Abstract
Hypertension is associated with endothelial dysfunction and vascular remodeling.
Objective
To assess effects of antihypertensive pharmacotherapy on eNOS and iNOS-dependent mechanisms and maximal vasodilator capacity in the cutaneous microvasculature.
Methods
Intradermal microdialysis fibers were placed in 15 normotensive (SBP 111±2 mmHg), 12 unmedicated hypertensive (SBP 142±2 mmHg), and 12 medicated hypertensive (SBP 120±2 mmHg) subjects. Treatments were control, iNOS-inhibited (1400w), and NOS-inhibited (L-NAME). Red cell flux, measured during local heating (42°C) and acetylcholine (ACh) dose-response protocols, was normalized to cutaneous vascular conductance (CVC=flux•MAP−1) and a percentage of maximal vasodilation (%CVCmax).
Results
Compared to normotensives, ACh-mediated vasodilation was attenuated in the hypertensive (p<0.001), but not medicated subjects (p=0.83). NOS inhibition attenuated ACh-mediated vasodilation in normotensives compared to hypertensive (p<0.001) and medicated (p<0.001) subjects. With iNOS inhibition there was no difference in ACh-mediated vasodilation between groups. Compared to the normotensives, local heat-induced vasodilation was attenuated in the hypertensives (p<0.001), but iNOS inhibition augmented vasodilation in the hypertensives so this attenuation was abolished (p=0.31). Compared to normotensives, maximal vasodilator capacity was reduced in the hypertensive (p=0.014) and medicated subjects (p=0.004).
Conclusion
In the cutaneous microvasculature, antihypertensive pharmacotherapy improved endothelial function through NO-dependent and independent mechanisms, but did not improve maximal vasodilator capacity.
Keywords: hypertension, renin-angiotensin system, cutaneous, microvasculature, endothelium
INTRODUCTION
Hypertension is a highly prevalent chronic disease that affects over one third of adults1, making high blood pressure a substantial public health problem2. Hypertension is associated with deleterious changes in the vasculature, including impairment of endothelial function3, 4 and increased vascular stiffness5, 6. These deleterious vascular changes occur ubiquitously across the vasculature7–9, but likely originate in the microcirculation, preceding detectable dysfunction observed in large conduit vessels10, 11. Because hypertension-associated vascular dysfunction is first apparent in the microvasculature, the microvasculature can be used to examine the effectiveness and physiological actions of antihypertensive medications.
The human cutaneous microvasculature is an accessible vascular bed that has been utilized to examine mechanisms underlying microvasculature dysfunction12–17. Vascular dysfunction in the skin is similar in mechanisms and magnitude to other nutritive vascular beds including the coronary, cerebral, and renal circulations7, 18–20. Upregulation of inducible nitric oxide synthase (iNOS) is one contributing factor to microvascular dysfunction in hypertension. iNOS can impair endothelial NOS (eNOS)-dependent vasodilation and increase oxidant stress21 by mediating the rapid accumulation of peroxynitrite which increases oxidant stress and decreases NO-bioavailability22. iNOS also upregulates the activity of arginase, an enzyme that preferentially utilizes L-arginine, the common substrate for eNOS23.
Additionally, minimum vascular resistance, representative of the vasculature’s maximum ability to dilate and an index of vascular structure, is elevated with hypertension24–26, suggestive of pathological vessel remodeling. Carberry and colleagues confirmed that minimum vascular resistance is elevated in the skin of hypertensive subject27. Our group has subsequently demonstrated that maximum conductance (the inverse of resistance) obtained in response to the nitric oxide (NO) donor sodium nitroprusside and elevated local skin temperature (43°C) is attenuated in hypertensive men and women compared to age-matched normotensive controls14, 15, further suggesting that pathological vessel remodeling occurs in the cutaneous microvasculature of hypertensive men and women.
Numerous classes of antihypertensive pharmacotherapy are available to treat high blood pressure. Commonly prescribed classes of antihypertensive medications include those targeting the renin-angiotensin-system (RAS) (angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs)). According to the JNC8 guidelines, ACE inhibitors and ARBs are considered first line antihypertensive pharmacotherapies28. Antihypertensive drugs targeting the RAS may have beneficial peripheral vascular effects29–32. RAS specific pharmacotherapy increases eNOS expression in rodent models33, 34, and improves endothelial function in humans35, 36. RAS targeted treatment also improves indices of microvessel structure, including reversing rarefaction29, 30, 37–39.
Whether RAS-inhibiting antihypertensive pharmacotherapy alters iNOS function and human cutaneous microvascular endothelial and smooth muscle function is currently unknown. RAS-inhibiting antihypertensive pharmacotherapies may alter iNOS expression40, 41; however whether iNOS expression is augmented or attenuated is equivocal. Systemic ACE inhibitor treatment with perindopril increased iNOS expression in human vascular smooth muscle cells40; whereas treatment with the ARB candesartan normalized iNOS protein expression41. These contrasting findings may be due to differing methodologies and hypertensive models. It is unknown if blood pressure normalization with pharmacotherapy that includes an ACE inhibitor or ARB alters iNOS function and attenuates microvascular dysfunction in humans with essential hypertension.
The purpose of this study was to determine if blood pressure control with pharmacotherapy improved cutaneous microvascular endothelial function and/or maximum cutaneous vascular conductance, an index of cutaneous microvascular structure. We further sought to determine if alterations in NO-bioavailability or iNOS were associated with changes in endothelial function. We chose a subject group treated with the common first-line pharmacotherapies of ACE inhibitors or ARBs as these treatments have demonstrated beneficial effects on the endothelium in other models of hypertension42, 43 and in other vascular beds35, 44, 45. We hypothesized that antihypertensive pharmacotherapy would improve cutaneous microvascular function through endothelial NO-dependent mechanisms and by limiting iNOS-mediated dysfunction. We also hypothesized that maximum cutaneous vascular conductance, an index of cutaneous microvascular structure, would not be changed.
