Abstract
Objective
Abnormal heart rate characteristics (HRC) of decreased variability and transient decelerations may occur in preterm infants with sepsis and other pathologic conditions. We sought to determine whether an early HRC index (HeRO score), measured in the first day and week after birth, predicts death and morbidities compared to established illness severity scores.
Study Design
For all very low birth weight infants in a single NICU from 2004–2014, the average first day HRC index was calculated within 24h of birth (aHRC-24h) and the average first week HRC index within 7 days of birth (aHRC-7d). The Score for Neonatal Acute Physiology (SNAP-II) and Clinical Risk Indicator for Babies (CRIB-II) were calculated when data were available. aHRC was compared to the Score for Neonatal Acute Physiology (SNAP-II) and Clinical Risk Indicator for Babies (CRIB-II) for predicting death, late onset septicemia (LOS), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), severe intraventricular hemorrhage (sIVH), or severe retinopathy of prematurity (sROP).
Results
All four scores were associated with death and sIVH(p< 0.01). The odds ratio and 95% CI for every one-point increase in aHRC for predicting mortality, adjusted for GA, was 1.59(1.25, 2.00) for aHRC-24h and 2.61(1.58, 4.33) for aHRC-7d. High aHRC-7d, SNAP-II and CRIB-II were associated with BPD(p<0.001). High aHRC-7d was associated with LOS(p<0.05). None of the scores predicted NEC or sROP.
Conclusion
HRC assessed in the first day or first week after birth compares favorably to established risk scores to predict death and morbidities in VLBW infants.
Keywords: heart rate characteristics, very low birth weight infants, illness severity score, septicemia, intraventricular hemorrhage, bronchopulmonary dysplasia, SNAP, CRIB
Introduction
Risk prediction scores based on illness severity are important for inter-institutional quality assessment and for clinical research. In the neonatal intensive care unit (NICU), gestational age (GA) alone is a strong predictor of adverse events and outcomes. Adding physiological and laboratory data can improve the accuracy of predictive models and could help identify infants who might benefit from heightened surveillance or preventative measures. Two established risk scores, the Score for Neonatal Acute Physiology (SNAP) and Clinical Risk Indicator for Babies (CRIB), rely on availability of clinical and blood gas data to calculate. A relatively new physiologic measure, the heart rate characteristics index, was designed to assess risk of imminent sepsis in very low birth weight (VLBW) infants and is continuously calculated from the bedside monitor electrocardiogram signal. Since depressed heart rate variability may occur in both acute and chronic pathologic conditions, we reasoned that a high HRC index shortly after birth might indicate high risk of death or morbidities in VLBW infants in the NICU.
Depressed heart rate variability reflects dysregulation of autonomic nervous system function and occurs in acute conditions such as sepsis or asphyxia (1)(2). In fetuses, abnormal HRC of transient decelerations on a background of depressed variability are a well-known sign of distress and are associated with adverse outcomes. In neonates, the most common cause of these abnormal HRC is sepsis (3)(4). A HRC monitor (HeRO Monitor, Medical Predictive Science Corporation) was developed as an early warning system for sepsis in VLBW infants in the NICU, and display of the HRC index (HeRO score) was shown to reduce all-cause mortality by 22% (5) and sepsis-associated mortality by 40% (6). Abnormal HRC are not specific for sepsis, and acute or chronic elevations in the HRC index have been reported in a number of other pathologic conditions, including necrotizing enterocolitis, respiratory failure, and brain hemorrhage(7)(8)(9).
Although not specifically designed for mortality prediction, it was shown that a high HRC index throughout the NICU stay was associated with high risk for death (10). SNAP-II and CRIB-II, which were designed to predict mortality, incorporate a limited number of vital sign metrics and laboratory values from the first 12 hours after birth (11)(12). Limited studies suggest that high SNAP or CRIB are associated with increased risk for other adverse outcomes, including IVH and BPD (13)(14)(15).
