SUMMARY
T follicular helper (Tfh) cells are a CD4+ T cell subset critical for long-lived humoral immunity. We hypothesized that integrins play a decisive role in Tfh cell biology. Here we show that Tfh cells expressed a highly active form of leukocyte function-associated antigen-1 (LFA-1) that was required for their survival within the germinal center niche. In addition, LFA-1 promoted expression of Bcl-6, a transcriptional repressor critical for Tfh cell differentiation, and inhibition of LFA-1 abolished Tfh cell generation and prevented protective humoral immunity to intestinal helminth infection. Furthermore, we demonstrated that expression of Talin-1, an adaptor protein that regulates LFA-1 affinity, dictated Tfh versus Th2 effector cell differentiation. Collectively, our results define unique functions for LFA-1 in the Tfh cell effector program and suggest that integrin activity is important in lineage decision-making events in the adaptive immune system.
Graphical abstract
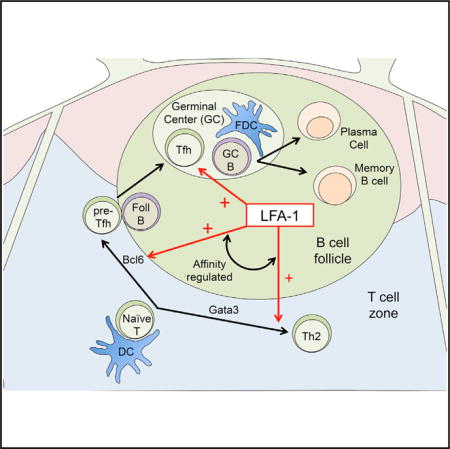
INTRODUCTION
T follicular helper (Tfh) cells are a distinct effector subset of the CD4+ T cell lineage that uniquely serve to promote long-lived humoral immune responses (Crotty, 2011). In contrast to other T effector (Teff) cells that egress from the secondary lymphoid organs (SLOs) following activation by dendritic cells, Tfh cells occupy a specialized niche within the SLOs by migrating deep into the B cell follicle. Within this niche, cognate interactions with antigen-presenting B cells drives the germinal center (GC) reaction and this response must be maintained to generate affinity matured memory B cells and plasma cells (Liu et al., 2015; Shulman et al., 2014). Although the Tfh effector program is critical for antibody-mediated protection against extracellular pathogens, uncontrolled Tfh cell responses can lead to immunopathology and autoimmune disease (Tangye et al., 2013). Thus it is essential to understand the regulatory mechanisms involved in Tfh cell differentiation as well as maintenance to promote health and prevent disease.
The transcription factor B cell lymphoma (Bcl)-6 is indispensable for Tfh cell differentiation and represses key signaling pathways that drive alternative CD4+ effector cell fates (Hatzi et al., 2015; Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). In contrast to other effector subsets in which cytokine signaling drives expression of lineage-specific transcription factors required for their differentiation, specific cytokines that selectively polarize Tfh cells have not been identified. By contrast, proteins associated with co-stimulation or intercellular adhesion such as CD28 and ICOS promote the initiation and persistence of Tfh cells (Choi et al., 2011; Linterman et al., 2014; Linterman et al., 2009). However, whether CD28 and ICOS signaling directly regulate Bcl-6 expression is not clear. In addition, SLAM family members are critical for sustained T-B cell interactions and GC formation, but are not required for initiation of Tfh cell differentiation (Cannons et al., 2010; Qi et al., 2008). Thus, factors that promote Tfh cell lineage specification remain to be determined.
Integrins are heterodimeric receptors expressed by leukocytes and play essential roles in leukocyte migration, tissue retention and immunological synapse formation (Evans et al., 2009). One member of the integrin family, leukocyte function-associated antigen (LFA)-1, is composed of the αL and β2 subunits and has been shown to be a potent intercellular adhesion and co-stimulatory molecule for T cell activation in vitro (Dubey et al., 1995; Dustin and Springer, 1989). These findings have been substantiated by in vivo studies in which deficiency in either subunit compromises T cell priming and is associated with decreased antigen-specific antibody production in humans and mice (Fischer et al., 1986; Kandula and Abraham, 2004; Morrison et al., 2015; Peters et al., 2012). Importantly, LFA-1 activity is not only regulated by its expression but also by its conformation on the cell surface. Indeed, conversion to an open conformation by T cell receptor (TCR) or chemokine-mediated signaling increases the binding affinity for various LFA-1 ligands of the intercellular adhesion molecule (ICAM) family (Hogg et al., 2011). However, the in vivo role of integrin activity in CD4+ T cell lineage commitment, in general, and Tfh cell biology specifically, has not been examined. Given the unique differentiation requirements of Tfh cells, we hypothesized that integrins play an important role in elaborating the Tfh effector cell program. Here we demonstrate that Tfh cells expressed a highly active form of LFA-1 that promoted maintenance of this effector subset within the GC niche. In addition, we found that LFA-1 activation enhanced CD4+ T cell expression of Bcl-6 in the context of TCR triggering. Inhibition of LFA-1 signaling compromised Tfh cell differentiation and prevented the generation of protective humoral immunity to intestinal helminth infection. Finally, we showed that deletion of Talin-1, an adaptor protein critical for generating the high-affinity conformation of LFA-1, selectively compromised Bcl-6 expression and Tfh cell development during infection. Our results reveal previously undefined functions for the integrin LFA-1 in controlling the initiation and persistence of Tfh cells and suggests an important target for controlling T-dependent humoral immune responses.
RESULTS
Tfh Cells Exhibit Elevated Expression of the Integrin LFA-1
We have previously shown that Tfh cells are the dominant producer of interleukin-4 (IL-4) and IL-21 following infection with the intestinal helminth Heligmosomoides polygyrus (Hp), (King et al., 2010; King and Mohrs, 2009). To assess the integrin profile expressed by Tfh cells compared to Teff cells, we used IL-4 dual reporter (4get/KN2) mice (King and Mohrs, 2009). As we have previously shown, GFP+huCD2−T cells represented CXCR5−PD-1−Teff cells that are enriched for the Th2 cell transcription factor Gata3, whereas GFP+huCD2+ T cells were CXCR5+PD-1+ Tfh cells expressing high amounts of Bcl-6 (King and Mohrs, 2009) (Figure 1A). Because integrins are a family of proteins composed of various combinations of 10 distinct α and β subunits (Barczyk et al., 2010), we examined the expression of these integrin subunits by naive and effector CD4+ T cell populations from the mesenteric lymph nodes (mLN) of Hp-infected mice. Our analysis revealed that GFP+huCD2−Teff cells expressed a wide array of integrins including β1, β2, and β3 family members. By contrast, GFP+huCD2+ Tfh cells expressed a very limited repertoire that was similar to naive GFP−T cells with the exception of elevated expression of both αL and β2 that together form the integrin LFA-1 (Figures 1B and 1C).
Figure 1. Mouse and Human Tfh Cells Highly Express the Integrin LFA-1.
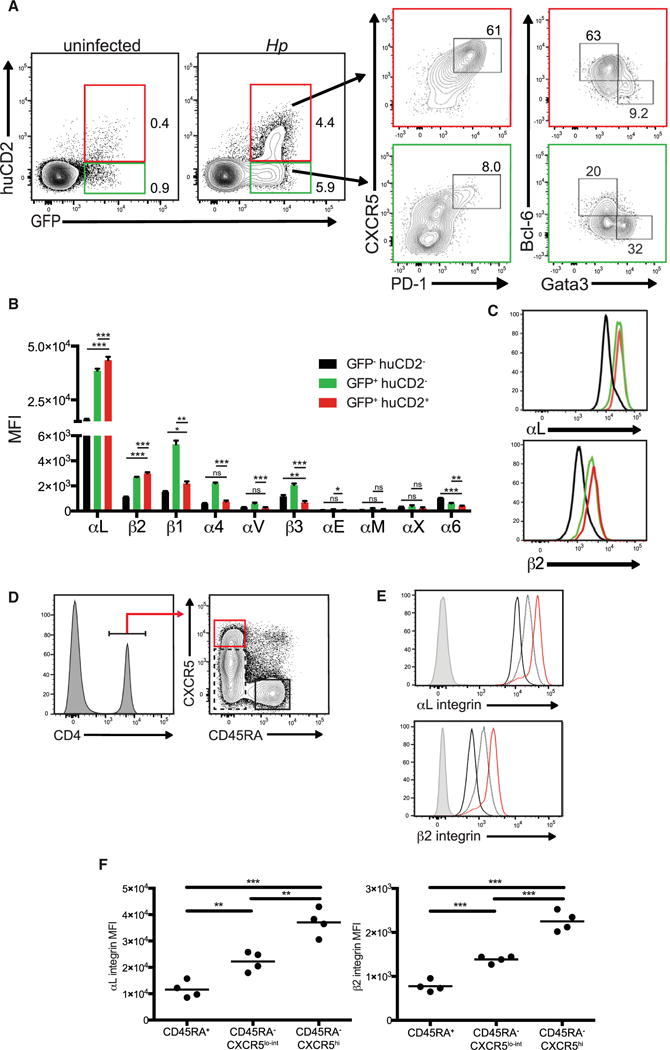
(A–C) 4get/KN2 mice were infected with 200 L3 Hp larvae and harvested 2 weeks post infection. (A) Flow cytometric analysis of GFP and huCD2 expression by CD4+ cells (left panels). CXCR5 and PD-1 (middle panels) and Bcl-6 and Gata3 (right panels) expression by CD4+GFP+huCD2−or CD4+GFP+huCD2+ T cell populations. Numbers within each representative dot plot indicate the frequency of each subset within the CD4+B220− (left panel) or CD4+GFP+huCD2−and CD4+GFP+huCD2+ (middle and right panels) T cells. (B and C) Integrin subunit expression (MFI) profile of subsets described in (A). Data shown are representative of at least two independent experiments with four mice per group.