MATERIALS AND METHODS
Subjects
Experimental procedures were approved by the institutional review board at The Pennsylvania State University and conformed to the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects before participation. Subject characteristics are presented in table 1. Groups consisted of 12 essential hypertensive subjects naïve to pharmacotherapy, 12 medicated hypertensive subjects with clinically controlled blood pressure, and 15 normotensive control subjects. Subjects were classified by JNC VIII blood pressure guidelines28. Blood pressure status was confirmed, and white-coat hypertension ruled out, through use of a 24-hour ambulatory blood pressure monitor (Ambulo 2400, Mortara Industries) that measured blood pressure once every hour. Due to the inclusion of sleeping hours, blood pressure measured via 24-hours monitors is lower than seated blood pressure measurements. Therefore, data from the ambulatory monitor confirmed hypertensive status if average SBP was ≥130 mmHg and/or DBP was ≥80 mmHg46. Subjects underwent a complete medical screening that included blood chemistry, HbA1C, lipid analysis, heart rate, medical history, anthropometrics, and resting ECG. Medicated hypertensive subjects were all taking mono- or poly-pharmacotherapy to treat hypertension and had been taking the same therapeutic regimen for a minimum of 3 months (average duration of treatment: 6 ± 2 years). All medicated subjects were taking at least one medication that influenced the RAS. The different antihypertensive drugs utilized by the subjects are presented in table 2. Of those taking multiple antihypertensive medications, three were co-prescribed diuretics, which do not alter endothelial function or vessel structure10, 47. The final subject was co-prescribed a calcium channel blocker, which has been shown to exert effects on the endothelium48. Unmedicated essential hypertensive and normotensive control subjects were not taking any antihypertensive medications. All subjects were free of other, non-antihypertensive medications which could alter blood flow. All subjects were generally healthy except for the presence of hypertension. All premenopausal women (N=3) were studied during the early follicular phase of their menstrual cycle, and postmenopausal women (N=17) reported that it had been ≥ 1 year since cessation of their last menses.
Table 1.
Subject characteristics
| Sex (M,F) | Age (y) | BMI (kg•m−2) | SBP (mmHg) | DBP (mmHg) | MAP (mmHg) | LDL (mg•dL−1) | HDL (mg•dL−1) | |
|---|---|---|---|---|---|---|---|---|
| Normotensive | 5, 10 | 52±1 | 25 ± 1 | 111±2 | 74±2 | 86±2 | 115±5 | 61±4 |
| Hypertensive | 9, 3 | 57±3 | 27±1 | 142±2* | 92±1* | 109±1* | 107±5 | 58±4 |
| Medicated | 5, 7 | 57±2* | 27±1 | 120±2*‡ | 77±1‡ | 91±1‡ | 113±6 | 61±6 |
p<0.05 compared to normotensive group;
p<0.05 compared to hypertensive group
Table 2.
Medicated subject pharmacotherapy.
| Medication | N |
|---|---|
| ACE Inhibitor | 7 |
| ARB | 1 |
| ACE Inhibitor + Diuretic | 3 |
| ACE Inhibitor + Calcium Channel Blocker | 1 |
Number of medicated hypertensive subjects receiving each class of mono- or poly-therapy.
Microdialysis Procedures
ACh Dose-Response Protocol
The purpose of the Acetylcholine (ACh) dose-response protocol was to pharmacologically induce endothelium-dependent vasodilation49, 50. All experiments were performed in a thermoneutral laboratory with the subject in a semi-supine position and the experimental arm at heart level. Three microdialysis fibers (10 mm, 20 kDa cutoff membrane, MD 2000; Bioanalytical Systems) were placed in the forearm skin as previously described14. Fibers were perfused with either lactated Ringer’s, a physiological saline to serve as control, 20 mM L-NAME (NG-nitro-L-arginine methyl ester, CalBiochem), a non-specific NOS antagonist, or 0.1 mM 1400w (N-(3-(Aminomehtyl)benzyl)acetamidine, AG Scientific) to selectively inhibit iNOS23, 51. All substances perfused through the microdialysis fibers were mixed immediately before use and sterilized with a syringe microfilter (0.2 μm pore size, Acrodisc). All substances were perfused at a rate of 2 μL•min−1 (Bioanalytical Systems Beehive and Baby Bee microinfusion pumps). After abatement of initial insertion trauma (60–90 minutes), local heaters were placed over each microdialysis site and skin temperature was clamped at 33°C. A laser Doppler flow probe (Moor Instruments) was placed into each heater to measure red blood cell flux, a relative index of skin blood flow. Blood pressure was measured every 5 minutes throughout the protocol via brachial auscultation (Cardiocap/5, General Electric). After stable baseline (20 min), seven increasing concentrations of ACh (0.01, 0.1, 1, 5, 10, 50, 100 mM) were perfused through the microdialysis fibers in 5 minute increments. Each dose of ACh was mixed with the appropriate NOS inhibitor (L-NAME, 1400w) or lactated Ringer’s to serve as a control. After completion of the ACh dose-response, the temperature of the local heaters was increased to 43°C and 28 mM SNP (sodium nitroprusside, U.S. Pharmacopia), a NO donor, was perfused through the fibers at a rate of 4 μL•min−1 to achieve maximal cutaneous vasodilation. Use of 28 mM SNP with simultaneous local heating is a commonly used protocol to induce maximum vasodilation in the human cutaneous microvasculature21, 50, 52, 53.
Local Heating Protocol
The purpose of the local heating protocol was to quantify endothelium-dependent vasodilation to a physiological stimulus54, 55. Two microdialysis fibers were placed in the skin of the forearm. One fiber was perfused with lactated Ringer’s solution (control) and the other with 0.1 mM 1400w to selectively inhibit iNOS. Substances were perfused through the fibers at a rate of 2 μL•min−1. After abatement of initial insertion trauma (60–90 minutes), local heaters were placed over each microdialysis site and skin temperature was clamped at 33°C. A laser Doppler probe was placed into each heater to measure red blood cell flux. Blood pressure was measured every 5 minutes throughout the protocol via brachial auscultation. Stable baseline flux was measured (20 min); local temperature was then increased at a rate of 0.5°C every 5 seconds to a temperature of 42°C. This protocol has been used extensively to examine physiologically-induced eNOS-dependent vasodilation21, 54, 56. Red cell flux was measured until a stable plateau had been reached (approximately 40 minutes), at which point 20 mM L-NAME (non-specific NOS antagonist) was perfused through both microdialysis fibers to quantify the contribution of NOS-derived NO to local heat-induced vasodilation. After a new post-L-NAME plateau was obtained, the local heater temperature was increased to 43°C and 28 mM SNP was perfused through the fibers at a rate of 4 μL•min−1 to obtain maximal cutaneous vasodilation.
