Abstract
Background and objectives
An understanding of the health care resource use associated with AKI is needed to frame the investment and cost-effectiveness of strategies to prevent AKI and promote kidney recovery.
Design, setting, participants, & measurements
We assembled population-based cohort of adults hospitalized in Alberta between November of 2002 and March of 2009 without ESRD or an eGFR<15 ml/min per 1.73 m2. Outpatient serum creatinine measurements 6 months preceding admission defined baseline kidney function, and serum creatinine during the first 14 days of hospitalization defined Acute Kidney Injury Network stage; kidney recovery defined as serum creatinine within 25% of baseline and independence from dialysis was assessed at 90 days after AKI. Health care utilization and costs (in 2015 Canadian dollars) were determined from inpatient, outpatient, and physician claims datasets during the index hospitalization, recovery period (90 days post-AKI assessment), and 3–12 months post-AKI. A fully adjusted generalized linear model regression analysis was used to estimate costs associated with AKI.
Results
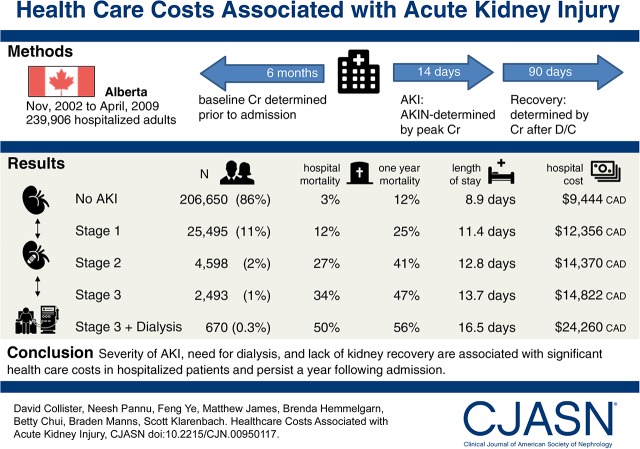
Of 239,906 hospitalized subjects, 25,495 (10.6%), 4598 (1.9%), 2493 (1.0%), and 670 (0.3%) had Acute Kidney Injury Network stages 1, 2, 3 without dialysis, and 3 with dialysis, respectively. Greater severity of AKI was associated with incremental increases in length of stay (+2.8; 95% confidence interval, 1.4 to 4.3 to +7.4; 95% confidence interval, 7.2 to 7.5 days) and costs (+$3779; 95% confidence interval, $3555 to $4004 to +$18,291; 95% confidence interval, $15,573 to $21,009 Canadian dollars) from admission to recovery assessment (3 months). At months 3–12 postadmission, compared with subjects without AKI, AKI with kidney recovery and AKI without kidney recovery were associated with incremental costs of +$2912–$3231 and +$6035–$8563 Canadian dollars, respectively. The estimated incremental cost of AKI in Canada is estimated to be over $200 million Canadian dollars per year.
Conclusions
Severity of AKI, need for dialysis, and lack of kidney recovery are associated with significant health care costs in hospitalized patients and persist a year after admission. Strategies to identify, prevent, and facilitate kidney recovery are needed.
Keywords: acute renal failure; chronic kidney disease; dialysis; costs; resource utilization; creatinine; Cost-Benefit Analysis; Inpatients; Outpatients; Linear Models; Alberta; Investments; Length of Stay; Acute Kidney Injury; Kidney Function Tests; Renal Insufficiency, Chronic; hospitalization; Health Care Costs; Kidney Failure, Chronic; Canada
Introduction
AKI is common, with an overall incidence of 22% in adult patients (1), which varies depending on the setting, population, and definition of AKI (2–6). AKI is strongly associated with an increased risk of morbidity, including cardiovascular events (7) and stroke (8), the development of both CKD (9) and ESRD (10), reduced health-related quality of life (11–14), and higher mortality (7,15).
The early detection, prevention, and treatment of AKI have been identified as research priorities (16). An understanding of the health care resource use associated with AKI is needed by decision makers to frame the investment and cost-effectiveness of potential strategies. Existing studies have limitations, including incomplete assessment of baseline kidney function before hospitalization, a lack of stratification by AKI severity and need for dialysis, a lack of consideration of kidney recovery, short-term follow-up limited to the index hospitalization, and focus on specific settings [e.g., intensive care unit (17,18)] or disease states [e.g., surgery (19), cardiovascular surgery (20–22), vascular surgery (23), malignancy (24,25), and infection (26,27)]; we did not identify studies that reflect a population-based perspective.
We sought to determine the economic implications of AKI from the health payer perspective using a population-based cohort in Alberta, Canada that has previously been used to described clinical outcomes (28,29). Specific inquiry of health care costs by AKI severity, need for dialysis, and kidney recovery status was performed.