(D) Gating strategy of human tonsillar CD45RA+CXCR5low CD4+, CD45RA−CXCR5low CD4+ and CD45RA−CXCR5hi CD4+ cell subsets.
(E) Representative histograms of the αL and β2 integrin subunit expression of T cell subsets outlined in (D). Gray histograms indicates isotype controls. (F) MFI of the αL and β2 integrin subunits from the populations indicated. Dots indicate individual donors. ***p < 0.001, **p < 0.01 and *p < 0.05. Error bars, SD; ns, not significant.
Many of the mechanisms underlying Tfh cell differentiation and function are conserved between mice and humans (Tangye et al., 2013). Therefore, we questioned whether a similar pattern of LFA-1 expression observed in our animal studies also applied to human Tfh cells. Previous studies indicate that tonsillar CD45RA−CXCR5hi CD4+ T cells have phenotypic and functional properties indicative of Tfh cells in comparison to CD45RA−CXCR5lo effector and CD45RA+ naive T cells (Ma et al., 2009). Therefore, CD4+ T cells were isolated from human tonsils and segregated into distinct subsets based on CD45RA and CXCR5 expression (Figure 1D). Whereas CD45RA+ naive T cells expressed low amounts of LFA-1 subunits, both αL and β2 integrin expression correlated with increasing CXCR5 expression with the highest amounts being present on the surface of CD45RA−CXCR5hi Tfh cells (Figures 1E and 1F). Thus, uniquely high expression of LFA-1 by Tfh cells is conserved across mouse and human species.
LFA-1 Promotes the Survival of Tfh Cells within the GC Niche
Tfh cell persistence drives the GC reaction and is critical for efficient selection of protective antibody clones (Baumjohann et al., 2013; Fahey et al., 2011). Thus, we questioned whether this pathway plays a role in maintaining the Tfh cell pool during an ongoing GC reaction. Both flow cytometry and confocal microscopy showed that acute blockade of either αL and β2 subunits of LFA-1 at the peak of the GC response resulted in a significant loss of mLN and GC Tfh cells 24 hr after treatment (Figures 2A and 2B). Loss of Tfh cells was not due to direct antibody-mediated cytotoxicity because in vitro incubation of mLN cells from Hp-infected mice with anti-αL had no impact on the frequency or number of CXCR5+huCD2+ T cells or their expression of active caspase-3, an indication of apoptosis (Figures S1A and S1B). These results were coupled with a small, but significant decrease in the number of GC B cells within this time period (Figure 2C). However, we also observed a small but significant loss of GFP+ huCD2−Teff cells (Figure 2A). As LFA-1 delays lymphocyte egress from the lymph nodes (Reichardt et al., 2013), we examined whether the decrease in mLN Teff and Tfh cells following acute LFA-1 blockade could be due to increased egress. We first measured CD69 expression which promotes T cell retention within lymph nodes (Shiow et al., 2006). Although CD69 expression did not change in the remaining Tfh cell population after acute LFA-1 blockade, decreased CD69 expression was observed in Teff cells following antibody treatment (Figure 2D). This result suggested that loss of LFA-1 engagement might promote egress of Teff, but not Tfh, cell subsets. To further test this possibility, we performed a series of studies in which Hp-infected 4get/KN2 mice were treated with anti-αL antibody in combination with FTY720, a sphingosine-1-phosphate receptor (S1P1) agonist that inhibits T cell exit from the lymph nodes (Matloubian et al., 2004). Although additional administration of FTY720 restored and even increased the number of Teff cells, the number of Tfh cells remained significantly decreased (Figure 2E).
Figure 2. LFA-1 Maintains Tfh Cells within the GC Niche by Limiting Apoptosis.
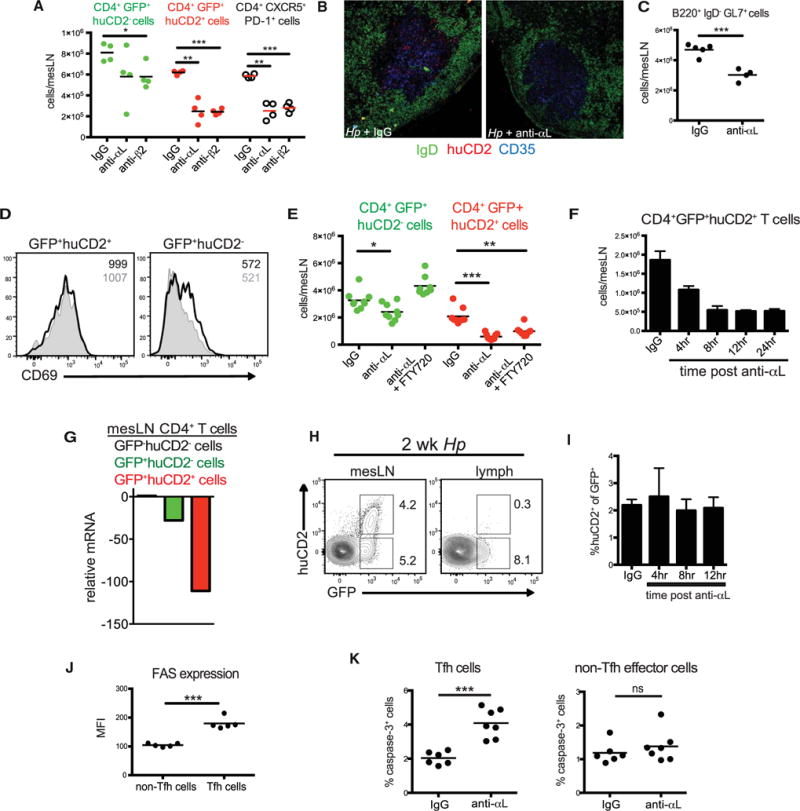
(A–D) 4get/KN2 mice were infected with Hp and administered anti-αL, anti-β2, or Rat IgG blocking antibodies i.p. at 2 weeks post-infection. mLNs were harvested 24 hr after antibody treatment and assessed by flow cytometry or confocal microscopy. (A) The number of GFP+huCD2−, GFP+huCD2+, and CXCR5+PD-1+ CD4+B220−mLN cells. (B) Immunofluorescent images of mLN sections stained with GL7 (green), huCD2 (red), and CD35 (blue). (C) The number of B220+ GL7+IgD−mLN cells 24 hr after anti-αL treatment. (D) Representative histograms of CD69 expression of the GFP+huCD2−and GFP+huCD2+ CD4+ mLN cell populations from rat IgG (unshaded histogram) or anti-αL (shaded histogram) treated 4get/KN2 mice 24 hr after treatment. Inset numbers represent MFI.
(E) The number of GFP−CD62L+, GFP+huCD2−, and GFP+huCD2+ CD4+ mLN cells from infected 4get/KN2 mice 24 hr after treatment with FTY720, anti-αL, or both. Rat IgG was used as a control.
(F) Total cell counts of GFP+huCD2+ CD4+ mLN cells at the indicated time points post-administration of αL antibody to 2 week Hp-infected 4get/KN2 mice.
(G) Expression of S1p1 mRNA in GFP+huCD2−and GFP+huCD2+ mLN CD4+ T cell populations sorted from 2 week Hp infected 4get/KN2 mice relative to the GFP−huCD2−population.