Data Analytical Approach
Red cell flux data were digitized at 40 Hz, recorded, and stored for offline analysis using Windaq software and a Dataq data acquisition system (Windaq: Dataq Instruments). For ACh dose-response data, skin blood flow was averaged over 2 minute segments of stable blood flux after each dose of ACh was perfused. For local heating data, red cell flux was averaged over stable 10 minute segments during the local heating plateau, post-L-NAME plateau, and SNP-induced maximal vasodilation. NO-dependent vasodilation was calculated from local heating data as vasodilation following local heating (42°C) minus vasodilation following NOS inhibition with L-NAME. In both protocols cutaneous vascular conductance (CVC: red cell flux•MAP−1) was calculated for ACh dose or local heating phase. Data were normalized to a percentage of maximum CVC (%CVCmax) obtained during 28 mM SNP perfusion and simultaneous 43°C heating ((CVC•CVCmax−1)•100).
All statistical analysis was conducted with SAS 9.3 software. A one-way ANOVA was used to compare physical characteristics between subject groups. There was no effect of localized drug treatment on absolute maximal CVC data obtained from 28 mM SNP/43°C heating (p=0.59), therefore maximal CVC data form the microdialysis sites were pooled by group and analyzed with a one-way ANOVA. Because group differences in maximal CVC were apparent, all data are presented as both absolute CVC and %CVCmax. A 3-way, mixed model, repeated-measures ANOVA (group*pharmacological site*ACh dose or local heating phase) was used to examine group, site, and dose or phase differences. Tukey’s multiple comparisons tests were used for specific planned comparisons. Significance was set at α=0.05. Results are presented as mean ± SEM.
RESULTS
Subject characteristics are presented in table 1. By design, subjects were well matched for characteristics other than blood pressure. SBP, DBP, and MAP were all significantly higher in the hypertensive group compared to both the normotensive and medicated groups (all p<0.001). Though still in the normal clinical range, SBP was slightly higher in the medicated group compared to the normotensive group (p=0.01).
Maximum CVC
Figure 1. shows maximal CVC data obtained during local heating (43°C) and perfusion of 28 mM SNP. Because there were no differences with individual localized microdialysis treatment, data are pooled within each group. Maximal CVC was lower in both the hypertensive (hypertensive 1.52 ± 0.06 flux•mmHg−1; p=0.014) and medicated (1.48 ± 0.06 flux•mmHg−1; p=0.004) groups compared to the normotensive group (normotensive 1.76 ± 0.06 flux•mmHg−1).
Figure 1.
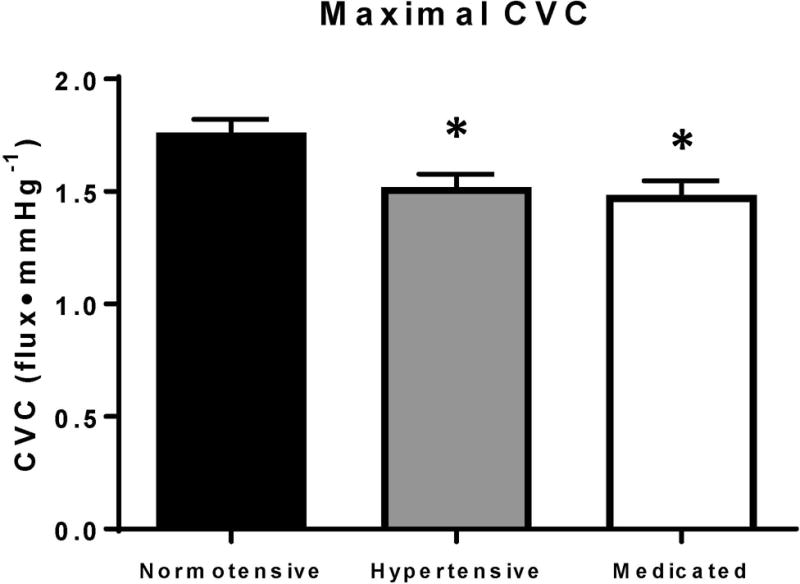
ACh dose-response
Figure 2 illustrates the ACh dose-response data for all three groups in each local treatment site expressed as both %CVCmax (Panel A) and absolute CVC (Panel B). At the control site, %CVCmax was attenuated in the hypertensive group compared to the normotensive and medicated groups at all ACh doses ≥0.1 mM (all p<0.05). Further, there was no difference in %CVCmax at the control site between the normotensive and medicated groups. Similarly, absolute CVC was also attenuated in the hypertensive group with all ACh doses ≥0.1 mM compared to the normotensive group at control (all p<0.05). However, absolute CVC was attenuated in the medicated group compared to the normotensive group at Ach doses 1–10 mM (all p<0.05).
Figure 2.
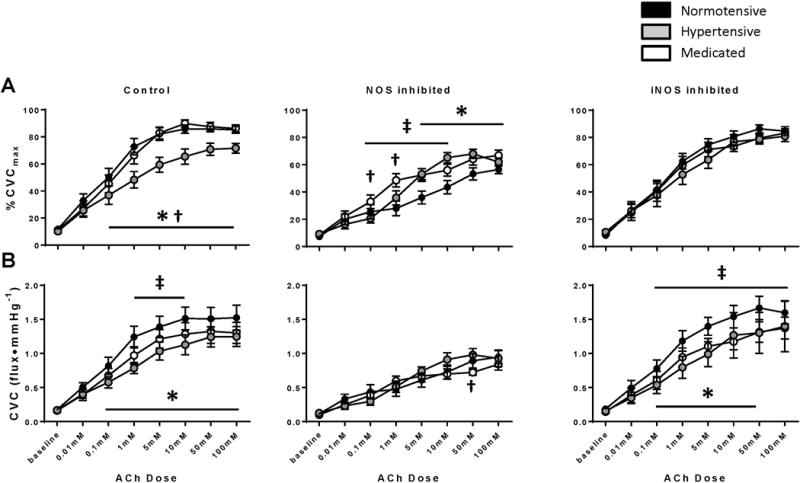
During NOS inhibition (L-NAME), %CVCmax was attenuated in the hypertensive (ACh doses 5–100 mM) and medicated (ACh doses 0.1–10 mM) groups compared to the normotensive group (all p<0.05). Absolute CVC, was lower in the medicated group compared to hypertensive group at 50 mM ACh (p=0.01).