Materials and Methods
The assessment of baseline kidney function, identification of AKI, assessment of comorbidity, and assessment of outcomes have been previously described (29). Briefly, discharge abstracts from a large integrated health region (catchment 1.8 million) within the province of Alberta, Canada were linked with provincial laboratory data to identify all inpatient and outpatient serum creatinine measurements. Patients with ESRD on dialysis as well as vital status (alive or dead) were determined by linkage with the Alberta Kidney Disease Network and Alberta Health databases, respectively, as previously described (30,31).
Study Cohort
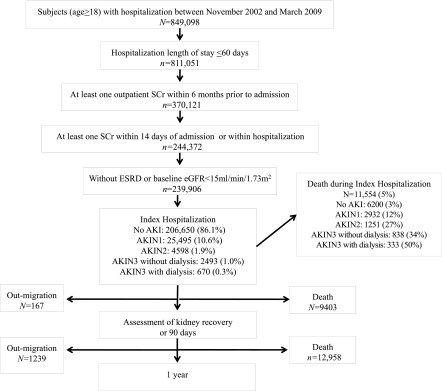
The study cohort included all adults >18 years old residing in Alberta who were admitted to the hospital between November 1, 2002 and March 31, 2009 with a least one outpatient serum creatinine measurement within 6 months before hospitalization to permit assessment of baseline kidney function, one or more measurements during the first 14 days of hospitalization, and one or more measurements in the period after hospitalization. If a patient had multiple hospitalizations, only the first hospitalization was considered (index hospitalization). Exclusion criteria included preexisting ESRD treated with dialysis or kidney transplant or baseline eGFR <15 ml/min per 1.73 m2. Index hospitalizations of >60 days were also excluded to avoid skewing of length of stay and costs by chronic care admissions (Figure 1).
Figure 1.
Study cohort. Study cohort size per time period as follows: N=239,906 for index hospitalization; N=239,739 for admission to assessment of kidney recovery or 90-day time period (239,906−167) exclusion of migration only; N=238,500 for admission to 1-year time period [239,906−(167+1239)] exclusion of migration only; and N=217,543 for assessment of kidney recovery or 90-day to 1-year time period [239,906−(167+1239)−(11,554+9403)] exclusion of migrations and deaths. AKIN, Acute Kidney Injury Network; SCr, serum creatinine.
Identification of AKI
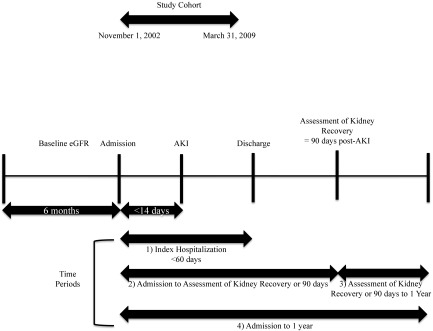
Serum creatinine measurements were standardized across laboratories, and eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation (32). Baseline kidney function was defined as the mean serum creatinine and eGFR obtained from all outpatient measurements within 6 months before the index hospitalization. Baseline kidney function was categorized as eGFR≥60, 45–59, 30–44, and <30 ml/min per 1.73 m2. AKI was identified by the change between baseline and peak serum creatinine observed within the first 14 days in hospital (Figure 2) and categorized using serum creatinine changes specified in Acute Kidney Injury Network (AKIN) criteria (33). The identification of AKI was limited to the first 14 days in hospital to focus on community- or early hospital–acquired AKI and late hospital–acquired AKI given the different patient populations. Patients requiring acute hemodialysis at any point during their index hospitalization with either intermittent hemodialysis or continuous RRT identified from procedure codes were classified as AKIN stage 3 with dialysis.
Figure 2.
Timeline.
Assessment of Recovery of Kidney Function
Kidney function among subjects who survived at least 90 days after hospital discharge was assessed using the serum creatinine drawn closest to 90 days after AKI (range =30–150 days) (Figure 2). Kidney recovery was defined as a post-AKI serum creatinine within 25% of the baseline (prehospitalization value) and independence from dialysis (33). The occurrence of ESRD treated with chronic dialysis posthospitalization was identified using the registries of the Northern and Southern Alberta Renal Programs as previously described (30,31). Previous sensitivity analyses for the assessment of baseline kidney function, AKI, and kidney recovery have been performed (29).
Time Periods
The following time periods were considered for analysis (Figure 2).
Index hospitalization from admission to discharge.
From the time of the index hospitalization admission to the assessment of kidney recovery or 90 days.
From the time of the assessment of kidney recovery or 90 days until 1 year after the index hospitalization admission.
One year after the index hospitalization admission.
Covariates
Demographic data, including age and sex, were determined from administrative data. Comorbid conditions were identified on the basis of validated International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision coding algorithms (34,35) applied to hospitalization discharge abstracts and physician claims data. Comorbidity was measured using the Charlson Comorbidity Index (36).
