Abstract
Purpose The purpose of this study was to determine the safety efficacy and outcomes of platelet-rich plasma (PRP) intra-articular injections for early stages of knee osteoarthritis (OA).
Methods Twenty-five patients affected by grade I and II knee primary OA according to the Kellgren–Lawrence scale received a single intra-articular PRP injection. Patients were prospectively evaluated for 6 months. Visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and Knee injury and Osteoarthritis Outcome Score (KOOS) scoring scales were used to evaluate clinical outcomes. Wilcoxon signed rank test was used to evaluate significance of improvement of WOMAC, KOOS, and VAS scores.
Results Twenty-one patients completed 6-months follow-up. The median WOMAC score improved from 29.1 points (range: 17.4–60.4; standard deviation [SD] = 13.0) at baseline to 42.41 (range: 24.3–71.2; SD = 12.5) at final follow-up. Improvements in median KOOS and VAS score have been also found, from 37.49 points and 64.2 mm before injection to 59.71 points and 42.8 mm, respectively. All these improvements were statistically significant ( p < 0.05). No adverse reactions have been observed.
Conclusion Treating knee OA with PRP injection is safe. A single dose of PRP seems to be effective in managing pain and improving quality of life in patients with low-grade knee OA.
Level of Evidence Level IV, therapeutic case series.
Keywords: platelet-rich plasma, growth factors, knee, osteoarthritis, intra-articular injection
Introduction
Osteoarthritis (OA) is the most common joint disease and a major cause of disability. According to the National Health Interview Survey, more than 50 million adults have been diagnosed with OA in the United States in 2012. 1 The knee is the one of the most commonly affected joint, and X-ray evidence of knee OA is diagnosed in up to 60% of people older than 45 years. 2
The etiology of OA is multifactorial and still not completely understood. Age, obesity, lower-limb malalignment, cartilage defects, joint instability, previous fractures, and meniscectomy surgery are all strongly correlated to knee OA. 3 Recently, literature reports suggested a female predisposition for developing knee OA. 4 Overall, any condition that can cause articular damages, and all unfavorable biomechanical conditions, which result in mechanical overload that exceeds the ability of a joint to maintain itself, predisposes the knee to OA. 5
During the past few decades, increased emphasis has been placed on the biochemical balance required for the health of the cartilage. It has become evident that the inflammatory mediators contribute significantly to the development and progression of structural changes in the OA joint. 6 Because the induction of proinflammatory mediators in cartilage, synovial membrane, and subchondral bone and their signaling pathways are interlinked and overlapped, it therefore remains controversial whether inflammatory mediators are primary or secondary regulators of cartilage damage and the defective repair mechanisms in OA. 7 Therefore, compounds that regulate cytokine and transglutaminases (TGs) synthesis and activity are considered as favorable targets for future OA therapy. Recent research focused on the anti-inflammatory effects of platelet-rich plasma (PRP). PRP is not only a rich source of growth factors but also contains leukocytes, some residual erythrocytes, metalloproteinases (MMPs), coagulation factors, and membrane glycoproteins. All these components seem to be able to influence inflammation by regulating the synthesis of other integrins, interleukins (ILs), chemokines, and cytokines. 8 However, PRP products can vary greatly, even in basic aspects such as their platelet concentration or the content of white blood cells, and this may influence the results.
In vitro studies showed that PRP, in particular leukocyte-rich plasma (L-PRP), is able to suppress inflammatory mediator concentration and gene expression in synovium and cartilage tissue, 9 and this could explain the pain improvement and promising results in some clinical series. Although there have been many clinical studies on the anti-inflammatory effects of corticosteroids and hyaluronic acid (HA) injections for knee OA, 10 clinical studies using PRP are limited.
The aim of this study was to determine the clinical effect and outcomes of PRP intra-articular injections for early stages of knee OA. The hypothesis of the study was that PRP can become an important pain and inflammation mediator, especially in early stages of knee OA.