Prior studies have examined the association of abnormal HRC (a high HRC index) throughout the NICU stay with acute and chronic conditions. In the current study, we sought to determine whether early measurement of the HRC index, in the first day and first week after birth, predicts later adverse events and conditions in VLBW infants, as compared to SNAP-II and CRIB-II.
Methods
Patient population and clinical outcomes
The University of Virginia (UVA) Institutional Review Board approved this study. We reviewed HRC monitoring data for all VLBW infants (<1500g birthweight) admitted to the UVA NICU between May 2004 and May 2014. Medical records were reviewed for occurrence of death before NICU discharge, severe intraventricular hemorrhage (IVH grade III-IV), late onset septicemia (LOS), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD, supplemental oxygen at 36 weeks postmenstrual age), and severe retinopathy of prematurity (ROP requiring laser or bevacizumab treatment). LOS was defined as clinical signs of sepsis after 3 days of age with a positive blood culture, and antibiotics given for at least 5 days. NEC included cases of medical or surgical NEC or intestinal perforation.
Heart rate characteristics index monitoring
A commercially available monitor (HeRO, Medical Predictive Science Corporation, Charlottesville, VA) calculates the HRC index for all infants throughout their stay in the UVA NICU. The monitor analyzes the electrocardiogram (ECG) signal from bedside monitors using an algorithm designed to detect imminent sepsis, incorporating measures of heart rate variability and decelerations. The three measures included in the algorithm are the standard deviation of R-R intervals, sample asymmetry (increased decelerations and few accelerations) and sample entropy (2)(1). The HRC index is calculated every hour from the previous 12h of ECG signal and represents the fold increase in risk that the infant will be diagnosed with sepsis in the ensuing 24 hours compared to the average risk of sepsis for all VLBW infants at all times during the NICU stay (16). The HRC index was externally validated for sepsis detection (2) and was tested in a large, multicenter randomized clinical trial (5).
Calculation of aHRC-24h, aHRC-7d, SNAP-II and CRIB-II
Average first day HRC index was calculated when at least 6 hours of HRC index data were available in the first 24 hours after birth (aHRC-24h). Average first week HRC index was calculated when at least 5 days (120 hours) of HRC index data were available in the first 7 days (aHRC-7d). For infants with either first day or first week HRC index calculated, medical records were reviewed for clinical and laboratory parameters in the first 12 hours after birth to calculate SNAP-II and CRIB-II Scores. The following components are included in SNAP-II: lowest temperature and blood pressure, lowest PaO2/FiO2 ratio, lowest pH, urine output, and seizures. A normal value was assumed for PaO2/FiO2 ratio if an arterial blood gas was not obtained. This has been suggested by the authors who developed SNAP and SNAP-II as an acceptable method of calculation when data is unavailable (11)(17). The following components are included in CRIB-II: Gestational age, birth weight, gender, admission temperature, and highest base deficit (12).
Statistical analysis
Differences in scores for infants with and without each adverse outcome were assessed by t-tests with and without logistic regression to adjust for gestational age. The additive value of each score compared to GA alone for risk prediction was quantified by the change of the area under receiver operator characteristic (ROC) curve as well as by the net reclassification improvement (NRI). NRI is a measure of the sum of proportions of patients reclassified into the correct risk group by a new risk marker compared to an established risk marker (17). For example, an NRI of 0.2 with respect to death would indicate that a high HRC index correctly identified survival in 20% more infants compared to the established risk marker of gestational age. As an additional measure of risk assessment, we calculated the odds ratio and 95% CI for every one-point increase in aHRC-24h and aHRC-7d over zero for predicting mortality. Statistical analyses were performed in MATLAB (MathWorks, Inc. Natick, MA) and all p-values are two-tailed.
