Peripheral neuropathy | 23 (34) | 2 (3) | 18 (28) | 1 (2) |
| Peripheral sensory neuropathy | 21 (31) | 2 (3) | 22 (34) | 2 (3) |
| Mucositis* | 19 (28) | 1 (2) | 10 (16) | 0 |
| Insomnia | 14 (21) | 0 | 12 (19) | 0 |
| Myalgia | 14 (21) | 1 (2) | 15 (23) | 2 (3) |
| Pain in extremity | 6 (9) | 0 | 17 (27) | 2 (3) |
| Febrile neutropenia** | 4(6) | 4 (6) | 1 (2) | 1 (2) |
| Adverse Event of Special Interest | ||||
| Infusion-related reactions*** | 17 (25) | 1 (2) | 5 (8) | 1 (2) |
| SAEs | ||||
| Any SAEs | 30 (45) | 27 (40) | 19 (30) | 17 (27) |
Consolidated term comprising the following synonymous MedDRA preferred terms: neutropenia (leukopenia, neutropenia, neutrophil count decreased, white blood cell count decreased); mucositis (mucosal inflammation, oropharyngeal pain, stomatitis).
These events are included here because they were considered clinically important.
Infusion-related reactions include a combination of specific preferred terms such as infusion-related reactions, anaphylaxis and signs and symptoms such as flushing and itching.
TEAEs and SAEs grade ≥3 were more frequent in patients treated with olaratumab + P/C arm as compared to P/C arm. (Table 2). TEAEs of grade ≥3 occurring at ≥5% (olaratumab + P/C) were thrombocytopenia (13% vs 5%) and fatigue (10% vs 3%). The rate of neutropenia was higher in olaratumab+P/C compared to the control arm: all grades (35: [(52%] vs 21 [(33%]) and grade ≥3 (25: [37%] vs 14 [22%]). The rate of febrile neutropenia was higher with olaratumab+P/C compared to the control arm (4 patients [6%] vs 1 patient [2%]), although the rate of serious infections was similar (7 patients [10%] vs 6 patients [9%], respectively).
Twenty-one patients (31%) in the olaratumab+P/C arm and 14 patients (22%) in the control arm experienced a TEAE leading to discontinuation of any study drug. Discontinuation of olaratumab occurred in 10 patients (15%) in the olaratumab+P/C arm. The most common TEAEs leading to discontinuation of olaratumab were IRR and fatigue (2 patients each). Eighteen patients (27%) in the olaratumab+P/C arm and 14 patients (22%) in the control arm experienced a TEAE leading to discontinuation of P/C chemotherapy. The most common TEAE leading to discontinuation of chemotherapy in either treatment arm was peripheral neuropathy (5 and 4, respectively in the olaratumab+P/C and P/C arm). TEAEs leading to discontinuation are shown in Supplemental Table 1.
Prespecified TEAEs of special interest included IRRs. The incidence of IRRs (related to olaratumab) was higher in the olaratumab+P/C arm (17 patients [25%]; 1 patient [2%] grade ≥3) than in the control arm (IRR related to chemotherapy: 5 patients [8%]; 1 patient [2%] grade ≥3). In addition, 2 patients (11%; 1 patient [6%] grade ≥3) who elected to receive olaratumab monotherapy after receiving P/C therapy experienced an IRR.
Of the 131 treated patients in the study, 53 (79.1%) of 67 patients in the olaratumab+P/C group and 47 (73.4%) of 64 patients in the P/C group had died in the study. In the olaratumab+P/C group, death was attributed to disease progression in 46 (68.7%) of 67 patients, other causes in 3 patients and AEs in 4 (6%) patients (myocardial infarction, bronchopleural fistula, dyspnea [interstitial lung disease], and sepsis). In the P/C group, death was attributed to disease progression in 42 (65.6%) of 64 patients, other causes in 2 (3.1%) patients, and AEs in 3 (4.7%) patients (hypoxic respiratory failure, pneumonia, and respiratory failure). With the exception of sepsis, all AEs were considered to be unrelated to study treatment.
3.5. Pharmacokinetics
Olaratumab serum concentration levels were available from 44 patients enrolled in the olaratumab+P/C arm. In the olaratumab+P/C arm, the geometric mean (CV%) olaratumab maximum observed serum concentration (Cmax) after the first infusion was 302 µg/mL (27.0%). Steady state was reached during Cycle 3, with mean steady state maximum and trough olaratumab serum concentration levels (Cmax,ss, Cmin,ss) of 420 µg/mL (57.5%) and 108 µg/mL (31.6%), respectively. In the patients who received olaratumab as monotherapy, Cmin,ss at the end of Cycle 3, with a mean value of 92.5 µg/mL (21.8%), was similar to that observed when olaratumab was combined with P/C. Due to the relatively short sampling time, post-end of infusion (168 hours), and relatively small number of samples collected, the terminal elimination half-life (t1/2) may not have been fully characterized; therefore, the t1/2 was not estimated.