There were no differences in %CVCmax with iNOS inhibition. Absolute CVC was attenuated in the hypertensive (ACh doses 0.1–50 mM) and medicated (ACh doses 0.1–100 mM) groups compared to the normotensive group (all p<0.05).
Within group changes were apparent between sites. In the normotensive group, %CVCmax was no different between the control and 1400w sites for any dose of ACh (all p>0.05), however %CVCmax was significantly lower at the L-NAME site with all ACh doses ≥0.1 mM compared to both the control and L-NAME sites. Similarly in the normotensive group, when data were expressed as CVC, there were no differences between the control and 1400w sites. Vasodilation was lower at the L-NAME site relative to control at all ACh doses ≥0.1 mM and lower relative to the 1400w site at all ACh doses ≥1 mM.
In the hypertensive group, %CVCmax was higher in the 1400w site compared to the control site with the 10 mM dose of ACh (control 70.8 ± 4.8, 1400w 76.9 ± 5.1 %CVCmax; p=0.02) and approached significance with the 100 mM dose of ACh (control 71.6 ± 3.6, 1400w 80.8 ± 3.9 %CVCmax; p=0.06). The control site was only different from the L-NAME site with the 0.1 mM dose of ACh (control 36.9 ± 6.8, L-NAME 20.5 ± 3.1 %CVCmax; p=0.03). %CVCmax was significantly greater in the 1400w site compared to L-NAME at all ACh doses ≥0.1 mM (all p<0.05). There were not between site differences in the hypertensive group when data were expressed as absolute CVC.
In the medicated group, %CVCmax was no different between the control and 1400w sites at any dose of ACh (all p>0.05). %CVCmax was significantly lower in the L-NAME site compared to the control site at all ACh doses ≥1 mM (all p<0.05) and lower compared to the 1400w site at all ACh doses ≥5 mM (all p<0.05). Likewise, when data were expressed as absolute CVC, there were no differences between the control and 1400w sites, while CVC was significantly lower in the L-NAME site compared to the control site at all ACh doses ≥1 mM (all p<0.05) and lower compared to the 1400w site at all ACh doses ≥5 mM (all p<0.05).
Local heating
Figure 3a. depicts local heating data, expressed as %CVCmax, for all three subject groups at the control site. %CVCmax was attenuated in the hypertensive group compared to the normotensive group (hypertensive: 84.0 ± 1.6 %CVCmax, normotensive: 93.7 ± 1.2 %CVCmax; p<0.001). %CVCmax in the medicated group did not differ from either the hypertensive (p=0.44) or control groups (89.6 ± 1.7 %CVCmax; p=0.06). NO-dependent vasodilation was similar among all groups (p> 0.05). Figure 3b. depicts local heating skin blood flow data expressed as absolute CVC. No significant differences in CVC were observed at the local heating plateau in the hypertensive (hypertensive: 1.48 ± 0.13 flux•mmHg−1, normotensive: 1.71 ± 0.23 flux•mmHg−1; p=0.06) or medicated (1.38 ± 0.18 flux•mmHg−1; p=0.08) groups compared to the normotensive group.
Figure 3.
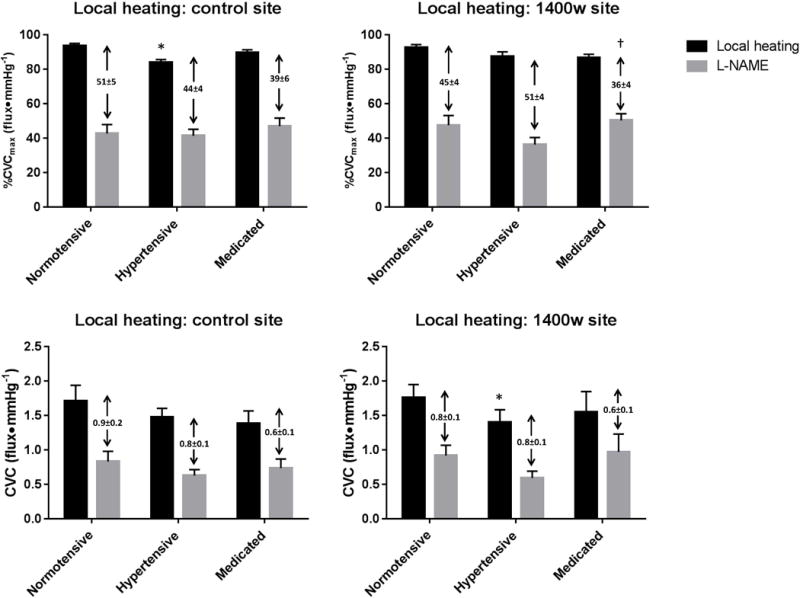
Figure 3c. depicts local heating data with iNOS inhibition expressed as %CVCmax. %CVCmax at the local heating plateau was not different among the groups (hypertensive: 87.5 ± 2. %CVCmax, normotensive: 92.7 ± 1.6 %CVCmax; p=0.31, medicated 86.7 ± 2.0 %CVCmax; p=0.30). There were no differences in NO-dependent vasodilation between the normotensive and hypertensive (p=0.24), or normotensive and medicated (p=0.16) groups. However, NO-dependent vasodilation was reduced in the medicated group compared to the hypertensive group (p=0.02). Figure 3d. depicts local heating data for the iNOS inhibited site expressed as CVC. Compared to normotensive subjects, the local heating plateau was reduced in the hypertensive group (hypertensive: 1.40 ± 0.18 flux•mmHg−1, normotensive: 1.76 ± 0.19 flux•mmHg−1; p=0.01), but not medicated group (1.55 ± 0.29 flux•mmHg−1; p=0.14). NO-dependent vasodilation did not differ between groups (all p>0.05).
In the normotensive group, when presented as %CVCmax, there were no differences between the control and 1400w sites either at the local heating plateau (control 93.7 ± 1.3, 1400w 92.7 ± 1.6 %CVCmax; p=0.54) or in NO-dependent vasodilation (control 51.1 ± 5.3, 1400w 45.1 ± 5.6 %CVCmax; p=0.23). Similarly, when data were expressed as CVC, there was no difference in the local heating plateau (control 1.71 ± 0.2, 1400w 1.76 ± 0.2 CVC; p=0.83) or NO-dependent vasodilation (control 0.87 ± 0.15, 1400w 0.84 ± 0.15 CVC; p=0.84).