Results
Patient demographics and outcomes
In the years of the study, 566 VLBW infants had at least 6h of HRC index data within 24h of birth for aHRC-24h analysis and data available for calculation of SNAP-II and CRIB-II, and 480 had >120h (5 days) of HRC monitoring data available in the first week for calculation of aHRC-7d. Mean GA was similar for infants in the aHRC-24h and aHRC-7d groups (28.6 ± 2.9 and 28.9 ± 2.8 weeks). Of the 566 infants, 52% were male and 32% were <1000g. During the same time period, approximately 1,300 VLBW infants were admitted to the UVA NICU (mean GA 27.5 ± 3.0 weeks, 51% male). Infants without early HRC data available for analysis were either outborn or did not have electrocardiogram leads placed in the first days after birth due to poor skin integrity. The incidences of death, sIVH, LOS, NEC, BPD and sROP of the 566 infants included in the analysis are shown in Table 1.
Table 1. Heart Rate Characteristics index, SNAP-II, and CRIB-II scores based on outcomes.
Scores were calculated for 566 VLBW infants (480 with aHRC-7d) and GA, survival, and morbidities are shown. Death and severe intraventricular hemorrhage (sIVH) were assessed for all infants, late onset septicemia (LOS) and necrotizing enterocolitis (NEC) for all infants except for those that died at less than 30 days old without LOS or NEC, and bronchopulmonary dysplasia (BPD) and severe retinopathy of prematurity (sROP) for infants still hospitalized in our unit at 36 weeks’ postmenstrual age. Median and IQR of aHRC in first 24 hours and first week (aHRC-24 and aHRC-7d) and SNAP-II and CRIB-II are shown.
| Outcome | N (%) | GA (mean, SD) |
aHRC-24hr median (IQR) |
aHRC-7d median (IQR) |
SNAP-II Median (IQR) |
CRIB-II median (IQR) |
|---|---|---|---|---|---|---|
| Died | 51 (9) | 27 ± 3 | 1.60 (0.84, 2.81) | 1.44 (0.68, 1.88) | 25 (14,44) | 9 (6,15) |
| Survived | 515 (91) | 29 ± 3 | 0.79 (0.53,1.37) | 0.77 (0.53, 1.28) | 14 (9,19) | 7 (5,9) |
| sIVH | 47 (8) | 27± 3 | 1.87 (1.02, 3.21) | 2.01 (0.98, 2.39) | 25 (14,38) | 9 (6,14) |
| No sIVH | 519 (92) | 29 ± 3 | 0.79 (0.53, 1.36) | 0.77 (0.53, 1.16) | 14 (9,19) | 6 (5,9) |
| LOS | 63 (12) | 27 ± 2 | 1.30 (0.68,1.8) | 1.00 (0.72, 1.75) | 14 (9,30) | 8 (6,10) |
| No LOS | 471 (88) | 29 ± 3 | 0.76 (0.51, 1.29) | 0.75 (0.51, 1.16) | 14 (8,19) | 6 (5,9) |
| NEC | 37 (7) | 28 ± 2 | 1.02 (0.64, 1.55) | 0.93 (0.59, 1.59) | 14 (9,22) | 7 (6,9) |
| No NEC | 497 (93) | 29 ± 3 | 0.79 (0.52, 1.37) | 0.77 (0.53, 1.17) | 14 (9,19) | 6 (5,9) |
| BPD | 98 (20) | 27 ± 2 | 1.15 (0.70, 2.08) | 1.28 (0.77, 1.74) | 20 (14,29) | 9 (7,13) |
| No BPD | 405 (80) | 29 ±3 | 0.73 (0.46, 1.21) | 0.69 (0.47, 1.00) | 9 (8,16) | 6 (5,7) |
| sROP | 15 (3) | 25 ± 2 | 1.48 (0.84, 2.46) | 1.65 (0.78, 2.11) | 30 (14,40) | 11 (8,14) |
| No sROP | 488 (97) | 29 ± 3 | 0.77 (0.51, 1.32) | 0.75 (0.51, 1.16) | 14 (8,19) | 6 (5,9) |
Predictive performance of aHRC-24h, aHRC-7d, SNAP-II and CRIB-II
Higher aHRC-24h and aHRC-7d were associated with death and severe IVH, with the difference remaining significant after accounting for GA (p≤0.01, Figure 1A). The odds ratio and 95% CI for every one point increase in aHRC for predicting mortality after adjustment for GA was 1.59 (1.25, 2.00) for aHRC-24h and 2.61 (1.58, 4.33) for aHRC-7d. The aHRC-7d was higher in those who developed both late onset septicemia and BPD (Figure 1B), but the aHRC-24h was not. GA was negatively correlated with aHRC-24h and aHRC-7d (Figure 1c). Higher SNAP-II was associated with death, severe IVH and BPD after adjustment for gestational age (p≤0.01), but not with LOS. None of the four scores was associated with development of NEC or ROP after adjustment for gestational age (all p>0.05). Median and interquartile ranges (IQR) of all four scores and GA for infants with and without each outcome are compared in Table 1.