Methods
Participants
Twenty-five patients matching strict inclusion and exclusion criteria received one single PRP injection each for knee OA at our institution and were enrolled in this study in 2014. The procedure was performed after the patients had signed a written consent and the study was approved by the local ethics committee.
Inclusion criteria were patients affected by grade I and II knee primary OA according to the Kellgren–Lawrence scale. Exclusion criteria were knee OA grade equal to or greater than III, history of previous knee surgery, posttraumatic knee OA, and previous infiltrative treatment of the affected knee. We also excluded patients affected by rheumatic diseases, diabetes mellitus, and hematological disease (coagulation disorder). Patients with platelet count of less than 150,000/mL were excluded from the treatment.
Radiographic examination performed prior to treatment (T0) included standard weight-bearing X-ray of the affected knee in anteroposterior and laterolateral views and Rosenberg view.
Interventions
PRP preparation was performed as follows. A 50-mL venous blood sample was collected from each patient and centrifuged with GPS II system (Biomet Biologics, Warsaw, Indiana, United States) for 15 minutes at a speed of 3,200 rpm. The mean volume obtained in this series was 7.43 mL of PRP for intra-articular administration. The whole process lasted approximately 20 minutes.
The patient was placed in a supine position with the knee in 90-degree flexion. The skin was disinfected with alcohol or iodine-based antiseptic solution and draped. PRP was injected very slowly in a sterile condition using a 22-gauge needle through the anterolateral “soft spot.” Immediately after administration, gentle passive flexion and extension exercises of the knee were encouraged. After the injection, the patients were monitored for 10 minutes before discharge. The patients were advised to not take any nonsteroidal anti-inflammatory drugs (NSAIDs) or to apply local ice for a week after injection to avoid reduction efficacy on PRP. In the patients with bilateral OA, both knees were injected. All knees received a single intra-articular PRP injection. None of the patients received physical therapy after the injection.
Outcome Measurements
All patients were clinically examined on follow-up by a physician who was not involved in PRP infiltration procedure. All the patients were evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Knee injury and Osteoarthritis Outcome Score (KOOS) scoring scales. 11 12 The questionnaires were administered at baseline (T0) and 6 months after PRP injection (T1). Visual analog scale (VAS) score was recorded before treatment and at final follow-up. 13 Demographic characteristics of the patients as well as complications and adverse events during treatment were recorded.
Data Analysis
Statistical analysis was performed using GraphPad Prism v 6.0 software (GraphPad Software Inc.). Data were expressed as the mean ± standard deviation. D'Agostino–Pearson normality test was used to test the normality of data distribution. Data were normally distributed, and paired comparisons were performed by two-tailed paired t -test. The significance level was set at p -value lower than 0.05.
Results
Fifteen females (60%) and ten males (40%) with mean age of 49.7 years (range: 79–42 years) were enrolled. Preoperative plain radiographs showed grade I and grade II primary OA in 8 and 17 patients, respectively. Of them, 21 were evaluated at after 6 months (T1), whereas 4 patients were lost at final follow-up ( Fig. 1 ). Mean WOMAC score at baseline was 29.1 ± 13.0, mean KOOS score before injection was 37.5 ± 18.1, and mean VAS was 64.2 ± 14.6. At 6 months follow-up, the mean WOMAC score improved to 42.4 ± 12.5, and the mean KOOS score was 59.7 ± 17.13. Improvements in WOMAC and KOOS score were statistically significant ( p = 0.0004 and p < 0.0001). We also found a significant improvement of the mean VAS score (42.8 ± 10.5; p < 0.0001), with all but one patient reporting pain relief 6 months after the procedure. We did not observe any adverse reactions or other possible serious complications such as infection after PRP injection.
Fig. 1.