In the hypertensive group, there were no between-site differences in %CVCmax at the local heating plateau (control 85.8 ± 1.9, 1400w 87.5 ± 2.7 %CVCmax; p=0.67) or in NO-dependent vasodilation (control 44.3 ± 4.3, 1400w 51.2 ± 4.2 %CVCmax, p=0.17). When expressed as CVC there were no between site differences in the local heating plateau (control 1.48 ± 0.13, 1400w 1.40 ± 0.18 flux•mmHg−1; p=0.69) or NO-dependent vasodilation (control 0.85 ± 0.14, 1400w 0.81 ± 0.11 flux•mmHg−1; p=0.82).
In the medicated group, there no between site differences in %CVCmax at the local heating plateau (control 90.1 ± 1.6, 1400w 87.0 ± 2.0 %CVCmax; p=0.25) or NO-dependent vasodilation (control 40.9 ± 5.1, 1400w 32.9 ± 4.3 %CVCmax; p=0.22). There were no between site difference when data were expressed as CVC at the local heating plateau (control 1.58 ± 0.19, 1400w 1.67 ± 0.31 flux•mmHg−1; p=0.65) or NO-dependent vasodilation (control 0.69 ± 0.12, 1400w 0.53 ± 0.08 flux•mmHg−1; p=0.28).
DISCUSSION
The principal finding of this study was that blood pressure control with antihypertensive pharmacotherapy that included either an ACE inhibitor or ARB modestly improved cutaneous microvascular function through endothelium-dependent mechanisms, potentially involving a reduction in iNOS-mediated dysfunction. However, in a cohort with well controlled blood pressure to clinically appropriate levels, treatment did not improve maximal vasodilator responsiveness, which is considered an index of microvascular structure. Taken together, these data suggest that blood pressure control with RAS-inhibiting antihypertensive medications exerts positive peripheral endothelial vascular effects, but do not affect indices of vessel remodeling in the cutaneous microcirculation.
In humans with elevated blood pressure, endothelial dysfunction and microvascular remodeling is present and detectable in the cutaneous microvasculature14,15. This is in-part through increased expression of iNOS21, 57, 58. Upregulation of iNOS produces large quantities of NO, which is rapidly converted into peroxynitrite57 and contributes to the high oxidant environment characteristic of the vasculature in hypertensive patients. Furthermore, upregulated iNOS increases arginase activity; arginase competes with eNOS for the common substrate L-arginine, thereby decreasing NO bioavailability23. Together, elevated arginase and peroxynitrite limit NO production, leading to microvascular endothelial dysfunction. We have demonstrated that inhibition of iNOS improves endothelial function in the cutaneous microvasculature of hypertensive men and women21.
iNOS is upregulated by angiotensin II acting on angiotensin 1 receptors59. Therefore, pharmacotherapy with effects on the RAS may reduce iNOS expression and restore endothelial function by increasing NO bioavailability. Our data show that when blood pressure is controlled with treatment that includes either an ACE inhibitor or ARB, vasodilation in response to the endothelium-dependent agonist ACh is improved. The augmented vasodilator response to ACh may have involved changes in iNOS-mediated dysfunction, as inhibition of iNOS did not augment vasodilation in the medicated group but did augment ACh-mediated vasodilation at certain doses in the hypertensive group.
There were only modest differences in the ACh dose-response (ACh doses 1–10mM), or local heating plateau, between normotensive and medicated groups at the control site. This suggests that blood pressure normalization with pharmacotherapy can improve endothelial function. Interestingly, when we blocked the contribution of NOS-produced NO to ACh-mediated vasodilation with L-NAME (figure 2), vasodilation decreased to a greater degree in the normotensive group compared to the medicated group, indicating more NO-bioavailability in the normotensive group. This suggests that the improvement in endothelial function that occurs with pharmacotherapy-mediated blood pressure normalization involves both NO and non-NO-dependent mechanisms.
While we observed improved endothelial function in response to ACh with medication, we did not observe an improvement in the local heating response (figure 3). While the local heating plateau in the medicated group was not statistically different from the normotensive group, the difference approached significance (p=0.06), indicating that an attenuation in the local heating response is likely in the medicated subjects. These disparate results are likely due to different mechanisms mediating the increase in skin blood flow to each stimulus. Local heating is predominantly mediated by eNOS-derived NO54, with the remainder attributed to endothelium-derived hyperpolarizing factors60. The mechanisms underlying ACh-mediated vasodilation differ depending on the protocol employed and potentially the dose of ACh61. ACh induces vasodilation through NO and prostanoids/cyclooxygenase (COX)-dependent mechanisms62, 63. However, the contribution of NO to ACh-mediated vasodilation is relatively less compared to local heating. It is possible that RAS-inhibiting antihypertensive pharmacotherapy increases both NO- and COX-dependent mechanisms, with the augmentation in COX only apparent during the ACh dose-response. There is evidence that ACE-inhibitors can increases COX-mediated vasodilation in the skin64. Our study did not utilize any specific COX inhibitors, but we did observe an increase in non-NO endothelium-dependent vasodilation in the medicated group (figure 2). Regardless of the specific mechanisms, both ACh and local heating induce vasodilation that is predominantly endothelium-dependent. Collectively, attenuated vasodilation in response to both ACh and local heating was apparent in the hypertensive group, but ACh-mediated vasodilation was improved in the medicated group. This suggests that blood pressure control through ACE inhibitor or ARB pharmacotherapy improved endothelial function through both NO-dependent and NO-independent mechanism(s).
Hypertension is not only associated with endothelial dysfunction, but also with pathological vessel remodeling, which is evident in the cutaneous microcirculation. Examination with nail fold capillaroscopy shows decreased capillary density in the skin of hypertensive men and women65–67. Minimum vascular resistance (or maximum conductance) is also indicative of vessel structure68. Our data show that maximal CVC, obtained through perfusion of SNP and simultaneous local heating (43°C), is attenuated in hypertensive men and women. Medicated subjects also exhibited attenuated maximal CVC compared to the normotensive group. Together, these data suggest that hypertension induces pathological vessel remodeling in the cutaneous microvasculature, and that blood pressure normalization with ACE inhibitor or ARB pharmacotherapy does not alter this index of microvessel structure in the cutaneous vascular bed. This index of maximal vasodilator capacity, when taken with our functional data, show that blood pressure normalization with RAS-inhibiting pharmacotherapy improved endothelial function, albeit within a narrowed structural limit.