Figure 1.
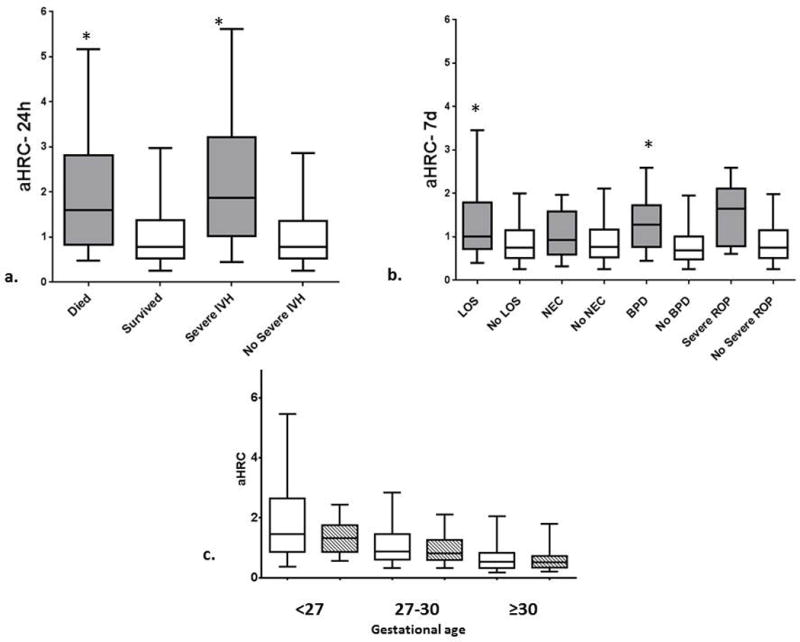
a. Median, IQR, 5th and 95th percentile aHRC-24h for infants who died or had severe IVH (gray) compared to infants who survived or did not have severe IVH (white). *P< 0.01 using logistic regression to adjust for gestational age
b. Median, IQR, 5th and 95th percentile aHRC-7d for infants who had late onset septicemia (LOS), necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD) or severe retinopathy of prematurity (ROP) (gray) compared with those who did not have the outcome (white). *P< 0.01 using logistic regression to adjust for gestational age
c. Median, IQR, 5th and 95th percentile aHRC-24h (white) and aHRC-7d (stripes) by gestational age. The correlation coefficient of aHRC-24h and aHRC-7d with gestational age was -.3999 and -.4276, respectively.
Gestational age (GA) alone was highly predictive of death and each morbidity (Table 2). Each score resulted in a statistically significant increase in the area under the ROC curve compared to GA alone for prediction of death and severe IVH (Table 2 and Figure 2). The change in ROC area was also significant for aHRC-7d predicting late onset septicemia and BPD (Table 2). SNAP-II and CRIB-II increased the ROC area over GA alone for predicting BPD but not LOS.
Table 2. Performance of scores and GA by area under the receiver operator characteristics curve (AUC).