(H) Representative contour plots gated on CD4+B220−cells from the mesenteric lymph node and mesenteric efferent lymphoid vessels of 2 week Hp infected 4get/KN2 mice. Numbers represent frequency of the gated subset from the total CD4+ population. (I) Frequency of huCD2+ cells from the total GFP+CD4+ cell population from the mesenteric efferent lymph at various time points following αL integrin blockade of 2 week Hp-infected 4get/KN2 mice.
(J) Fas expression (MFI) by Tfh (CD4+B220−CD62L−CD44+CXCR5+huCD2+) and non-Tfh effector cells (CD4+B220−CD62L−CD44+CXCR5−huCD2−) from the mLNs of 4get/KN2 mice 8 hr post αL blockade.
(K) Frequency of active caspase-3+ Tfh (CD4+B220−CD62L−CD44+CXCR5+huCD2+ or CD4+B220−CD62L−CD44+CXCR5+PD-1+) and non-Tfh effector cells (CD4+B220−CD62L−CD44+CXCR5−huCD2−or CD4+B220−CD62L−CD44+CXCR5−PD−1−) from the mLNs of 4get/KN2 mice 8 hr post αL blockade. Dots in graphs represent individual mice.
(A)–(J) are representative of at least two independent experiments and (K) shows representative data compiled from four independent experiments. ***p < 0.001, **p < 0.01, and *p < 0.05. Error bars, SD ns, not significant. See also Figure S1.
To further dissect the mechanism by which LFA-1 signaling maintains the Tfh cell population, we determined the kinetics of Tfh cell depletion after αL blockade. As shown in Figure 2F, Tfh cell numbers began to decrease as early as 4 hr after anti-αL treatment and were maximally depleted by 12 hr. Although inhibiting S1P-dependent lymph node egress during LFA-1 blockade did not restore Tfh cell numbers, Tfh cells expressed low amounts of S1p1 compared to naive or Teff cells (Figure 2G). Thus, it remained possible that these cells were exiting the draining mLN in an S1P-independent manner. To directly test this possibility, we cannulated the efferent lymph vessels draining the mLN and collected lymph fluid after LFA-1 blockade. Under control conditions, egressing GFP+huCD2−T cells were readily detectable. In contrast, very few huCD2+ Tfh cells could be observed at any time point examined after anti-αL treatment (Figures 2H and 2I). Thus, LFA-1 maintains the Tfh cell compartment independent of leukocyte egress from reactive lymph nodes.
LFA-1 signaling has been previously shown to promote the survival of CD4+ T cells (Winter et al., 2001). Consistent with human studies (Ma et al., 2009), Tfh cells showed increased expression of Fas, a dominant pro-apoptotic pathway in T lymphocytes (Krueger et al., 2003), compared to Teff cells following Hp infection (Figure 2J). To test whether LFA-1 is important in Tfh cell survival, we assessed active caspase-3 expression at 8 hr post-LFA-1 blockade, a time point in which Tfh cells were decreasing from the mLNs (Figure 2F). Consistently, there was a significant increase in the frequency of active caspase-3+ Tfh cells after 8 hr of LFA-1 blockade, whereas the frequency of caspase-3+ Teff cells did not change (Figures 2K and S1C and S1D). Thus, LFA-1 acts as pro-survival signal maintaining the Tfh cell population within the GC niche.
LFA-1 Is Required for Tfh Cell Differentiation and Protective Humoral Immunity
We next questioned whether LFA-1 activation may also impact Tfh cell differentiation during infection. Peyer’s patches (pp), which constitutively harbor a robust population of Tfh and GC B cells due to persistent stimulation by microbiota-derived antigens, were first compared in WT and αL-deficient mice (Kubinak et al., 2015). Compared to WT controls, pp from αL-deficient mice had significantly fewer CXCR5+Bcl-6+ Tfh cells (Figure 3A). To examine the loss of LFA-1 activity in an acute manner, we infected 4get/KN2 mice with Hp and administered a blocking antibody against αL at the time of infection and every other day thereafter. At 7 days post-infection, LFA-1 blockade resulted in a significant decrease in the frequency and number of Tfh cells compared to control-treated mice (Figures 3B and 3C). Although GFP+huCD2−effector cells were generated, their numbers were similarly decreased (Figure 3B). Within the Treg cell population, a small population of Foxp3+CXCR5+PD-1+ T follicular regulatory (Tfr) cells could be detected that was similarly decreased after LFA-1 blockade (Figure 3D and S2A) (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011). In addition, exclusion of Foxp3+ T cells from our analysis confirmed the selective loss of Tfh cells following LFA-1 blockade (Figure S2B).
Figure 3. LFA-1 Is Required for Tfh Cell Differentiation and Protective Humoral Immunity.
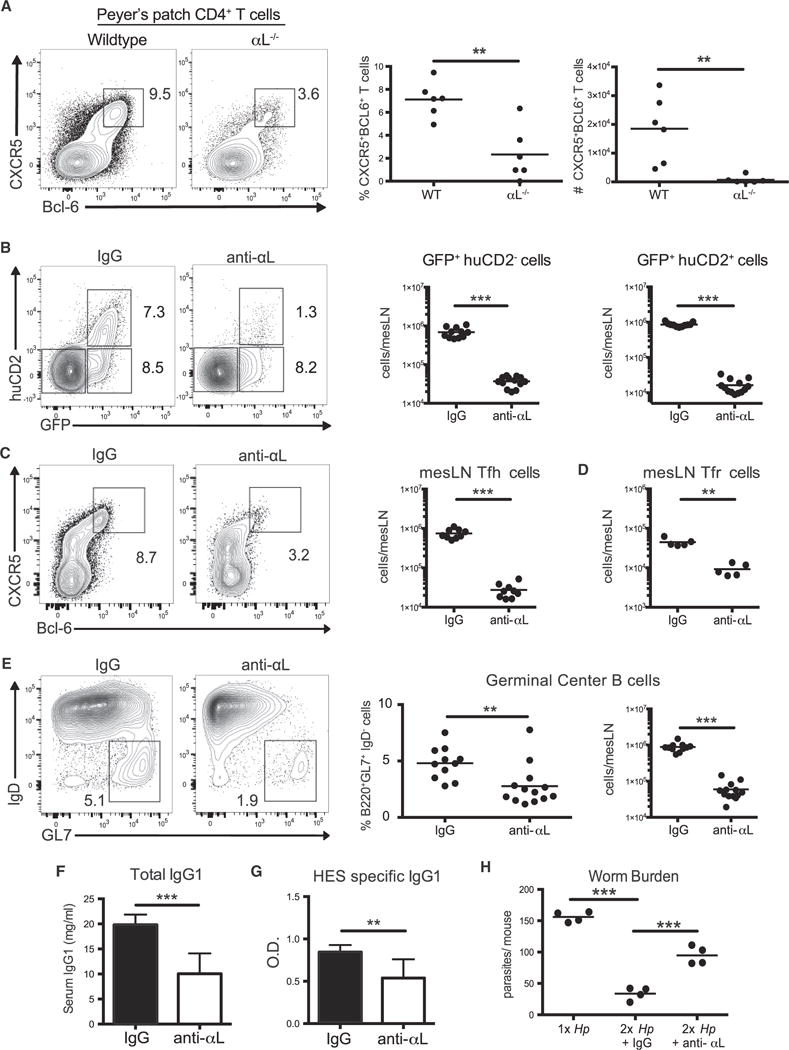
(A) Frequency and number of CXCR5+Bcl-6+ CD4+ cells from the Peyer’s patches of uninfected WT and αL-deficient mice. Representative contour plots are gated on CD4+B220−T cells.
(B–E) 4get/KN2 mice were administered 100 μg of anti-αL antibody or rat IgG i.p. from days 0–7 post-Hp infection. mLN cells were analyzed at day 7 post-infection. (B and C) Representative contour plots and cell counts of (B) GFP+huCD2−, GFP+huCD2+, and (C) CXCR5+Bcl-6+ mLN cells gated on the CD4+B220−population. (D) Total mLN CD4+Foxp3+CXCR5+PD-1+ cell counts.
(E) Representative contour plots, frequencies and cell counts of GL7+IgD−mLN cells gated on B220+CD4−mLN cells. (F and G) Total and HES-specific serum IgG1 from Hp-infected 4get/KN2 mice treated with rat IgG or anti-αL antibody from days 0–14 post infection.