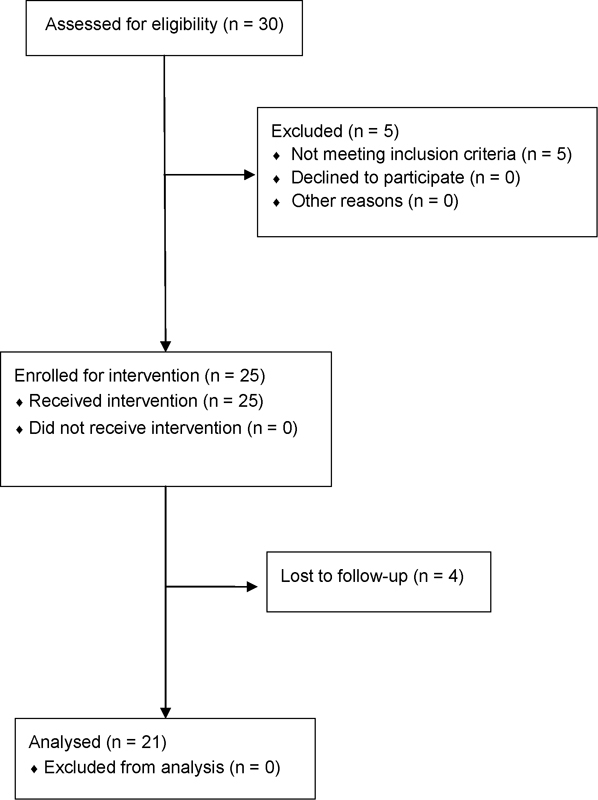
Flow diagram of the study.
Discussion
The main finding of this study is that PRP injection is an effective and safe treatment for low-grade knee OA in terms of improving function and reducing pain at short-term follow-up. This contributes to achieve the established goals for the treatment of knee OA, such as relieving pain, improving function, increasing quality of life, and, finally, reducing disability. 14 15 A variety of modalities have been used in the treatment of knee OA, including both conservative methods and surgical methods. PRP has showed promising results for the treatment of various musculoskeletal injuries and has gained popularity in the treatment of knee OA due to its ease of use, low cost, and minimally invasive nature. 16 However, the exact mechanisms underneath its efficacy still have to be fully understood, and often its administration is suggested on empiric rather than scientifically validated basis.
The etiopathogenesis of OA is complex and involves many mechanical and biochemical processes. Aging is the most important single risk factor of OA, and any unfavorable biomechanical environment results in mechanical demand that predispose to articular cartilage damage. 3 However, OA is not related to only mechanical stress, but many cellular and biochemical processes are also involved in its pathogenesis. 17 In normal conditions, cartilage extracellular matrix is in a dynamic equilibrium. In particular, the balance between anabolic and catabolic activities of chondrocytes maintains the structural and functional integrity of cartilage. 18 In OA, a deregulated balance between proteinases degrading the extracellular matrix and their inhibitors may be responsible for cartilage degeneration. 19
Histomorphometrical and biomolecular investigations showed an increase of inflammatory mediators and of TG-2 expression in human and experimental OA. 20 TGs have been implicated in the formation and development of hard tissue, extracellular matrix maturation, and mineralization in growth plate cartilage. They are also involved in organogenesis, tissue repair, and many pathological process. 21 TG-2, also known as tissue TG, is implicated in joint tissue remodeling, with particular reference to the interplay with inflammatory mediators of OA. The capacity of transamidation by TG-2 to regulate activation of latent transforming growth factor beta (TGF-β) seems to have a potential impact on the regulation of inflammatory response in osteoarthritic tissues. 14 TG-2 is also able to activate the crystal-promoting factor TGF- β1, and an overexpression of TGF-β1 was found in laboratory of OA models. 22 TGF-β1 seems to play an important role in maintaining chondrocyte hypertrophy, which is typically present near sites of cartilage surface damage, and it has also been recognized as a crucial factor in the process of osteophyte formation. 23 Finally, inflammatory mediators implicated in OA, including IL-1, stimulate chondrocyte matrix calcification. 24 Others inflammatory mediators such as matrix MMP-1, IL-6, IL-8, and chemokine (C-C motif) ligand 5 are present and significantly higher in the synovial fluid of patients with OA compared with normal patients. 6 These data confirm the complex role of TG-2 and inflammatory mediators during osteoarthritic joint tissue remodeling and may play an important role in the development of OA. However, it is not well known that if they are starters or just the consequence of degenerative process of articular cartilage, and certainly, they are not the only pathogenetic mechanism in the osteoarthritic process.