3.6. Biomarker analysis
Pre-treatment tumor biopsy samples were submitted by a total of 44 enrolled patients (Table 3). Of these, only 33 had sufficient tumor for evaluation (18 in the olaratumab+P/C arm, 15 in the control arm). Of the 33 evaluable samples, 22 were submitted as adenocarcinoma and 11 as squamous cell carcinoma. No PDGFRA amplification was observed in 33 tested samples. None of the 33 samples with sufficient tumor for evaluation had PDGFRα expression detectable by immunohistochemistry. Sixteen adenocarcinomas and 7 squamous cell carcinomas had sufficient quantities of tumor stroma for evaluation (12: olaratumab+P/C; 11: control). Tumor stromal fibroblasts were positive for PDGFRα by immunohistochemistry in 13 of 16 (81%) of adenocarcinomas and 5 of 7 (71%) of squamous cell carcinomas (Figure 3). In the olaratumab+P/C arm, 10 of 12 total samples had PDGFRα-positive stroma; in the control arm, 8 of 11 samples were immunohistochemistry positive. While approximately balanced between arms, the number of submitted samples (particularly the number of samples adequate for evaluation) were too small for meaningful statistical correlation of tumor staining, tumor stromal staining or histologic subtype to efficacy endpoints. Baseline plasma PDGF-AA levels in this study were 100- to 1000-fold higher compared to historically reported data and correlated with platelet levels, suggesting that PDGF-AA levels observed in this study reflected platelet activation that occurred in vitro, at the time of plasma collection, rather than endogenous PDGF-AA levels. There was no significant association (assessed at adjusted alpha level of 0.008) between baseline PDGF-AA and VEGF-A levels and PFS or OS assessed by binary cut-point analyses (dichotomized at 25th, 50th, and 75th percentiles of the marker distribution separately) in either interaction or main effects model.
Table 3.
Non-small cell lung carcinoma histology and results of cases submitted for evaluation PDGFRα expression by immunohistochemistry
| Adenocarcinoma N (%) |
Squamous Cell Carcinoma N (%) |
|
|---|---|---|
| Total evaluable cases | 22 | 11 |
| Tumor positive | 0 | 0 |
| Tumor negative | 22 (100) | 11 (100) |
| Stroma positive | 13* (81) | 5* (71) |
| Stroma negative | 3 | 2 |
Legend: Results of immunohistochemical staining for PDGFRα by subtype of NSCLC. Only 33 cases of 44 total specimens submitted for evaluation had sufficient tissue for interpretation, as most submitted tissues were core biopsy specimens following on-site work-up for diagnosis and NSCLC sub-typing.
Of the 33 submitted cases, only a subset had sufficient tumor stroma for evaluation due to needle sampling bias and some cytology cell block specimens submitted.
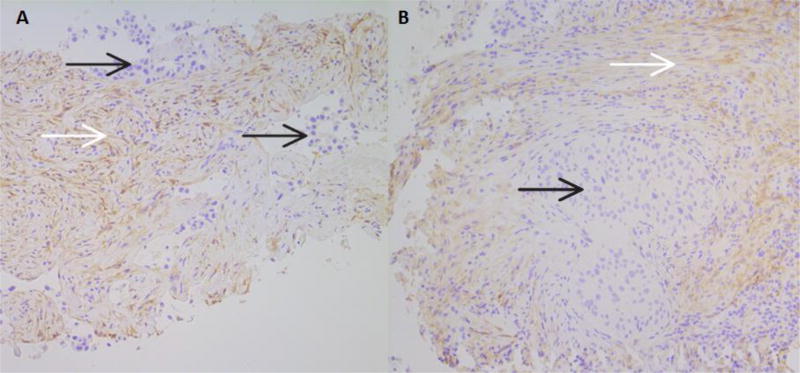
Figure 3.
A. Results of PDGFRα IHC in a core biopsy of an adenocarcinoma. The tumor is negative, and moderate cell membrane and cytoplasmic staining of stromal cells can be seen.
B. Results of PDGFRα IHC in a core biopsy of a squamous cell carcinoma. The tumor is negative, and surrounding stromal fibroblasts show light to moderate cell membrane and cytoplasmic staining, beginning to intersperse among lymphocytes in the lower right corner. Both images are at 100× magnification.
In both A and B, black arrows identify tumor, while white arrows identify positively staining tumor-associated stroma.