These findings, which indicate no structural alteration occurred with blood pressure normalization are inconsistent with other published findings36, 69, 70, likely due to the different vessels examined and methods used to determine vessel structure. Currently, technology is in its infancy for direct measurements of cutaneous microvessel structure71 outside of the nail fold. To our knowledge, no studies have been performed to measure cutaneous microvascular diameter in hypertensive subjects. Instead, maximum conductance was achieved in response to high doses of SNP and simultaneous local heating as an index of vessel structure. Further research is warranted to elucidate the changes in cutaneous microvascular structure with pharmacotherapy.
Our data show that endothelial function is improved with pharmacologically-mediated blood pressure normalization. Endothelial dysfunction is a hallmark of hypertension and many other chronic diseases7. Determining the mechanisms through which controlling hypertension restores endothelial function could lead to new treatments not only for hypertension, but for other pathologies involving vascular dysfunction. All medicated subjects in this study took one medication that influenced the RAS. Numerous putative mechanisms for the pleotropic effects of RAS inhibition exist, such as increased ACE2/Ang 1–772, increased Ac-SDKP/decreased lysyl oxidase73, increased bradykinin74, and decreased production of reactive oxygen species75. Further investigation into these signaling pathways will allow greater understanding of chronic disease progression and help tailor targeted treatments.
The main limitation of this study is that the findings cannot be attributed solely to either specific effects of ACE inhibitors/ARBs, general effects of antihypertensive pharmacotherapy, or blood pressure normalization. However, our subjects with blood pressure controlled through ACE inhibitor or ARB antihypertensive pharmacotherapy represent a clinically valid and real-world subject pool. A second limitation is that we used only an index of microvascular structure and did not employ a direct measure of the microvasculature. It is conceivable that the lower maximum vasodilation observed in the hypertensive and medicated groups were due to impaired smooth muscle function and not structural deficits.
Conclusion
Controlling blood pressure with antihypertensive medications improves microvascular endothelial function, but not structure, in men and women with essential hypertension. This augmentation in endothelial function occurs through both NO and non-NO mechanisms, and may be mediated by a reduction in iNOS. These data confirm that blood pressure normalization with antihypertensive pharmacotherapy elicits vascular benefits, and extends our understanding to include the cutaneous microvasculature.
Perspectives
The results of the present study suggest that blood pressure controlled with pharmacotherapy that includes either an ACE inhibitor or ARB improves endothelial function and this improvement in endothelial function involves NO-dependent and –independent mechanisms. Despite the improvement in endothelial function, maximal vasodilatory capacity of the cutaneous microvasculature is not restored with pharmacotherapy. From a practical standpoint, we conclude that blood pressure control with RAS-inhibiting pharmacotherapy improves microvascular endothelial function, which is an important component of overall vascular health.
Acknowledgments
The authors would like to acknowledge the time and effort put in by all of the volunteers. We would also like to thank Sue Slimak RN and Jane Pierzga MS for their assistance with data collection.
Grants: NIH R01 HL089302
Abbreviations
- ACE
Angiotensin converting enzyme
- ACh
Acetylcholine
- ARB
Angiotensin receptor blocker
- COX
Cyclooxygenase
- CVC
Cutaneous vascular conductance
- %CVCmax
Percentage of maximal CVC
- DBP
Diastolic blood pressure
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
- RAS
Renin-angiotensin system
- SBP
Systolic blood pressure
- SNP
Sodium nitroprusside
- 1400w
N-(3-(Aminomehtyl)benzyl)acetamidine
Footnotes
MR. DANIEL H CRAIGHEAD (Orcid ID : 0000-0002-8744-5349)
Disclosures: None
References
- 1.Nwankwo TYS, Burt V, Gu Q. Hypertension among adults in the united states: National health and nutrition examination survey, 2011–2012. NCHS Data Brief. 2013;113:1–8. [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Muiesan ML, Salvetti M, Monteduro C, Corbellini C, Guelfi D, Rizzoni D, Castellano M, Agabiti-Rosei E. Flow-mediated dilatation of the brachial artery and left ventricular geometry in hypertensive patients. Journal of hypertension. 2001;19:641–647. doi: 10.1097/00004872-200103001-00018. [DOI] [PubMed] [Google Scholar]
- 4.Ghiadoni L, Huang Y, Magagna A, Buralli S, Taddei S, Salvetti A. Effect of acute blood pressure reduction on endothelial function in the brachial artery of patients with essential hypertension. Journal of hypertension. 2001;19:547–551. doi: 10.1097/00004872-200103001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bouthier JD, De Luca N, Safar ME, Simon AC. Cardiac hypertrophy and arterial distensibility in essential hypertension. American heart journal. 1985;109:1345–1352. doi: 10.1016/0002-8703(85)90364-3. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa M, Nagao K, Kinoshita Y, Rodbard D, Asahina A. Increased pulse wave velocity and shortened pulse wave transmission time in hypertension and aging. Cardiology. 1997;88:147–151. doi: 10.1159/000177321. [DOI] [PubMed] [Google Scholar]
- 7.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 8.IJ RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. European journal of clinical investigation. 2003;33:536–542. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 9.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circulation Cardiovascular interventions. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 10.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HAJ. Microcirculation in hypertension - a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 11.Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 12.Smith CJ, Santhanam L, Alexander LM. Rho-kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res. 2013;87:58–64. doi: 10.1016/j.mvr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holowatz LA, Thompson CS, Kenney WL. L-arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;574:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holowatz LA, Kenney WL. Local ascorbate administration augments no- and non-no-dependent reflex cutaneous vasodilation in hypertensive humans. American journal of physiology Heart and circulatory physiology. 2007;293:H1090–1096. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 15.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–872. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas K, Kolossvary E, Jarai Z, Nemcsik J, Farsang C. Non-invasive assessment of microvascular endothelial function by laser doppler flowmetry in patients with essential hypertension. Atherosclerosis. 2004;173:97–102. doi: 10.1016/j.atherosclerosis.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 18.IJzerman RG, de Jongh RT, Beijk MAM, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CDA. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. European journal of clinical investigation. 2003;33:536–542. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossi M, Carpi A, Galetta F, Franzoni F, Santoro G. The investigation of skin blood flowmotion: A new approach to study the microcirculatory impairment in vascular diseases? Biomed Pharmacother. 2006;60:437–442. doi: 10.1016/j.biopha.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. American journal of physiology Heart and circulatory physiology. 2004;287:H2687–2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- 21.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension. 2011;58:935–942. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Inducible nitric oxide synthase as a possible target in hypertension. Current drug targets. 2014;15:164–174. doi: 10.2174/13894501113146660227. [DOI] [PubMed] [Google Scholar]
- 23.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible no synthase-dependent s-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 24.Stead EA, Kunkel P. Nature of peripheral resistance in arterial hypertension. J Clin Invest. 1940;19:25–33. doi: 10.1172/JCI101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkow B, Grimby G, Thulesius O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand. 1958;44:255–272. doi: 10.1111/j.1748-1716.1958.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita A, Mark AL. Decreased vasodilator capacity of forearm resistance vessels in borderline hypertension. Hypertension. 1980;2:610–616. doi: 10.1161/01.hyp.2.5.610. [DOI] [PubMed] [Google Scholar]
- 27.Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension. 1992;20:349–355. doi: 10.1161/01.hyp.20.3.349. [DOI] [PubMed] [Google Scholar]
- 28.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 29.Dahlof B, Hansson L. The influence of antihypertensive therapy on the structural arteriolar changes in essential hypertension: Different effects of enalapril and hydrochlorothiazide. Journal of internal medicine. 1993;234:271–279. doi: 10.1111/j.1365-2796.1993.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 30.Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension. 1995;25:474–481. doi: 10.1161/01.hyp.25.4.474. [DOI] [PubMed] [Google Scholar]
- 31.Buus NH, Bottcher M, Jorgensen CG, Christensen KL, Thygesen K, Nielsen TT, Mulvany MJ. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension. 2004;44:465–470. doi: 10.1161/01.HYP.0000141273.72768.b7. [DOI] [PubMed] [Google Scholar]
- 32.Mancini M, Scavone A, Sartorio CL, Baccaro R, Kleinert C, Pernazza A, Buia V, Leopizzi M, D’Amati G, Camici PG. Effect of different drug classes on reverse remodeling of intramural coronary arterioles in the spontaneously hypertensive rat. Microcirculation. 2016 doi: 10.1111/micc.12298. [DOI] [PubMed] [Google Scholar]
- 33.Fujii M, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Bradykinin improves left ventricular diastolic function under long-term angiotensin-converting enzyme inhibition in heart failure. Hypertension. 2002;39:952–957. doi: 10.1161/01.hyp.0000015613.78314.9e. [DOI] [PubMed] [Google Scholar]
- 34.Bachetti T, Comini L, Pasini E, Cargnoni A, Curello S, Ferrari R. Ace-inhibition with quinapril modulates the nitric oxide pathway in normotensive rats. J Mol Cell Cardiol. 2001;33:395–403. doi: 10.1006/jmcc.2000.1311. [DOI] [PubMed] [Google Scholar]
- 35.Mancini GB, Henry GC, Macaya C, O’Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Luscher TF, Klibaner MI, Haber HE, Uprichard AC, Pepine CJ, Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The trend (trial on reversing endothelial dysfunction) study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 36.Rizzoni D, Muiesan ML, Porteri E, Castellano M, Zulli R, Bettoni G, Salvetti M, Monteduro C, Agabiti-Rosei E. Effects of long-term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ventricular hypertrophy. Journal of hypertension. 1997;15:197–204. doi: 10.1097/00004872-199715020-00011. [DOI] [PubMed] [Google Scholar]
- 37.Toyama T, Sato C, Koyama K, Kasama S, Murakami J, Yamashita E, Kawaguchi R, Adachi H, Hoshizaki H, Oshima S. Olmesartan improves coronary flow reserve of hypertensive patients using coronary magnetic resonance imaging compared with amlodipine. Cardiology. 2012;122:230–236. doi: 10.1159/000339762. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi T, Abletshauser C, Nekolla SG, Schwaiger M, Bengel FM. Effect of the angiotensin receptor blocker valsartan on coronary microvascular flow reserve in moderately hypertensive patients with stable coronary artery disease. Microcirculation. 2007;14:805–812. doi: 10.1080/10739680701410827. [DOI] [PubMed] [Google Scholar]
- 39.Unger T, Mattfeldt T, Lamberty V, Bock P, Mall G, Linz W, Scholkens BA, Gohlke P. Effect of early onset angiotensin converting enzyme inhibition on myocardial capillaries. Hypertension. 1992;20:478–482. doi: 10.1161/01.hyp.20.4.478. [DOI] [PubMed] [Google Scholar]
- 40.Zhuo JL, Mendelsohn FA, Ohishi M. Perindopril alters vascular angiotensin-converting enzyme, at(1) receptor, and nitric oxide synthase expression in patients with coronary heart disease. Hypertension. 2002;39:634–638. doi: 10.1161/hy0202.103417. [DOI] [PubMed] [Google Scholar]
- 41.Palaniyappan A, Uwiera RR, Idikio H, Jugdutt BI. Comparison of vasopeptidase inhibitor omapatrilat and angiotensin receptor blocker candesartan on extracellular matrix, myeloperoxidase, cytokines, and ventricular remodeling during healing after reperfused myocardial infarction. Mol Cell Biochem. 2009;321:9–22. doi: 10.1007/s11010-008-9905-3. [DOI] [PubMed] [Google Scholar]
- 42.Potenza MA, Marasciulo FL, Tarquinio M, Quon MJ, Montagnani M. Treatment of spontaneously hypertensive rats with rosiglitazone and/or enalapril restores balance between vasodilator and vasoconstrictor actions of insulin with simultaneous improvement in hypertension and insulin resistance. Diabetes. 2006;55:3594–3603. doi: 10.2337/db06-0667. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo E, Maeso R, Munoz-Garcia R, Navarro-Cid J, Ruilope LM, Cachofeiro V, Lahera V. Endothelial dysfunction in spontaneously hypertensive rats: Consequences of chronic treatment with losartan or captopril. Journal of hypertension. 1997;15:613–618. doi: 10.1097/00004872-199715060-00007. [DOI] [PubMed] [Google Scholar]