AUC of GA and aHRC-24hr, aHRC-7d, SNAP-II and CRIB-II, adjusted for GA.
| GA | aHRC-24hr | aHRC-7d | SNAP-II | CRIB-II | |
|---|---|---|---|---|---|
| Death | 0.725 | 0.741* | 0.719* | 0.771* | 0.723* |
| sIVH | 0.726 | 0.778* | 0.782* | 0.765* | 0.724 |
| LOS | 0.734 | 0.738 | 0.738* | 0.733 | 0.737 |
| NEC | 0.630 | 0.628 | 0.627 | 0.642 | 0.643 |
| BPD | 0.820 | 0.827 | 0.827* | 0.839* | 0.840* |
| sROP | 0.931 | 0.930 | 0.931 | 0.935 | 0.933 |
Significant change in ROC area compared to GA alone (p≤0.01)
Figure 2.
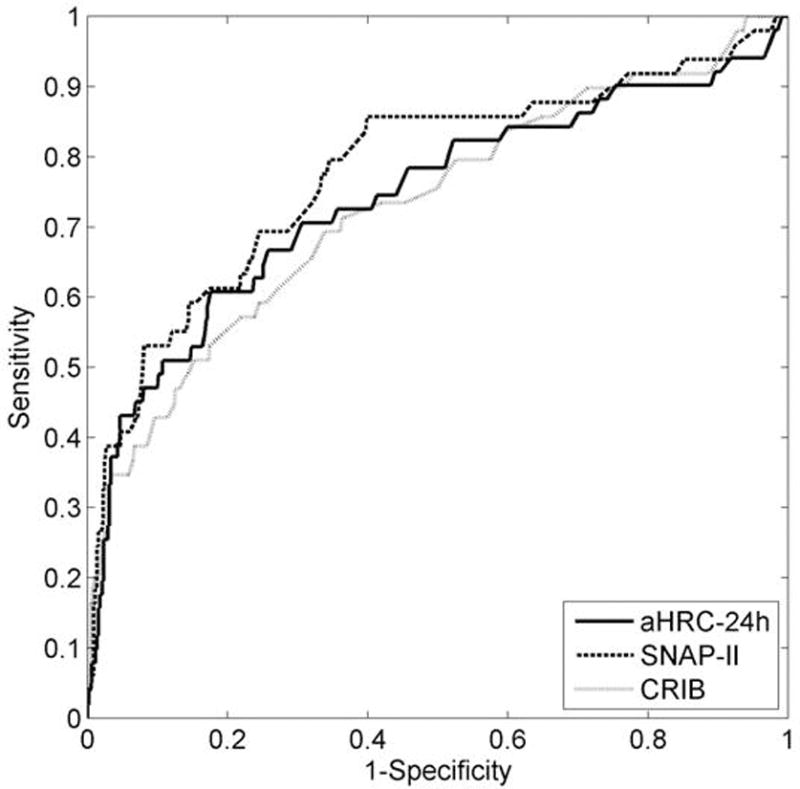
ROC curves for predicting death using aHRC-24h, SNAP-II and CRIB-II, all adjusted for gestational age.
We also used a relatively new metric, the net reclassification improvement (NRI) to compare the four risk indices with gestational age, which is a well-established, major risk predictor for death and morbidities in neonates. The NRI is a measure of fraction of cases reclassified into the correct category with the new test compared to the old. The NRI demonstrated that aHRC-24h, aHRC-7d, and SNAP-II but not CRIB-II added information to risk prediction for death and IVH compared to GA alone (NRI > 0.30, p≤ 0.01). The NRI was also significant for BPD prediction using aHRC-7d, SNAP-II and CRIB-II (NRI >0.40, p< 0.001) and for septicemia prediction using aHRC-7d (NRI= 0.30, p= 0.03).
There was no additive value to combining aHRC with SNAP-II or CRIB-II for risk assessment based on change in ROC area or NRI.