4. Discussion
The PDGF-PDGFR axis is implicated in local tumor invasiveness, modulation of tumor interstitial pressure, and angiogenesis[13]. In approximately 1% of NSCLC cell lines, PDGFA amplification results in in vitro sensitivity to PDGFR inhibitors[20]. Additionally, independent of tumor PDGF or PDGFR expression, inhibition of stromal PDGFR signaling inhibits tumor growth and enhances the effect of chemotherapy in multiple lung cancer xenograft models[21]. Based on these preclinical observations and the favorable safety profile in monotherapy studies of the anti-PDGFRα monoclonal antibody olaratumab[22,24], we performed this randomized phase II clinical trial of carboplatin-paclitaxel±olaratumab in previously untreated advanced NSCLC.
Despite this scientific rationale, the addition of olaratumab to chemotherapy did not improve the primary endpoint of PFS or secondary endpoints of response rate and OS. Only 32 samples had sufficient tumor content for tumor immunohistochemical analysis, and 23 samples (12 in the olaratumab treatment arm) with sufficient stroma for evaluation. We identified no cases with tumor cell PDGFRα protein expression or PDGFRA gene amplification, consistent with earlier reports that such events in NSCLC are quite rare[20], while stromal positivity for PDGFRα was observed in the majority of cases. While limited sample numbers preclude reaching any conclusions, one potential explanation for the negative results in this clinical study may be related to the absence of tumor PDGFRα expression. Whether NSCLC tumors also fail to constitutively produce PDGFRα-related ligands (which could hypothetically promote stromal/tumor interactions in the absence of tumor PDGFRα expression) was not examined in this study. Interestingly, in contrast to NSCLC, tumor cell PDGFR activation by autocrine and paracrine mechanisms has been reported in sarcomas[28–30], a tumor type in which olaratumab has recently shown activity in combination with doxorubicin[31].
Although this is the first clinical trial in NSCLC examining the efficacy of an anti-PDGFRα monoclonal antibody, a number of PDGFR-targeting small molecule inhibitors have been studied in this setting. These drugs differ from olaratumab not only in their dual inhibition of PDGFRα and PDGFRβ, but also in their activity against a number of other kinase targets, among them VEGFR, cKIT, and FGFR. While studies with imatinib and sunitinib have not clearly demonstrated improvement over chemotherapy alone[32,33], the addition of nintedanib to docetaxel chemotherapy resulted in some improvement in the general NSCLC population (median PFS 3.4 months versus 2.7 months; HR 0.79; P=0.002). Modest activity in median OS was seen in adenocarcinoma patients (12.6 months versus 10.3 months; HR 0.83; P=0.04)[34]. Whether the moderate efficacy of this agent reflects targeting of both α and β PDGFR isoforms, VEGFR, or FGFR is not known.
Pharmacokinetic analyses demonstrated olaratumab 15 mg/kg administered on a day-1 and-8 schedule achieved target serum concentrations expected from preclinical modeling[24]. Furthermore, serum concentrations in this trial were comparable to those associated with efficacy in a recently disclosed trial of olaratumab in soft tissue sarcoma[31]. Accordingly, it seems unlikely that higher doses of olaratumab would have improved clinical efficacy in NSCLC.
In general, the addition of olaratumab to P/C had an acceptable safety profile and consisted of mainly toxicities due to P/C. Although there were higher rates of neutropenia in the olaratumab-containing arm, there was only a slight increase in febrile neutropenia and serious infections, and no increase in discontinuation due to neutropenia or infections. By contrast, in early NSCLC studies combining imatinib with chemotherapy, dose-limiting hematologic toxicity led to subsequent trials employing a pulsed, intercalated dosing regimen[13,32,35]. The greater myelosuppression observed with imatinib compared to olaratumab may reflect effects on c-KIT (stem cell factor receptor), which has a key functional role in hematopoiesis[36,37]. Similarly, NSCLC studies of sunitinib+combination chemotherapy have demonstrated unacceptable rates and severity of hematologic toxicity[33].
In conclusion, the addition of the anti-PDGFRα monoclonal antibody olaratumab to P/C chemotherapy did not improve clinical efficacy in advanced NSCLC. This trial confirms preclinical observations that tumor cell PDGFRα expression or PDGFRA amplification are very rare events in NSCLC. Although further investigation in NSCLC is not currently planned, olaratumab studies in other patient populations—including soft tissue sarcoma, pancreatic cancer, and pediatric malignancies—are underway.
Supplementary Material
highlights.