(H) Hp-infected 4get/KN2 mice were treated as in (F) and at 4 weeks post-infection, mice were cured and challenged 2 weeks later with 200 L3 Hp larvae. 14 days post-secondary infection, mice were sacrificed and worm burden in the small intestine was determined.
(A–G) Representative data compiled from two or three independent experiments. (H) Representative data from two independent experiments. Dots in graph represent individual mice. ***p < 0.001, **p < 0.01, and *p < 0.05. Error bars, SD; ns, not significant. See also Figure S2.
The generation of both GC B cells and protective antibodies requires Tfh-derived signals (Crotty, 2011). Consistent with a loss of Tfh cell differentiation during LFA-1 blockade, the frequency and number of B220+GL7+IgD−GC B cells was significantly decreased compared to control-treated mice (Figure 3E). As parasite-specific immunoglobulin G1 (IgG1) is critical for protective immunity against Hp (Pritchard et al., 1983), we assessed the production of antibodies reactive against Hp larvae excretory-secretory (HES) antigens (Hewitson et al., 2011). Under these conditions, generation of both total and HES-specific IgG1 required LFA-1 (Figures 3F and 3G). We next tested whether LFA-1 blockade during primary infection would compromise protection upon reinfection. Indeed, mice treated with anti-LFA-1 during primary infection carried a significantly higher worm burden after reinfection (Figure 3H). Therefore, LFA-1 signaling during the primary immune response is indispensable for Tfh cell differentiation and the generation of protective humoral immunity to intestinal parasitic infection.
Tfh Cell Differentiation following Immunization Requires LFA-1
Because we found that LFA-1 mediates Tfh cell differentiation following Hp infection, we next evaluated another model of immune challenge in which entry and egress to the site of immune priming is independent of LFA-1 (Nolte et al., 2002). Parenteral sheep red blood cell (SRBC) immunization induces a Tfh cell-dependent GC response in the spleen 7 to 9 days post-challenge (Yu et al., 2009). Consistent with our Hp infection studies, LFA-1 blockade resulted in a substantial decrease in the frequency and number of Tfh cells at day 9 post-immunization (Figure 4A–4C). By contrast, there was an increase in the number of Teff cells (Figure 4C). Additionally, there was a complete inhibition of a GC reaction in anti-αL treated mice (Figure 4D). To determine whether the effect of LFA-1 blockade on Tfh cell differentiation was T cell-intrinsic, we purified CD4+ T cells from WT or αL-deficient mice and transferred them into TCR-β-deficient mice prior to SRBC immunization. In this setting, Tfh cell differentiation and GC B cell formation, but not Teff cell generation, were compromised in the absence of T cell expression of LFA-1 (Figures 4E and 4F). Thus, LFA-1 is required for the differentiation of Tfh cells across multiple settings of immune challenge and occurs in a CD4+ T cell-intrinsic manner.
Figure 4. Tfh Cell Differentiation following Immunization Requires LFA-1 in a Cell-Intrinsic Manner.
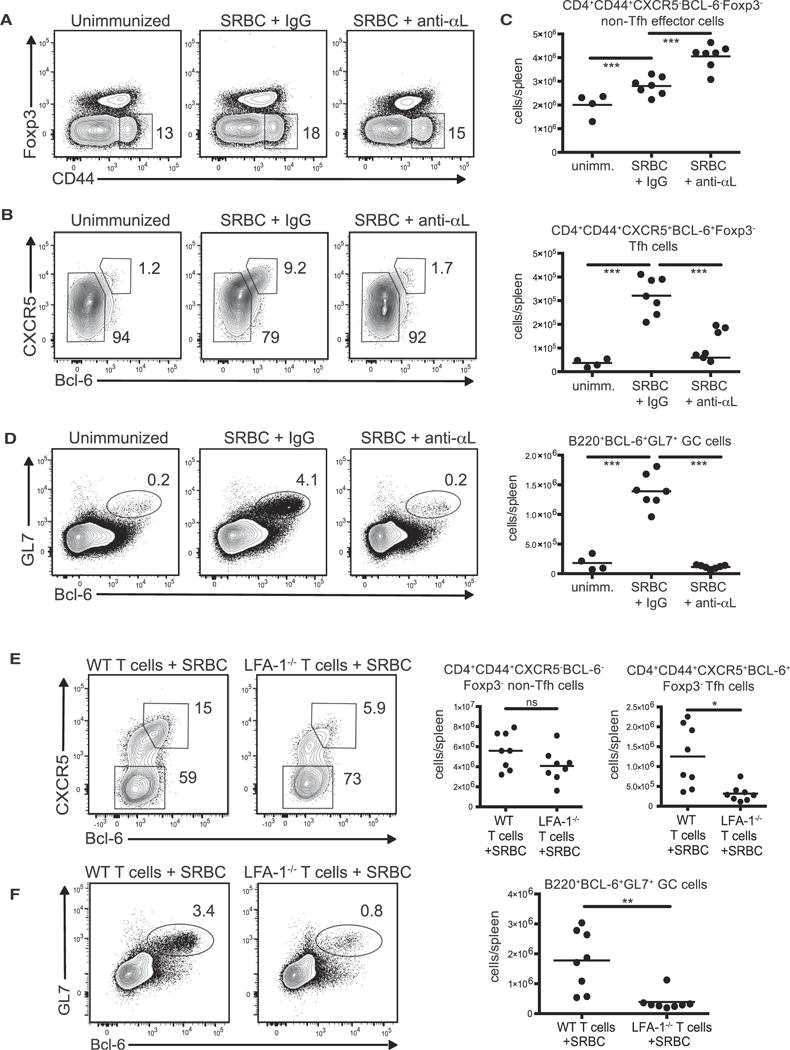
(A–D) C57/BL6 mice were immunized with 2 × 109 SRBCs i.p. and treated with anti-αL antibody or rat IgG every other day until harvest at day 9 post-immunization. (A) Representative contour plots of CD4+B220−splenocytes gating on the Foxp3−CD44hi population. (B) Contour plots from the CD44+Foxp3−gate shown in (A). (C) Cell counts of CD4+CD44hiBcl-6−CXCR5−Foxp3−non-Tfh effector subset (top graph) and the CD4+CD44hiBcl-6+CXCR5+Foxp3−Tfh cell subset (bottom graph) based on the gating in (B). (D) Representative contour plots and cell counts of GL7+Bcl-6+ splenocytes gated from B220+ cells.
(E and F) 2 × 106 purified CD4+ T cells from either naive C57/BL6 or Itgal−/−mice were transferred into TCRβ-deficient mice. 24 hr later, recipient mice were immunized with 2 × 109 SRBCs i.p. and spleens were harvested 9 days post-immunization. Representative contour plots and cell counts are shown of the (E) CD4+CD44hiBcl-6+CXCR5+Foxp3−Tfh or CD4+CD44hiBcl-6−CXCR5−Foxp3−non-Tfh and (F) B220+GL7+Bcl-6+ GC B cells. The numbers in each contour plot represent cell frequency. All the data shown are pooled from two independent experiments with 3–7 mice per group per experiment. Dots in graph represent individual mice. ***p < 0.001, **p < 0.01, and *p < 0.05. ns, not significant.
LFA-1 Is Required for Early Tfh and Th2 Cell Differentiation following Hp Infection
Our initial assessment of the Tfh cell response was performed at the time of the peak GC response. However, prior studies have shown a defect in early IL-2 and IFN-γ production from CD4+ T cells lacking the β2 integrin subunit of LFA-1 (Kandula and Abraham, 2004). Therefore, we questioned whether early events in T cell differentiation prior to GC formation were also dictated by LFA-1 signals. For these studies, we returned to Hp infection of 4get/KN2 mice where both Th2 and Tfh cell expansion can be observed. A kinetic analysis indicated that day 5 post-infection was the earliest time point in which an increase in GFP and huCD2 as well as lineage-specific transcription factor expression was detectable in CD44+CD4+ T cells (Figures 5A and S3A–S3F). Of note, this time point preceded GC development, allowing us to separate the impact of LFA-1 on T cell differentiation from T-GC B cell dynamics (Figures 5B and S3C). Consistent with a prior study examining IL-2 production as a readout for early T cell activation following immunization (Kandula and Abraham, 2004), we similarly found that LFA-1 blockade abrogated the generation of IL-2 producing CD44hiGFP+ Teff cells at day 5 post-infection (Figure 5C). In addition, development of both IL-4 producing Tfh cells and Gata3+ Th2 cells was abrogated in animals treated with anti-αL antibodies from the onset of infection (Figures 5D–5F). To confirm that LFA-1 impinges on DC-dependent T cell priming, we repeated these experiments in 4get/KN2/μMT mice lacking mature B cells. The absence of B cells compromised the total number of GFP+huCD2+ and CXCR5+Bcl-6+ Tfh cells without affecting the number of Gata3+ Th2 cells generated during infection. However, LFA-1 blockade further decreased both early Tfh and Th2 cell differentiation (Figures 5G–5I). Collectively, these results indicate that whereas acute LFA-1 blockade at the peak of the Tfh cell response plays a selective role in Tfh cell maintenance, LFA-1 plays a broader role in early B cell-independent Tfh and Th2 differentiation events during infection.