Recent in vitro studies showed an anti-inflammatory effect of PRP. 9 PRP is not only a rich source of growth factors, but it also contains antibacterial and fungicidal proteins, MMPs, coagulation factors, and membrane glycoproteins that influence inflammation by inducing the synthesis of other integrins, ILs, chemokines, and cytokines. 25 El-Sharkawy et al 26 used monocyte culture to assess cytokine and chemokine levels, as well as monocyte chemotactic migration, in the presence and absence of PRP. They showed that monocyte chemotactic protein-1, which is released by monocytes in response to proinflammatory stimuli, was significantly decreased by PRP in monocyte culture compared with untreated cells, suggesting that PRP can act as an anti-inflammatory agent by producing endogenous anti-inflammatory factors and by affecting monocyte cytokine release. Mazzocca et al 27 developed an in vitro study to assess the anti-inflammatory effects of PRP on stimulated human umbilical vein endothelial cells either alone or in combination with the corticosteroid or NSAIDs. The authors concluded that PRP reduces cellular inflammation compared with control. However, only one study has directly addressed effects of PRP on chondrocytes. Sundman et al 28 assessed the anti-inflammatory effects of PRP in an ex vivo coculture model for OA using human cartilage and synovium and concluded that PRP can stimulate endogenous HA synthesis while decreasing cartilage catabolism and that it can also act to suppress inflammatory mediator concentration in synovium and cartilage tissue. A recent review concluded that although the effectors mediating the beneficial effects of PRPs have not been identified, PRP could act as an endogenous source of chondroprotection by interfering with the early catabolic and inflammatory events and by subsequently promoting anabolic responses. 29
Another important fact to be considered, is that many PRP formulation are available for clinical uses and products can vary greatly, and it may influences the efficacy of treatment and the results of clinical trials. Ehrenfest et al 30 proposed a classification based on platelet, fibrin, and leukocyte concentration: pure PRP (P-PRP), leukocyte- and platelet-rich plasma, pure platelet-rich fibrin, and leukocyte- and platelet-rich fibrin. In particular, the content and different concentration of leukocytes in PRP products may affect the anti-inflammatory effects of PRP. We used L-PRP type because it has been identified to improve cellular chemotaxis, proliferation and differentiation, angiogenesis, and production of extracellular matrix, and also being responsible for stimulating defense mechanisms against infections. The role of leucocytes in PRP is a controversial issue in literature, and it has not been proven that taking leucocytes from a PRP sample could either benefit or result better outcomes for the patient.
McCarrel et al 31 demonstrated using tendonlike cells harvested and cultured from horse flexor digitorum superficialis tendons that an increase in leukocyte content of PRP products is positively correlated with an increased expression of inflammatory cytokines and that platelet/leukocyte ratio had no influence on this effect. Among PRP formulations, a further division can be made between those activated ex vivo with thrombin and/or calcium and those inactivated, which rely on in vivo activation through endogenous collagen. 32
Several authors have noticed reduction in pain after PRP application as reported in our study. However, an explanation of this phenomenon has not always been given. Crane and Everts believe that serotonin release from activated platelets might be responsible for decreased pain. 33 Except for the growth factors in the alpha-granules, large amounts of serotonin are contained within the dense platelet granules. 34
Clinical studies on the anti-inflammatory effects of PRP injections are limited. A recent systematic review on the use of PRP in intra-articular knee injections for OA has been published. Eight studies published between 2010 and 2013 were included, and all of them showed the efficacy of PRP in improving function and quality of life and in reducing pain. 35 When PRP injections were compared with HA, the results of the studies did not reveal superiority of PRP. Cerza et al 36 in their randomized controlled trial compared 120 patients treated with PRP (60 patients) and HA (60 patients) at a final follow-up at 24 weeks. Each patient received four intra-articular injections, and the authors found significantly better clinical outcome in PRP group compared with HA group, with lower WOMAC scores. In contrast, other authors did not find any statistically significant improvement with PRP compared with HA. 7
The long-term efficacy of PRP and the number of doses to administer is another debating matter because all studies published in literature to date have a short-term follow-up (maximum 2 years), and only one long-term study suggests that the benefits of PRP are not sustainable. 37 We also choose to use a single L-PRP administration because there is evidence of no significant differences between a single dose and multiple doses, and therefore a less invasive treatment could be performed. 37 This should represent an advantage compared with three HA injections or more and also in terms of cost-efficacy.