Olaratumab (IMC-3G3) is a fully human anti-PDGFR monoclonal antibody
Olaratumab plus carboplatin-paclitaxel does not prolong PFS or OS in NSCLC
PDGFR expression is rare in NSCLC tumor cells but common in NSCLC stroma
The addition of olaratumab to chemotherapy is well tolerated
Acknowledgments
The authors thank Eli Lilly and Company employees Gerard Joseph Oakley III for useful discussion and retrieval of archived immunohistochemistry results, and Anastasia Perkowski for writing assistance. D.E.G. is funded in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentations:
Presented in abstract form at the 50th Annual Meeting of the American Society of Clinical Oncology, May 31-June 3, 2014, Chicago, IL.
Trial registration
Conflict of interest statement
Damien M Cronier, Amy Qin, Robert Ilaria Jr, Jan Cosaert, and Ashwin Shahir are employees of Eli Lillyand Company. The other authors have no relevant disclosures.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 9.Heldin CH, Ostman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 10.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 11.Ostman A, Heldin CH. Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv Cancer Res. 2001;80:1–38. doi: 10.1016/s0065-230x(01)80010-5. [DOI] [PubMed] [Google Scholar]
- 12.Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 13.Bauman JE, Eaton KD, Martins RG. Antagonism of platelet-derived growth factor receptor in non small cell lung cancer: rationale and investigations. Clin Cancer Res. 2007;13:s4632–s4636. doi: 10.1158/1078-0432.CCR-07-0212. [DOI] [PubMed] [Google Scholar]
- 14.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 15.Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 17.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- 18.Ramos AH, Dutt A, Mermel C, et al. Amplification of chromosomal segment 4q12 in non-small cell lung cancer. Cancer Biol Ther. 2009;8:2042–2050. doi: 10.4161/cbt.8.21.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol. 2008;3:963–970. doi: 10.1097/JTO.0b013e3181834f52. [DOI] [PubMed] [Google Scholar]
- 20.McDermott U, Ames RY, Iafrate AJ, et al. Ligand-dependent platelet-derived growth factor receptor (PDGFR)-alpha activation sensitizes rare lung cancer and sarcoma cells to PDGFR kinase inhibitors. Cancer Res. 2009;69:3937–3946. doi: 10.1158/0008-5472.CAN-08-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber DE, Gupta P, Dellinger MT, et al. Stromal platelet-derived growth factor receptor a (PDGFRα) provides a therapeutic target independent of tumor cell PDGFRα expression in lung cancer xenografts. Mol Cancer Ther. 2012;11:2473–2482. doi: 10.1158/1535-7163.MCT-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116:1018–1026. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- 23.Loizos N, Xu Y, Huber J, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4:369–379. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 24.Chiorean EG, Sweeney C, Youssoufian H, et al. A phase 1 study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:595–604. doi: 10.1007/s00280-014-2389-9. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138:1432–1443. doi: 10.5858/arpa.2013-0610-CP. [DOI] [PubMed] [Google Scholar]
- 28.Sturzl M, Roth WK, Brockmeyer NH, Zietz C, Speiser B, Hofschneider PH. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992;89:7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas U, Barwad A, Pubbaraju SV. Complete response of monoblastic myeloid sarcoma with FIP1L1-PDGFRA rearrangement to imatinib monotherapy. Br J Haematol. 2014;165:583. doi: 10.1111/bjh.12742. [DOI] [PubMed] [Google Scholar]
- 30.Ehnman M, Missiaglia E, Folestad E, et al. Distinct effects of ligand-induced PDGFRα and PDGFRβ signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res. 2013;73:2139–2149. doi: 10.1158/0008-5472.CAN-12-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tap WD, Jones RL, Van tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauman JE, Eaton KD, Wallace SG, et al. A Phase II study of pulse dose imatinib mesylate and weekly paclitaxel in patients aged 70 and over with advanced non-small cell lung cancer. BMC Cancer. 2012;12:449. doi: 10.1186/1471-2407-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Socinski MA, Scappaticci FA, Samant M, Kolb MM, Kozloff MF. Safety and efficacy of combining sunitinib with bevacizumab + paclitaxel/carboplatin in non-small cell lung cancer. J Thorac Oncol. 2010;5:354–360. doi: 10.1097/JTO.0b013e3181c7307e. [DOI] [PubMed] [Google Scholar]
- 34.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 35.Tsao AS, Liu S, Fujimoto J, et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J Thorac Oncol. 2011;6:2104–2111. doi: 10.1097/JTO.0b013e31822e7256. [DOI] [PubMed] [Google Scholar]
- 36.Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 37.Geissler EN, Liao M, Brook JD, et al. Stem cell factor (SCF), a novel hematopoietic growth factor and ligand for c-kit tyrosine kinase receptor, maps on human chromosome 12 between 12q14.3 and 12qter. Somatic Cell Mol Genet. 1991;17:207–214. doi: 10.1007/BF01232978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.