- 44.Pasini AF, Garbin U, Nava MC, Stranieri C, Pellegrini M, Boccioletti V, Luchetta ML, Fabrizzi P, Lo Cascio V, Cominacini L. Effect of sulfhydryl and non-sulfhydryl angiotensin-converting enzyme inhibitors on endothelial function in essential hypertensive patients. American journal of hypertension. 2007;20:443–450. doi: 10.1016/j.amjhyper.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, Tamaki N, Tsutsui H. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. Journal of the American College of Cardiology. 2007;50:1144–1149. doi: 10.1016/j.jacc.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Hypertension EETFftMoA. 2013 practice guidelines for the management of arterial hypertension of the european society of hypertension (esh) and the european society of cardiology (esc): Esh/esc task force for the management of arterial hypertension. Journal of hypertension. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 47.Dorresteijn JA, Schrover IM, Visseren FL, Scheffer PG, Oey PL, Danser AH, Spiering W. Differential effects of renin-angiotensin-aldosterone system inhibition, sympathoinhibition and diuretic therapy on endothelial function and blood pressure in obesity-related hypertension: A double-blind, placebo-controlled cross-over trial. Journal of hypertension. 2013;31:393–403. doi: 10.1097/HJH.0b013e32835b6c02. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi T, Yamaguchi T, Sakakibara Y, Taguchi K, Maeda M, Kuzuya M, Hattori Y. Enos-dependent antisenscence effect of a calcium channel blocker in human endothelial cells. PloS one. 2014;9:e88391. doi: 10.1371/journal.pone.0088391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. American journal of physiology Heart and circulatory physiology. 2008;295:H123–129. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, Knowles RG. 1400w is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. The Journal of biological chemistry. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 52.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Experimental physiology. 2010;95:946–954. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 53.Dupont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or l-arginine improves cutaneous microvascular function in chronic kidney disease. Journal of applied physiology. 2011;111:1561–1567. doi: 10.1152/japplphysiol.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. Journal of applied physiology. 2012;112:2019–2026. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson JM, Kellogg DL., Jr Local thermal control of the human cutaneous circulation. Journal of applied physiology. 2010;109:1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. Journal of applied physiology. 2009;107:1438–1444. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. British journal of pharmacology. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar U, Chen J, Sapoznikhov V, Canteros G, White BH, Sidhu A. Overexpression of inducible nitric oxide synthase in the kidney of the spontaneously hypertensive rat. Clinical and experimental hypertension. 2005;27:17–31. doi: 10.1081/ceh-200044249. [DOI] [PubMed] [Google Scholar]
- 59.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin ii-induced cardiac hypertrophy. Hypertension. 2004;43:1195–1201. doi: 10.1161/01.HYP.0000128621.68160.dd. [DOI] [PubMed] [Google Scholar]
- 60.Brunt VE, Minson CT. Kca channels and epoxyeicosatrienoic acids: Major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590:3523–3534. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunt VE, Fujii N, Minson CT. Endothelial-derived hyperpolarization contributes to acetylcholine-mediated vasodilation in human skin in a dose-dependent manner. Journal of applied physiology. 2015;119:1015–1022. doi: 10.1152/japplphysiol.00201.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. Journal of applied physiology. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 63.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008;15:569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warren JB, Loi RK. Captopril increases skin microvascular blood flow secondary to bradykinin, nitric oxide, and prostaglandins. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9:411–418. doi: 10.1096/fasebj.9.5.7896012. [DOI] [PubMed] [Google Scholar]
- 65.Serne EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38:238–242. doi: 10.1161/01.hyp.38.2.238. [DOI] [PubMed] [Google Scholar]
- 66.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 67.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34:655–658. doi: 10.1161/01.hyp.34.4.655. [DOI] [PubMed] [Google Scholar]
- 68.Rosei EA, Rizzoni D, Castellano M, Porteri E, Zulli R, Muiesan ML, Bettoni G, Salvetti M, Muiesan P, Giulini SM. Media: Lumen ratio in human small resistance arteries is related to forearm minimal vascular resistance. Journal of hypertension. 1995;13:341–347. [PubMed] [Google Scholar]
- 69.De Ciuceis C, Savoia C, Arrabito E, Porteri E, Mazza M, Rossini C, Duse S, Semeraro F, Agabiti Rosei C, Alonzo A, Sada L, La Boria E, Sarkar A, Petroboni B, Mercantini P, Volpe M, Rizzoni D, Agabiti Rosei E. Effects of a long-term treatment with aliskiren or ramipril on structural alterations of subcutaneous small-resistance arteries of diabetic hypertensive patients. Hypertension. 2014;64:717–724. doi: 10.1161/HYPERTENSIONAHA.114.03380. [DOI] [PubMed] [Google Scholar]
- 70.Shargorodsky M, Leibovitz E, Lubimov L, Gavish D, Zimlichman R. Prolonged treatment with the at1 receptor blocker, valsartan, increases small and large artery compliance in uncomplicated essential hypertension. American journal of hypertension. 2002;15:1087–1091. doi: 10.1016/s0895-7061(02)03134-5. [DOI] [PubMed] [Google Scholar]
- 71.Carter HH, Gong P, Kirk RW, Es’haghian S, Atkinson CL, Sampson DD, Green DJ, McLaughlin RA. Optical coherence tomography in the assessment of acute changes in cutaneous vascular diameter induced by heat stress. Journal of applied physiology. 2016;121:965–972. doi: 10.1152/japplphysiol.00918.2015. [DOI] [PubMed] [Google Scholar]
- 72.Zimmerman MA, Harris RA, Sullivan JC. Female spontaneously hypertensive rats are more dependent on ang (1–7) to mediate effects of low-dose at1 receptor blockade than males. American journal of physiology Renal physiology. 2014;306:F1136–1142. doi: 10.1152/ajprenal.00677.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonzalez GE, Rhaleb NE, Nakagawa P, Liao TD, Liu Y, Leung P, Dai X, Yang XP, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline reduces cardiac collagen cross-linking and inflammation in angiotensin ii-induced hypertensive rats. Clinical science. 2014;126:85–94. doi: 10.1042/CS20120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gauthier KM, Cepura CJ, Campbell WB. Ace inhibition enhances bradykinin relaxations through nitric oxide and b1 receptor activation in bovine coronary arteries. Biological chemistry. 2013;394:1205–1212. doi: 10.1515/hsz-2012-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Giet M, Erinola M, Zidek W, Tepel M. Captopril and quinapril reduce reactive oxygen species. European journal of clinical investigation. 2002;32:732–737. doi: 10.1046/j.1365-2362.2002.01064.x. [DOI] [PubMed] [Google Scholar]