Figure 5. LFA-1 Is Required for Early Tfh and Th2 Cell Differentiation following Hp Infection.
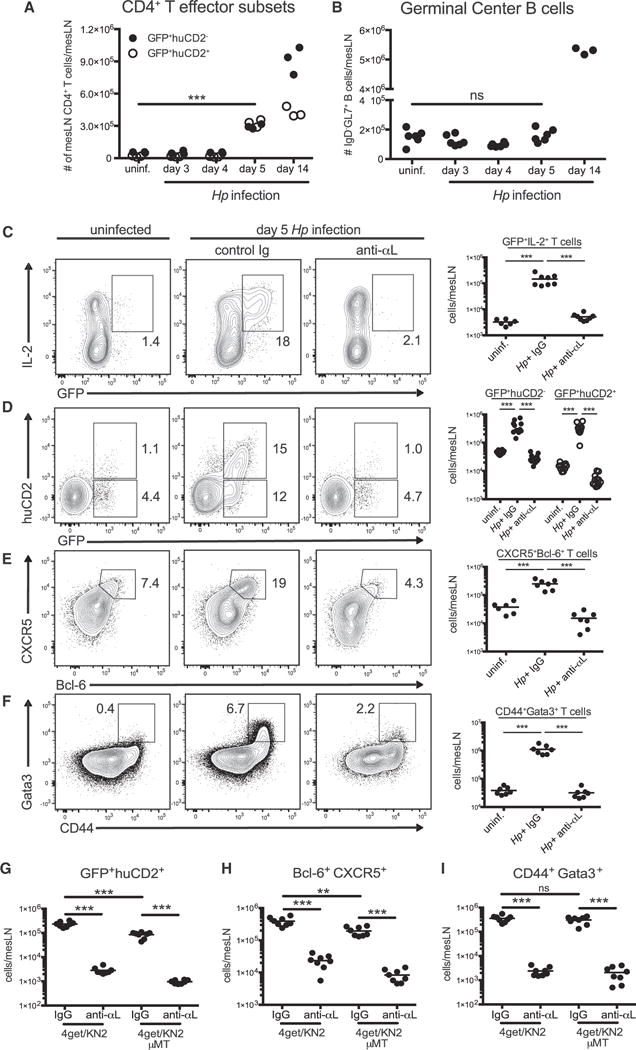
4get/KN2 mice were infected with 200 L3 Hp larvae and mLNs were harvested at various time points post infection. (A) CD4+GFP+huCD2−, CD4+GFP+huCD2+, and (B) B220+IgD−GL7+ mLN cell counts at the time points indicated post infection.
(C–I) 4get/KN2 or 4get/KN2 μMT−/−mice were administered 100 μg of anti-αL antibody or rat IgG i.p. from days 0–5 post-Hp infection and mLN cells were analyzed at day 5 post-infection. mLNs from uninfected 4get/KN2 were included as controls. Representative contour plots and total cell counts of (C) GFP+IL-2+ cells, (D) GFP+huCD2−, and GFP+huCD2+, (E) CXCR5+Bcl-6+ subsets from the CD44hiCD4+Foxp3−B220−population and (F) CD44+Gata3+ T cell subset. The numbers in each contour plot represent cell frequency.
(G–I) Total cell counts of the indicated T cell subsets from 5 day Hp-infected 4get/KN2 or 4get/KN2/μMT mice treated as above. Representative data compiled from two or three independent experiments with 3–5 mice per group. Dots in graph represent individual mice. ***p < 0.001, **p < 0.01. ns, not significant.
Tfh Cells Express a Highly Active Form of LFA-1
TCR and/or chemokine receptor stimulation triggers conformational changes to LFA-1, referred to as inside-out signaling, that increase affinity for ligands such as ICAM-1 (Evans et al., 2009). Because both Teff and Tfh cells expressed high amounts of LFA-1 subunits, we tested whether LFA-1 affinity was enhanced in Tfh cells compared to other CD4+ T cell subsets. To this end, mLN cells were isolated from Hp-infected 4get/KN2 mice and incubated with soluble ICAM1-Fc. Tfh cells bound significantly more ICAM-1 compared to both GFP− naive T cells and GFP+huCD2−T cells in a dose-dependent fashion. This interaction was specific as the addition of anti-αL blocking antibodies neutralized ICAM-1 binding (Figure 6A). Given the ability of TCR signaling to induce inside-out activation of LFA-1, we questioned whether TCR signal strength might be regulating ICAM-1 binding. However, expression of Nur77, an early response gene induced by TCR signaling, did not differ between GFP+huCD2−and GFP+huCD2+ T cells (Figure 6B). Similar results were obtained when examining TCR-β downregulation and CD5 expression as additional indicators of TCR signal strength (Lee et al., 1997; Mandl et al., 2013) (Figure 6B). However, a previous study demonstrated that low affinity LFA1-ICAM1 interactions stimulates activation of Zap70, a kinase also downstream of TCR signals, leading to a high-affinity conformation of LFA-1 (Evans et al., 2011). Consistent with this work, we found significantly greater Zap70 phosphorylation in Bcl-6+ Tfh cells compared to Teff and naive T cells (Figure 6C). These results suggest that TCR signal strength alone does not explain the increased LFA-1 affinity in Tfh cells, but that these pathways might synergize in a Zap70-dependent manner to enhance LFA-1 affinity and promote Tfh cell differentiation and maintenance.
Figure 6. Talin-1 Dependent LFA-1 Activation Enhances Bcl-6 Production by CD4+ T cells.
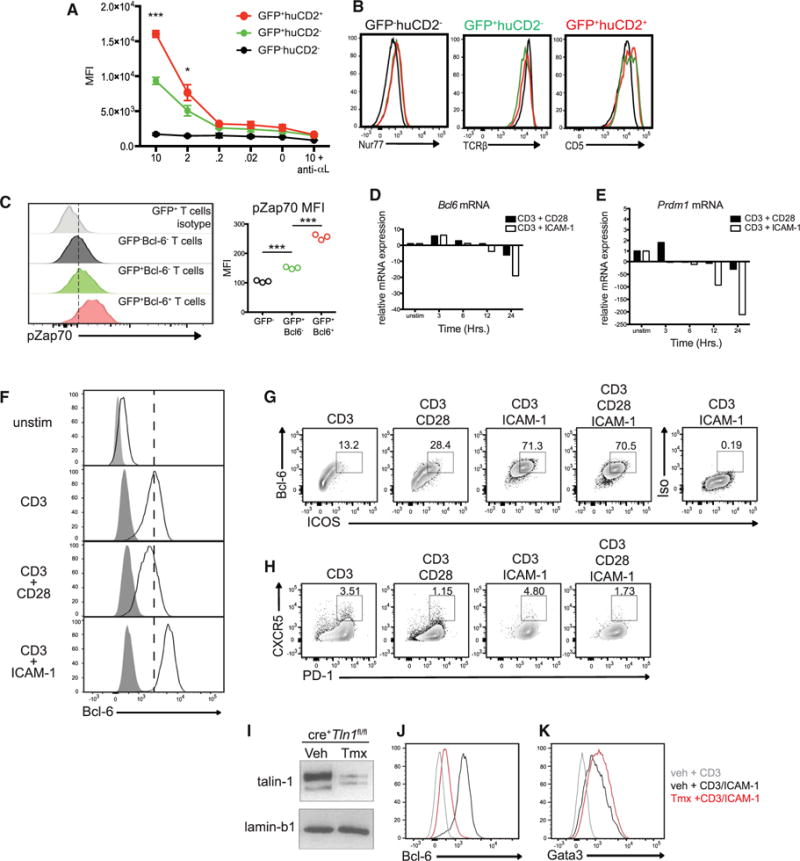
(A–C) 4get/KN2 mice were infected with 200 L3 Hp larvae and mLNs were harvested at 2 weeks post-Hp infection. (A) mLN cells were incubated in the presence of varying concentrations of ICAM-1-Fc ± anti-αL blocking antibody. ICAM-1 binding by the indicated T cell subsets was quantified by detection of bound ICAM-1-Fc by anti-human Fc-PE using flow cytometry. The x axis indicates the concentration of rICAM-Fc (μg/ml). Asterisks represent p values between GFP+huCD2− and GFP+huCD2+ populations. (B) Representative histograms of Nur77, TCRβ, and CD5 expression by the indicated CD4+ T cell populations. (C) Quantification of ZAP-70 activation (pZAP70) of the indicated CD4+ cell subsets using phosflow cytometry.