Therefore, authors agree that PRP could be an effective treatment in the short term to improve patients' function, quality of life, and reduce pain, 38 as it is reflected in the results of this study. According to literature, results are worse in patients with gonarthrosis greater than grade II. 16
The most relevant and clinically significant findings of this study were a notable improvement in symptoms related to knee OA process, especially pain, demonstrated in the results of the VAS scale in this study, and an improvement in quality of life, with a high rate of satisfaction as evidenced by the results of KOOS and WOMAC questionnaires. Moreover, we proved that a single dose of L-PRP can achieve satisfactory results, reducing procedure-related morbidity and costs in comparison to multiple doses schemes that have been used traditionally in previous studies.
The advantage of this treatment is that even if the mechanisms leading to clinical effects are still to be understood, current literature agrees on safety of PRP, with no serious complications reported. 37 Minor adverse events associated with repeated intra-articular injections include moderate pain, swelling, and mild effusion that lasted a few days, 3 as shown in this study, where no adverse effects were reported. 39
We acknowledge the limitations of this study. One limitation of this study is a relatively short follow-up that cannot assess long-term results of intra-articular knee PRP, which would determine whether there is some influence on the progression of the ostheoarthritic disease or just a relieving of symptoms. The small sample size is also another limitation of the study. The absence of a control group does not allow to draw a clear superiority of PRP injection compared with the other available treatments. Thus, knee OA is a chronic disease, and long-term outcomes should be an important consideration in evaluating new treatments. Therefore, our short-term follow-up (6 months) does not allow us to state if these results are maintained along time and to draw definitive conclusions.
In conclusion, a single dose of PRP in patients with knee OA grade I or II is a safe an effective treatment for managing the symptoms associated with this pathology, especially pain, and achieving improvements in quality of life of patients.
References
- 1.Barbour K E, Helmick C G, Theis K A et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010-2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Arfaj A, Al-Boukai A A. Prevalence of radiographic knee osteoarthritis in Saudi Arabia. Clin Rheumatol. 2002;21(02):142–145. doi: 10.1007/s10067-002-8273-8. [DOI] [PubMed] [Google Scholar]
- 3.Heijink A, Gomoll A H, Madry H et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(03):423–435. doi: 10.1007/s00167-011-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H A, Kim S, Seo Y I et al. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford) 2008;47(01):88–91. doi: 10.1093/rheumatology/kem308. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter J A, Martin J A, Brown T D.Perspectives on chondrocyte mechanobiology and osteoarthritis Biorheology 200643(3-4):603–609. [PubMed] [Google Scholar]
- 6.Monibi F, Roller B L, Stoker A, Garner B, Bal S, Cook J L. Identification of synovial fluid biomarkers for knee osteoarthritis and correlation with radiographic assessment. J Knee Surg. 2016;29(03):242–247. doi: 10.1055/s-0035-1549022. [DOI] [PubMed] [Google Scholar]
- 7.Filardo G, Kon E, Di Martino Aet al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial BMC Musculoskelet Disord 201213229. Doi: 10.1186/1471-2474-13-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole B J, Seroyer S T, Filardo G, Bajaj S, Fortier L A. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010;2(03):203–210. doi: 10.1177/1941738110366385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beitzel K, McCarthy M B, Russell R P, Apostolakos J, Cote M P, Mazzocca A D. Learning about PRP using cell-based models. Muscles Ligaments Tendons J. 2014;4(01):38–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9(02):493–500. doi: 10.3892/etm.2014.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard B, Johnston M, Dixon D.Exploring differential item functioning in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) BMC Musculoskelet Disord 201213265. Doi: 10.1186/1471-2474-13-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos E M, Lohmander L S.The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis Health Qual Life Outcomes 2003164. Doi: 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLoach L J, Higgins M S, Caplan A B, Stiff J L. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86(01):102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(09):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raeissadat S A, Rayegani S M, Hassanabadi H et al. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial) Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu W K, Mishra A, Rodeo S R et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739–748. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 17.Tarantino U, Ferlosio A, Arcuri G, Spagnoli L G, Orlandi A. Transglutaminase 2 as a biomarker of osteoarthritis: an update. Amino Acids. 2013;44(01):199–207. doi: 10.1007/s00726-011-1181-y. [DOI] [PubMed] [Google Scholar]
- 18.Goldring M B, Goldring S R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 19.Söder S, Hakimiyan A, Rueger D C, Kuettner K E, Aigner T, Chubinskaya S. Antisense inhibition of osteogenic protein 1 disturbs human articular cartilage integrity. Arthritis Rheum. 2005;52(02):468–478. doi: 10.1002/art.20856. [DOI] [PubMed] [Google Scholar]
- 20.Orlandi A, Oliva F, Taurisano G et al. Transglutaminase-2 differently regulates cartilage destruction and osteophyte formation in a surgical model of osteoarthritis. Amino Acids. 2009;36(04):755–763. doi: 10.1007/s00726-008-0129-3. [DOI] [PubMed] [Google Scholar]
- 21.Via A G, De Cupis M, Spoliti M, Oliva F. Clinical and biological aspects of rotator cuff tears. Muscles Ligaments Tendons J. 2013;3(02):70–79. doi: 10.11138/mltj/2013.3.2.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenthal A K, Gohr C M, Henry L A, Le M. Participation of transglutaminase in the activation of latent transforming growth factor beta1 in aging articular cartilage. Arthritis Rheum. 2000;43(08):1729–1733. doi: 10.1002/1529-0131(200008)43:8<1729::AID-ANR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Scharstuhl A, Glansbeek H L, van Beuningen H M, Vitters E L, van der Kraan P M, van den Berg W B. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169(01):507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 24.Johnson K, Hashimoto S, Lotz M, Pritzker K, Terkeltaub R. Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am J Pathol. 2001;159(01):149–163. doi: 10.1016/S0002-9440(10)61682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91(08):987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 26.El-Sharkawy H, Kantarci A, Deady J et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78(04):661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 27.Mazzocca A D, McCarthy M B, Intravia J et al. An in vitro evaluation of the anti-inflammatory effects of platelet-rich plasma, ketorolac, and methylprednisolone. Arthroscopy. 2013;29(04):675–683. doi: 10.1016/j.arthro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Sundman E A, Cole B J, Karas V et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(01):35–41. doi: 10.1177/0363546513507766. [DOI] [PubMed] [Google Scholar]
- 29.Dohan Ehrenfest D M, Andia I, Zumstein M A, Zhang C Q, Pinto N R, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(01):3–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Dohan Ehrenfest D M, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(03):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 31.McCarrel T M, Minas T, Fortier L A.Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy J Bone Joint Surg Am 20129419e143, 1–8 [DOI] [PubMed] [Google Scholar]
- 32.Mishra A, Randelli P, Barr C, Talamonti T, Ragone V, Cabitza P. Platelet-rich plasma and the upper extremity. Hand Clin. 2012;28(04):481–491. doi: 10.1016/j.hcl.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8:12–26. [Google Scholar]
- 34.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 35.Lai L P, Stitik T P, Foye P M, Georgy J S, Patibanda V, Chen B. Use of platelet rich plasma in intra-articular knee injections for osteoarthritis: a Systematic review. PM R. 2015;7(06):637–648. doi: 10.1016/j.pmrj.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Cerza F, Carnì S, Carcangiu A et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 37.Patel S, Dhillon M S, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(02):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Yuan M, Meng H Y et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(03):351–361. doi: 10.5312/wjo.v5.i3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]