Discussion
In this large study of VLBW infants, we found that a heart rate characteristics index assessed in the first day or first week after birth predicts adverse outcomes. The average HRC index compared favorably to SNAP-II and CRIB-II scores and, based on assessment of change in ROC area and Net Reclassification Improvement, added information beyond gestational age alone for predicting death, severe IVH, and development of BPD. The first week aHRC index was also associated with late-onset septicemia, but none of the scores was associated with development of NEC or ROP.
The HRC index was designed to detect imminent sepsis, incorporating measures of heart rate variability and transient heart rate decelerations, which can be indicative of an acute inflammatory response (18). An earlier study also showed that a higher HRC index throughout the NICU stay was associated with death (10), and the current study shows that abnormal HRC shortly after birth also indicate increased risk of death. In contrast to the HRC index, SNAP and CRIB scores were developed for mortality risk prediction and incorporate both physiologic and laboratory data in the first 12h after birth. We found that first day HRC performed similarly to SNAP-II and similarly or slightly better than CRIB-II for predicting mortality in this population, though previous studies have reported higher AUCs for both SNAP-II and CRIB-II (11) (12)(19)(20)(21)(22). Since gestational age alone strongly influences mortality and morbidity risk with a high AUC, we analyzed the change in AUC and a newer measure, the Net Reclassification Improvement, and showed that each score added to GA, but HRC did not add to SNAP-II or CRIB-II for predicting death or morbidities.
Our results add to the limited number of studies evaluating the ability of illness severity scores to predict outcomes other than death in preterm infants (13)(14) (15). SNAP-II incorporates measures of oxygen administration and blood oxygen levels (lowest PaO2/FiO2 ratio) and was shown to predict development of BPD in a large study of preterm infants <32 weeks (13). Although the HRC index does not factor in tests directly related to respiratory status, we previously found that an acute increase in the HRC index may occur in preterm infants with acute respiratory deterioration (8), which may be related to effects of hypoxia or acidosis on heart rate variability, or frequent heart rate decelerations during apnea spells. The aHRC-7d was predictive of BPD whereas the aHRC-24h was not, which may reflect transient abnormalities of heart rate characteristics shortly after birth or procedures or medications given in the in the first day. For example, if atropine is given for intubation it dramatically and transiently depresses heart rate variability.
We found that infants with severe IVH have higher HRC index values in the first day and first week. IVH occurs most often during the first three days after birth and we did not have serial brain ultrasounds during this period to determine whether a high HRC index actually predicts or simply reflects occurrence of severe IVH. In a prior study we found severe IVH to be associated with chronically abnormal HRC for the first month, which may represent ongoing autonomic nervous system dysfunction or neuroinflammation (9). Chronic elevation of the HRC index during the NICU stay was also found to be associated with neurodevelopmental impairment at one year of age in two studies (9)(23). Association of high SNAP and CRIB scores with adverse neurodevelopmental outcomes has been reported in some studies but not in others (24)(25).
None of the early illness severity scores analyzed in this study correlated with increased risk of NEC or severe ROP after adjustment for gestational age. Gestational age alone is a very strong predictor of severe ROP. With regard to NEC, we previously showed that an acute rise in the HRC index occurs in some infants in the 24h period before NEC diagnosis (26), but in the current study we did not find that first day or first week HRC index predicted later development of NEC or intestinal perforation. A prior small study found that low heart rate variability in the high-frequency spectrum (thought to represent parasympathetic tone) occurring during the first week identifies preterm infants at increased risk for development of NEC (27). Our results found no association between SNAP-II or CRIB-II and NEC, similar to another study showing that neither first day nor daily assessment of SNAP-II scores predicts NEC (15). Since NEC is uncommon and its pathophysiology complex, larger studies would be needed to develop physiologic predictive scores.