(D and E) Expression of (D) Bcl6 and (E) Prdm1 mRNA from purified CD62L+CD44low CD4+ T cells stimulated with anti-CD3 and anti-CD28 or anti-CD3 and ICAM-1 for various times measured by real-time RT-PCR. Data are shown relative to unstimulated CD62L+CD44low CD4+ T cells and normalized to Hprt.
(F–H) Purified CD62L+CD44low CD4+ T cells from WT mice were cultured for 72 hr with various combinations of plate-bound anti-CD3, anti-CD28, and/or recombinant ICAM-1 and assessed by flow cytometry for the indicated markers. In (F), open histograms indicate Bcl-6 expression and shaded histograms represent isotype control staining. Numbers shown in (G) and (H) indicate frequency of Bcl-6+ICOS+ or CXCR5+PD-1+ cells.
(I) Immunoblot of Talin-1 (225 and 190 kDa) and Lamin B1 (67 kDa) from total protein extracted from purified CD62L+CD44low CD4+ T cells from cre+Tln1fl/fl mice 7 days post-tamoxifen or vehicle treatment.
(J and K) CD62L+CD44low CD4+ T cells purified from ert2-cre+Tln1fl/fl mice 7 days post-tamoxifen or vehicle treatment were cultured for 72 hr with various combinations of plate bound anti-CD3 and/or recombinant ICAM-1. Histograms show Bcl-6 (J) or Gata3 (K) expression under the indicated conditions. All data shown are representative of two or three independent experiments. ***p < 0.001 and *p < 0.05.
Talin-1 Dependent LFA-1 Activation Enhances Bcl-6 Production by CD4+ T Cells
The importance of LFA-1 in early T cell activation events led us to perform a series of in vitro studies to determine whether T cell activation in the presence of LFA-1 stimulation was sufficient to induce Bcl-6 expression. To this end, naive T cells were stimulated with anti-CD3 in the presence of the LFA-1 ligand, ICAM-1, and Bcl6 mRNA expression was examined over time. Bcl6 transcription increased early and transiently regardless of stimulating conditions (Figure 6D). At later time points, however, stimulation with anti-CD3 and ICAM-1 lead to a greater reduction of Bcl6 mRNA compared to anti-CD3 and anti-CD28 stimulation, consistent with the self-regulating capacity of Bcl-6 protein (Hatzi et al., 2015). As Bcl-6-mediated antagonism of Blimp-1 is required for Tfh cell differentiation (Johnston et al., 2009), we measured Prdm1 mRNA (the gene encoding Blimp-1) in parallel. As shown in Figure 6E, Prdm1 mRNA expression increased slightly upon anti-CD3 and anti-CD28 stimulation early, but was not sustained. By contrast, ICAM-1 co-stimulation rapidly inhibited Prdm1 transcription over time in comparison to CD28 costimulation (Figure 6E). These transcription profiles suggested that ICAM-1 co-stimulation might result in greater Bcl-6 protein expression. Indeed, naive T cells stimulated with anti-CD3 and ICAM-1 enhanced Bcl-6 protein expression in comparison to anti-CD3 alone or anti-CD3 and anti-CD28 stimulation (Figure 6F) and this occurred in an LFA-1-dependent manner (Figure S4A). The addition of ICOS staining revealed that LFA-1 stimulation led to a greater frequency of Bcl-6+ICOS+ T cells compared to other groups (Figure 6G). Although somewhat variable, expression of the classical Tfh cell markers CXCR5 or PD-1 did not differ between CD28 or LFA-1 stimulated cells (Figure 6H). Notably, ICAM-1 stimulation alone was not sufficient to upregulate Bcl-6, indicating that the activity of LFA-1 is linked to TCR-dependent activation (Figures S4B and S4C). Thus, T cell stimulation in the context of LFA-1 activation enhances Bcl-6 expression.
Given the selective expression of a highly active form of LFA-1 by Tfh cells compared to Teff cells, we questioned whether directly modulating integrin affinity might impact Bcl-6 expression. To this end, we took advantage of mice expressing conditional alleles for the gene encoding Talin-1 (Tln1fl/fl), a cytoplasmic scaffolding protein required for the change of LFA-1 to its high-affinity conformation (Petrich et al., 2007). These mice were crossed to animals expressing a tamoxifen-inducible cre recombinase under control of the ubiquitously expressed Rosa26 locus (hereafter referred to as ert2-cre+Tln1fl/fl). Oral tamoxifen treatment of ert2-cre+ Tln1fl/fl mice resulted in efficient deletion of Talin-1 in CD4+ cells in the spleen by 7 days post-treatment (Figures 6I and S4D). Naive T cells from the spleens of tamoxifen or vehicle-treated ert2-cre+ Tln1fl/fl mice were cultured in the presence of anti-CD3 and ICAM-1 and Bcl-6 expression was assessed 72 hr later. Consistently, control T cells increased Bcl-6 expression upon anti-CD3 and ICAM-1 stimulation (Figure 6J). However, Talin-1-deficient T cells failed to express Bcl-6 under the same conditions. By contrast, loss of Talin-1 did not compromise the ability of T cells to upregulate the Th2 transcription factor Gata3 despite a slight decrease in the proliferation of Talin-1 deficient T cells (Figures 6K and S4E). These results indicate that LFA-1 stimulation in the context of TCR signaling specifically enhances Bcl-6 expression in a Talin-1 dependent manner.
Talin-1 Dependent Integrin Activation Is Selectively Required for Tfh Cell Differentiation during Helminth Infection
To determine whether our in vitro results translated to Th cell differentiation in vivo, purified CD4+ T cells were isolated from peripheral lymph nodes of ert2-cre+ Tln1fl/fl or ert2-cre+ Tln1fl/+ controls and transferred into TCRβ-deficient mice. Recipient mice were administered tamoxifen and 1 week later infected with Hp. Consistent with our anti-αL blockade data, Tfh cell differentiation was significantly reduced in ert2-cre+ Tln1fl/fl mice compared to ert2-cre+ Tln1fl/+ controls (Figures 7A and 7B). However, Talin-1 was not required for the induction of other CD4+ effector subsets including Gata3+ Th2 cells (Figures 7C and 7D; Figure S5F). Notably, Talin-1 deletion in CD4+ T cells also compromised the humoral response as determined by a significant decrease in the number of GC B cells, as well as total and parasite-specific IgG1 titers (Figures 7E–7G). Collectively, these data indicate the Talin-1 dependent integrin activation is an important determinant in selectively promoting Tfh cell differentiation compared to other Teff cells during Hp infection.
Figure 7. Talin-1 Is Selectively Required for Tfh Cell Differentiation during Hp Infection.