Interestingly, a high average HRC index in the first week was associated with later development of septicemia, occurring on average about four weeks after birth. It is possible that decreased heart rate variability soon after birth reflects autonomic nervous system dysfunction and since the ANS plays a role in host defense this might indicate vulnerability to later infection (28). Alternatively, abnormal HRC in the early postnatal period may simply serve as a marker of high overall illness severity and need for interventions such as prolonged mechanical ventilation or intravascular catheters, which increase sepsis risk. Prior small studies have reported a higher incidence of late onset septicemia in neonates with high first day SNAP (1) and SNAPPE-II (15), but in our larger study sample we did not find this association. We also did not find the first day aHRC index to be predictive of LOS, which may reflect events or procedures occurring at or shortly after birth that transiently affect heart rate characteristics.
Generally, the HRC index and SNAP-II and CRIB-II scores performed similarly for risk prediction in this study, and there was no additive benefit. There are some differences in acquisition and other aspects of the scores that deserve consideration. Measurement of the HRC index requires specialized equipment not available in most NICUs, and requires placement of ECG leads immediately after birth, which may not always be done for extremely preterm infants with very fragile skin. Also, administration of anticholinergic medication prior to endotracheal intubation, which is common practice in some NICUs, dramatically depresses heart rate variability and raises the HRC index. An advantage of SNAP-II and CRIB-II is that they integrate demographic and laboratory data with vital sign data to identify infants with high mortality risk. Another advantage to these scores is that no specialized equipment is required. On the other hand, these illness severity scores require time and effort to calculate and thus are not readily or continuously available. A consideration for all of these scores is that they were not specifically designed to identify infants at high risk for many of the serious adverse events and outcomes common to very preterm infants, such as NEC and ROP. Addition of physiologic data beyond heart rate characteristics such as pulse oximetry data might result in more sensitive and specific predictive algorithms. A small study found that a “PhysiScore” incorporating multiple vital signs and oxygen saturation in the first 3 hours after birth performed well for identifying preterm infants at high risk for various morbidities (19). Advances in “big data” analytics may allow development of optimized scores incorporating both vital signs and demographic and laboratory data to help clinicians identify high risk or deteriorating patients, and this in turn might lead to earlier therapies and improved patient outcomes.
Conclusion
Abnormal heart rate characteristics soon after birth are associated with mortality and multiple morbidities in VLBW infants in the NICU. Risk scores such as the HRC index may be useful for inter-institutional outcomes research, for stratification in clinical trials, and for identifying infants that might benefit from heightened surveillance or targeted therapies.
Acknowledgments
Funding Source: NIH R01 HD072071-02
Abbreviations and acronyms
- HRC
heart rate characteristics
- VLBW
very low birth weight
- NICU
neonatal intensive care unit
- aHRC-24h
average first day HRC index
- aHRC-7d
average first week HRC index
- SNAP-II
Score for Neonatal Acute Physiology II
- CRIB-II
Clinical Risk Indicator for Babies II
- GA
gestational age
- IVH
intraventricular hemorrhage
- BPD
bronchopulmonary dysplasia
- ROP
retinopathy of prematurity
- NEC
necrotizing enterocolitis
- LOS
late onset septicemia
- ROC
receiver operator characteristics
- AUC
area under the ROC curve
- NRI
net reclassification improvement
Footnotes
Disclosure: DEL and JRM have equity shares in Medical Predictive Science. The company had no input into study design, data analysis, or manuscript preparation
References
- 1.Griffin MP, Moorman JR. Toward the Early Diagnosis of Neonatal Sepsis and Sepsis-Like Illness Using Novel Heart Rate Analysis. Pediatrics. 2001;107:97–104. doi: 10.1542/peds.107.1.97. [DOI] [PubMed] [Google Scholar]