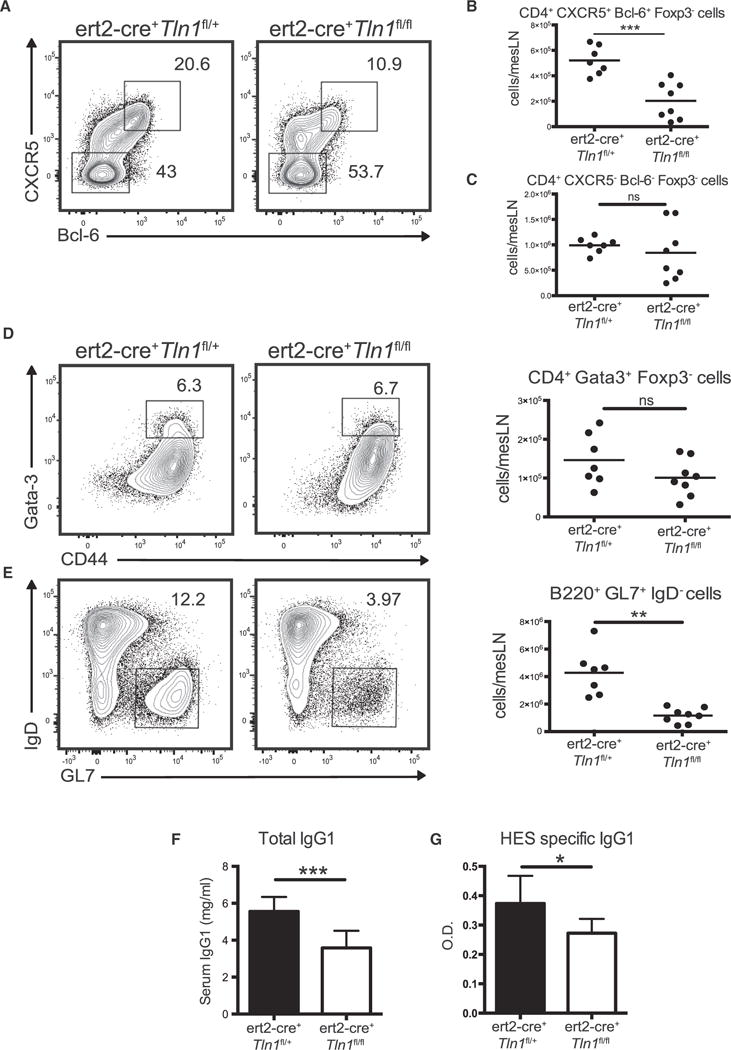
(A–G) 2 × 106 purified CD4+ T cells from either naive ert2-cre+Tln1fl/fl or ert2-cre+Tln1fl/+ mice were transferred to TCRβ-deficient mice. Recipient mice were administered tamoxifen and 7 days later infected with Hp. The data shown are from 2 weeks after Hp infection. (A) Representative contour plots of CD44hiFoxp3−B220−CD4+ T cells gating on CXCR5+Bcl-6+ and CXCR5−Bcl-6−subsets. (B and C) Total cell counts of populations gated in (A). (D) Representative contour plots and total cell counts of CD44hiGata3+ cells from CD4+Foxp3−B220−mLN cells. (E) Representative contour plots and total cell counts of GL7+IgD−cells from the B220+ population. (F) Total and (G) HES-specific serum IgG1. All graphs consist of data compiled from two independent experiments with three or four mice per group. The numbers in each contour plot represent cell frequency. Dots in graphs represent individual mice. ***p < 0.001, **p < 0.01, and *p < 0.05. Error bars, SD ns, not significant.
DISCUSSION
By examining T-dependent humoral immune responses in the context of both intestinal parasite infection and parenteral immunization, we have detailed the impact that integrins, particularly LFA-1, have on Tfh cell differentiation and maintenance within the lymph node microenvironment. Although CD4+ T cell differentiation is a multi-factorial process strongly influenced by the presence of polarizing cytokines in conjunction with TCR engagement, our data reinforce and provide new insight into the importance of co-stimulatory molecules as an integral determinant of T cell differentiation. Furthermore, our findings indicate that integrin activity, not only expression per se, influences lineage commitment during intestinal parasitic infection.
Our results build on prior studies showing that CD4+ T cells from mice with dysfunctional LFA-1 signaling have a compromised ability to promote antigen-specific antibody responses (Semmrich et al., 2005). Using Hp infection, which induces a robust Tfh and Th2 cell response, we demonstrate that LFA-1 plays a critical role in both Tfh and Th2 cell differentiation during the early stages of T cell differentiation while, at later stages of the humoral immune response, it was required for Tfh cell survival. One explanation for the latter result is the unique localization of Tfh cells within the GC. The constant integration of signals by Tfh cells resulting from persistent antigen stimulation and intercellular adhesion are essential to ensure proper localization and help for GC B cell selection and differentiation. Indeed, MHCII-restricted engagement with B cells is required to maintain T cell expression of the master regulator of Tfh cell differentiation, Bcl-6 (Goenka et al., 2011). Another component unique to the GC is the presence of follicular dendritic cells (FDCs), a mesenchymal-derived stromal population important for antigen retention in the light zone of the germinal center. Previous work in humans and mice has indicated a combined role for both LFA-1 and VLA-4 integrins in regulating B cell conjugate formation with FDCs to promote GC B cell survival (Koopman et al., 1991; Wang et al., 2014). In contrast, we find that LFA-1 alone plays a nonredundant role in maintaining Tfh cells. Future studies should determine the critical LFA-1 ligands expressed by both hematopoietic and non-hematopoietic cells that regulate Tfh cell function.
Many groups have investigated the role of cytokines as critical determinants of Tfh cell differentiation in a manner similar to other T cell lineages. Although specific cytokines have been clearly shown to promote Tfh cell differentiation in vitro and in vivo (Eto et al., 2011; Ma et al., 2009; Schmitt et al., 2014), the importance of pathways other than those related to cytokine signaling might be particularly relevant when considering mechanisms underlying Tfh cell differentiation. To be sure, the cytokine repertoire is context-dependent, whereas diverse types of immune challenges give rise to T-dependent humoral immune responses suggesting that polarizing cytokines cannot be the only determinants of Tfh cell differentiation. Indeed, co-stimulatory molecules also play distinct roles that influence Tfh cell differentiation including CD28, ICOS, and SLAM family members (Bachmann et al., 1997; Linterman et al., 2014; Qi et al., 2008; Xu et al., 2013).
During early T cell priming, we determined that neutralization of LFA-1 at the T cell priming stage compromises early DC-dependent differentiation events required for Th2 and Tfh lineage commitment following intestinal parasitic infection. Although we found that LFA-1 was critical for Tfh cell differentiation across different types of immune challenge, the importance of LFA-1 in early T cell activation events might depend on the setting of antigen encounter. Indeed, we observed a limited role for LFA-1 in non-Tfh effector cell generation in the spleen of systemically immunized animals. Understanding how tissue-specific signals can modulate T cell responsiveness to integrins is an exciting area of future investigation.
Integrins change conformation via inside-out TCR signaling and enhance TCR signal strength via increased adherence to antigen-presenting DCs or B cells. Thus, a TCR signal of sufficient strength would drive an efficient increase in LFA-1 affinity leading to a feed-forward mechanism amplifying T-APC interactions and Tfh cell differentiation. Indeed, persistent and/or repeated interaction with antigen-presenting cells is required for maintenance of Bcl-6 expression and commitment to the Tfh cell lineage (Deenick et al., 2010). In support of this model, TCR-triggered binding of LFA-1 to ICAM-1 led to enhanced expression of Bcl-6 in vitro compared to CD28-mediated costimulation. However, deletion of Talin-1, a cytosolic protein recruited to the β2 integrin subunit following inside-out activation and required for acquisition of the high-affinity conformation of LFA-1, compromised Bcl-6 expression in vitro and Tfh cell differentiation in vivo, but did not impact the activation of T cells and their ability to commit to other Teff lineages such as Gata3+ Th2 cells (Kinashi, 2012). As Talin-1 is additionally required for the high-affinity conformation of β1 and β3 integrins (Petrich et al., 2007), we cannot exclude the possibility that loss of activity in these integrins contribute to the phenotype observed in our in vivo experiments. However, β1 and β3 are not highly expressed by Tfh cells during Hp infection. Thus our data reveal a previously unknown role for Talin-1 and integrin activation in influencing T helper cell differentiation and lineage commitment.
In conclusion, this work provides new insight into the role of integrins in regulating CD4+ T cell differentiation and humoral immune responses. Given the conserved expression profile between mouse and human Tfh cells, LFA-1 might be a relevant target for modulating human T-dependent humoral immune responses in scenarios where this cell type plays an important role (Tangye et al., 2013). Although developing reagents that promote LFA-1 activity and its associated signaling pathways might be beneficial for vaccine development, inhibition of integrin signaling might also be useful to limit Tfh cell-dependent antibody production in the context of antibody-mediated autoimmune disorders or transplant rejection.
EXPERIMENTAL PROCEDURES
Mice
4get/KN2, Tcrb−/−, ert2-cre+ Tln1fl/fl, ert2-cre+ Tln1fl/+, and Itgal−/− (αL-deficient, LFA-1−/−) mice on a C57BL/6 background were bred and housed under specific-pathogen-free conditions. Mice were used at 6–16 weeks of age, and all experiments were approved by the McGill University Animal Care Committee.
Flow Cytometry
Murine single cell suspensions of mLN, spleen, and pp were processed as described (Meli and King, 2015). Data were acquired with a FACS Canto II or LSR Fortessa (BD Biosciences) and analyzed using FlowJo software.
Human Tonsil Samples
Human tonsils were obtained from routine tonsillectomy at the Mater Hospital (North Sydney, NSW, Australia). Mononuclear cells were isolated from human tonsils and labeled as previously described (Ma et al., 2009). Anti-αL (clone HI111, Affymetrix) and anti-β2 antibodies (clone L130, BD) were used for integrin expression analysis. Data were acquired on an LSR-II flow cytometer and analyzed using FlowJo (Treestar, Ashland, OR). Institutional Human Research Ethics Committees approved all studies.
Hp Infection and Antibody Treatment
Mice were infected by gavage with 200 L3 Hp larvae and harvested at the indicated time points. For protection experiments, adult Hp were eliminated 4 weeks post infection with two doses of 100 mg/kg pyrantel pamoate and subsequently challenged with 200 larvae. In some experiments, mice were administered anti-αL (M17/4), anti-β2 (GAME-46), rat IgG (100 μg), or FTY720 (20 μg, Cayman Chemical) i.p. at various time points post-Hp infection or SRBC immunization.
SRBC Immunization
10% packed sheep red blood cells (Cedarlane Technologies) were washed and resuspended in PBS and 2 × 109 SRBCs were injected into the peritoneum of adult mice.
Confocal Microscopy
mLNs were harvested from 2 week infected 4get/KN2 mice and processed as previously described (King and Mohrs, 2009). Images were taken using an Olympus FV1000 confocal microscope and analyzed using ImageJ software.
Cell Culture
Naive CD62L+CD4+ T cells from the peripheral lymph nodes or splenocytes from C57BL/6 or ert2-cre+Tln1fl/fl mice were processed into single cell suspensions and stimulated as described.
Immunoblotting
Total lymphocytes or cell-sorted naive CD4+ T cell lysates (15 μg) were resolved by 5% SDS-PAGE and transferred to nitrocellulose (BioRad). Membranes were probed with primary antibodies against Talin-1 (Sigma-Aldrich) or Lamin B1 (Santa Cruz) overnight at 4°C. Signals were detected using a horseradish peroxidase–labeled anti-mouse IgG (BioRad) and enhanced chemiluminescence (ECL; PerkinElmer) on HyBlot CL film (Harvard Apparatus).
ELISAs
Total serum IgG1 was measured using anti-mouse IgG1 antibody pairs as previously described (King et al., 2010). HES protein was prepared as previously described (Valanparambil et al., 2014). To detect HES-specific IgG1, we coated 96-well flat-bottom plates with 1 μg/ml of HES protein and incubated at 4°C overnight. Serum samples were subsequently added and antibodies were detected using rat anti-mouse IgG1-Biotin (SB77E) and Streptavidin-HRP (Southern Biotech).
qPCR
mRNA was extracted using TRIzol (Sigma) and cDNA samples were prepared as previously described (King and Mohrs, 2009). Quantitative real-time RT-PCR was performed using sybr green detection (Bcl6, Prdm1, and Hprt) or taqman probes (S1p1 and Gapdh) using the Biorad CFX96™ Real-Time System or Applied Biosystems Taqman 7500 Fast System and software. Fold expression was calculated using the ΔΔCT method and Gapdh or Hprt as reference genes.
Supplementary Material
Highlights.
Mouse and human Tfh cells have high expression of LFA-1
Survival of Tfh cells during the germinal center reaction requires LFA-1
LFA-1 is required for Tfh cell development and immunity to helminth infection
Tfh cell differentiation selectively requires Talin-1-dependent integrin activation
Acknowledgments
The authors would like to thank Camille Stegen for her management of the MIMM flow cytometry facility and members of the King laboratory for their technical input. This work was supported by the Canadian Institute of Health Research (CIHR) operating grant (MOP-130579) and McGill University Faculty of Medicine. I.L.K. also holds a Canada Research Chair in Humoral Immunity. A.P.M. received support from the Fonds de recherche du Québec – Santé and Fonds de recherche du Québec – Nature et Technologies.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2016.09.018.
AUTHOR CONTRIBUTIONS
A.P.M. performed experiments, analyzed data, and wrote the manuscript. G.F., D.T.A., S.A.L., and A.B.-T. performed experiments and analyzed data. M.T. and M.M.S. provided reagents and technical input. M.E. provided tonsil samples. J.M., D.J.F., and S.G.T. provided reagents, data, and conceptual input. I.L.K. designed the project, performed experiments, analyzed data, and wrote the manuscript.
References
- Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey C, Croft M, Swain SL. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- Evans R, Lellouch AC, Svensson L, McDowall A, Hogg N. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood. 2011;117:3331–3342. doi: 10.1182/blood-2010-06-289140. [DOI] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Durandy A, Sterkers G, Griscelli C. Role of the LFA-1 molecule in cellular interactions required for antibody production in humans. J Immunol. 1986;136:3198–3203. [PubMed] [Google Scholar]
- Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, Laufer TM. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011;187:1091–1095. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson JP, Filbey KJ, Grainger JR, Dowle AA, Pearson M, Murray J, Harcus Y, Maizels RM. Heligmosomoides polygyrus elicits a dominant nonprotective antibody response directed against restricted glycan and peptide epitopes. J Immunol. 2011;187:4764–4777. doi: 10.4049/jimmunol.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–4451. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- Kinashi T. Overview of integrin signaling in the immune system. Methods Mol Biol. 2012;757:261–278. doi: 10.1007/978-1-61779-166-6_17. [DOI] [PubMed] [Google Scholar]
- King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Mohrs K, Mohrs M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J Immunol. 2010;185:6138–6145. doi: 10.4049/jimmunol.1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, Round JL. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe. 2015;17:153–163. doi: 10.1016/j.chom.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Cossoy MB, Chau LA, Singh B, Madrenas J. Inactivation of lck and loss of TCR-mediated signaling upon persistent engagement with complexes of peptide:MHC molecules. J Immunol. 1997;159:61–69. [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vinuesa CG. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Denton AE, Divekar DP, Zvetkova I, Kane L, Ferreira C, Veldhoen M, Clare S, Dougan G, Espéli M, Smith KG. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. eLife. 2014;3:3. doi: 10.7554/eLife.03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naϊve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Meli AP, King IL. Identification of mouse T follicular helper cells by flow cytometry. Methods Mol Biol. 2015;1291:3–11. doi: 10.1007/978-1-4939-2498-1_1. [DOI] [PubMed] [Google Scholar]
- Morrison VL, Uotila LM, Llort Asens M, Savinko T, Fagerholm SC. Optimal T Cell Activation and B Cell Antibody Responses In Vivo Require the Interaction between Leukocyte Function-Associated Antigen-1 and Kindlin-3. Journal of Immunology. 2015;195:105–115. doi: 10.4049/jimmunol.1402741. [DOI] [PubMed] [Google Scholar]
- Nolte MA, Hamann A, Kraal G, Mebius RE. The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunology. 2002;106:299–307. doi: 10.1046/j.1365-2567.2002.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T, Bloch W, Pabst O, Wickenhauser C, Uthoff-Hachenberg C, Schmidt SV, Varga G, Grabbe S, Kess D, Oreshkova T, et al. Adaptive immune response to model antigens is impaired in murine leukocyte-adhesion deficiency-1 revealing elevated activation thresholds in vivo. Clin Dev Immunol. 2012;2012:450738. doi: 10.1155/2012/450738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard DI, Williams DJ, Behnke JM, Lee TD. The role of IgG1 hypergammaglobulinaemia in immunity to the gastrointestinal nematode Nematospiroides dubius. The immunochemical purification, antigen-specificity and in vivo anti-parasite effect of IgG1 from immune serum. Immunology. 1983;49:353–365. [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt P, Patzak I, Jones K, Etemire E, Gunzer M, Hogg N. A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes. EMBO J. 2013;32:829–843. doi: 10.1038/emboj.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA, Craft JE, Nussenzweig MC. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- Valanparambil RM, Segura M, Tam M, Jardim A, Geary TG, Stevenson MM. Production and analysis of immunomodulatory excretory-secretory products from the mouse gastrointestinal nematode Heligmosomoides polygyrus bakeri. Nat Protoc. 2014;9:2740–2754. doi: 10.1038/nprot.2014.184. [DOI] [PubMed] [Google Scholar]
- Wang X, Rodda LB, Bannard O, Cyster JG. Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness. J Immunol. 2014;192:4601–4609. doi: 10.4049/jimmunol.1400090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SS, Sweatman JJ, Lawrence MB, Rhoades TH, Hart AL, Larson RS. Enhanced T-lineage acute lymphoblastic leukaemia cell survival on bone marrow stroma requires involvement of LFA-1 and ICAM-1. Br J Haematol. 2001;115:862–871. doi: 10.1046/j.1365-2141.2001.03182.x. [DOI] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