- 2.Griffin MP, O’Shea TM, Bissonette EA, Harrell FE, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53:920–6. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 3.Griffin MP, Lake DE, Bissonette EA, Harrell FE, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–4. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 4.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2002;283:R789–97. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- 5.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159:900–6. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairchild K, Schelonka R, Kaufman D. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res. 2013;74:570–5. doi: 10.1038/pr.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone ML, Tatum PM, Weitkamp J-H, Mukherjee AB, Attridge J, McGahren ED, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33:847–50. doi: 10.1038/jp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J Pediatr. 2014;164:775–80. doi: 10.1016/j.jpeds.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairchild KD, Sinkin RA, Davalian F, Blackman AE, Swanson JR, Matsumoto JA, et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. J Perinatol. 2014;34:375–9. doi: 10.1038/jp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin MP, O’Shea TM, Bissonette EA, Harrell FE, Lake DE, Moorman JR. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res. 2004;55:782–8. doi: 10.1203/01.PDR.0000119366.21770.9E. [DOI] [PubMed] [Google Scholar]
- 11.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 12.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;36:1789–91. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 13.Chien L-Y, Whyte R, Thiessen P, Walker R, Brabyn D, Lee SK. Snap-II predicts severe intraventricular hemorrhage and chronic lung disease in the neonatal intensive care unit. J Perinatol. 2002;22:26–30. doi: 10.1038/sj.jp.7210585. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Yan J, Li M, Xiao Z, Zhu X, Pan J, et al. Addition of SNAP to perinatal risk factors improves the prediction of bronchopulmonary dysplasia or death in critically ill preterm infants. BMC Pediatr. 2013;13:138. doi: 10.1186/1471-2431-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim L, Rozycki HJ. Postnatal SNAP-II scores in neonatal intensive care unit patients: relationship to sepsis, necrotizing enterocolitis, and death. J Matern Fetal Neonatal Med. 2008;21:415–9. doi: 10.1080/14767050802046481. [DOI] [PubMed] [Google Scholar]
- 16.Fairchild KD. Predictive monitoring for early detection of sepsis in neonatal ICU patients. Curr Opin Pediatr. 2013;25:172–9. doi: 10.1097/MOP.0b013e32835e8fe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson D, Gray J. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91:617–23. [PubMed] [Google Scholar]
- 18.Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1019–27. doi: 10.1152/ajpregu.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2:48–65. doi: 10.1126/scitranslmed.3001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagliardi L, Cavazza A, Brunelli A, Battaglioli M, Merazzi D, Tandoi F, et al. Assessing mortality risk in very low birthweight infants: a comparison of CRIB, CRIB-II, and SNAPPE-II. Arch Dis Child Fetal Neonatal Ed. 2004;89:F419–22. doi: 10.1136/adc.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Felice C, Del Vecchio A, Latini G. Evaluating illness severity for very low birth weight infants: CRIB or CRIB-II? J Matern Fetal Neonatal Med. 2005;17:257–60. doi: 10.1080/14767050500072557. [DOI] [PubMed] [Google Scholar]
- 22.Manktelow BN, Draper ES, Field DJ. Predicting neonatal mortality among very preterm infants: a comparison of three versions of the CRIB score. Arch Dis Child Fetal Neonatal Ed. 2010;95:9–13. doi: 10.1136/adc.2008.148015. [DOI] [PubMed] [Google Scholar]
- 23.Addison K, Griffin MP, Moorman JR, Lake DE, O’Shea TM. Heart rate characteristics and neurodevelopmental outcome in very low birth weight infants. J Perinatol. 2009;29:750–6. doi: 10.1038/jp.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson M, Bodin L, Finnström O, Schollin J. Can severity‐of‐illness indices for neonatal intensive care predict outcome at 4 years of age? Acta Paediatr. 2002;91:1093–100. doi: 10.1080/080352502760311601. [DOI] [PubMed] [Google Scholar]
- 25.Dorling JS, Field DJ, Manktelow B. Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed. 2005;90:F11–16. doi: 10.1136/adc.2003.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone ML, Tatum PM, Weitkamp J-H, Mukherjee a B, Attridge J, McGahren ED, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33:847–50. doi: 10.1038/jp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doheny KK, Palmer C, Browning KN, Jairath P, Liao D, He F, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol Motil. 2014;26:832–40. doi: 10.1111/nmo.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RPA, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol. 2011;300:R330–9. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