Mortality
Subjects who died before the time of assessment of kidney recovery were not included in the analyses of costs for the assessment of kidney recovery or 90 days until 1 year after the index hospitalization admission time period. Health care resources were captured for all patients, including those who died during all other time periods.
Costs
Alberta Health delivers publicly funded health services (such as physician services, hospital care, and laboratory testing) under universal health care coverage in the province of Alberta and captures patient-specific health care resource utilization (31). The primary datasets used to calculate costs of resource utilization included the following.
Alberta Health Care Insurance Plan Payment database for physician claims.
Ambulatory Care Classification System dataset. This is a high-quality dataset that captures all outpatient health resource use, including specialist clinic visits, visits to allied health care professionals (physiotherapists and occupational therapists), laboratory testing, diagnostic imaging, and outpatient medical or surgical procedures, including dialysis.
Inpatient database captures patient-specific encounters, including length of stay, in-hospital procedures, and resource utilization, on the basis of Canadian Institutes for Health Information (37) (CIHI) methodology (incorporating resource intensity weights and complexity), which allows for calculation of total costs.
Outpatient medication is provided by Alberta Blue Cross Drug Insurance but only to select patient groups, such as patients ≥65 years old and patients with ESRD; because of incomplete capture during this study period, outpatient medication costs were excluded from the analysis.
A unique patient identifier was used to link datasets, enabling estimation of total cumulative costs for each patient. Costs of inpatient care were determined using CIHI methods, which were developed to estimate average cost of services delivered to patients in all acute care facilities. The estimated cost corresponds to clinical services provided to the typical patients modified by complexity (38). The National Ambulatory Care Reporting System (39), developed by CIHI, includes cost data for all hospital- and community-based ambulatory care, including day surgery, outpatient and community-based clinics, and emergency departments. Physician fees were on the basis of compensation amounts for claims in Alberta that are paid for each service that they deliver. A subcategory of resource use specific to provision of nephrology care (physician claims by nephrologist, dialysis procedures, admission codes, and ambulatory care codes) was also determined (Supplemental Table 1).
Statistical Analyses
We constructed multivariable linear regression models to determine the association of AKI status with cumulative cost. We used generalized linear model regression with bootstrapping (40) to determine 95% confidence intervals (95% CIs) (41) to examine the association of AKI status with cost for each time period. All cost models were adjusted for age, sex, Charlson comorbidities, hospitalization primary diagnosis (case mix group), baseline eGFR, and albuminuria. A generalized linear model with log link was used to estimate the mean cost ratios, a ratio of the mean cost for those in the reference group (no AKI) compared with those in exposure groups, and the potential distribution of the costs was identified by the modified Park test. Effects of logarithmic transformations of costs used in multivariable regression models were explored in sensitivity analysis (Supplemental Table 2). A sensitivity analysis removing individuals who died focusing only on survivors was performed given the competing risk of death in AKI, which could bias costs in survivors versus nonsurvivors (Supplemental Table 3). Hospitalization length of stay was estimated using Poisson regression, and a negative binomial regression was used if the primary assumption that variance equals the mean was not met. The recycled prediction method (42), which estimates marginal mean, was used to estimate mean cost in each AKI and kidney recovery group. Because the data were from population-based registries, missing data were not present, and participants lost to follow-up due to migration out of province were tracked and removed from the analysis.
We estimated the cost associated with AKI in Canada per year, assuming that the proportion of adult patients with various AKI outcomes and costs from our population-based cohort would occur in Canada (43) using data from CIHI. This analysis took the perspective of the health care purchaser and did not include related nonmedical costs (such as costs in losses in productivity and informal care). All costs are reported in 2015 Canadian dollars (CAD). The institutional review boards of the University of Alberta and the University of Calgary approved the study and granted waiver of patient consent.
Results
The cohort consisted of 239,906 subjects with mean age of 62.7 years old; 47% were men, and the mean baseline eGFR was 78 ml/min per 1.73 m2 (Table 1). The most common reasons for hospitalization in decreasing frequency were cardiovascular (18%) and gastrointestinal (13%), and 13% of hospitalizations involved admission to a critical care setting; 25,495 (10.6%), 4598 (1.9%), 2493 (1.0%), and 670 (0.3%) patients developed AKIN stages 1, 2, 3 without dialysis, and 3 with dialysis, respectively. Index hospitalization mortality of the overall cohort was 4.8%, with 3% for no AKI and 12%, 27%, 34%, and 50% for the AKI categories, respectively (Supplemental Table 4). One-year mortality of the overall cohort was 14%, with 12% for no AKI and 25%, 41%, 47%, and 56% for the AKI categories, respectively (Supplemental Table 4).
Table 1.
Patient characteristics by AKI status in adults hospitalized in Alberta between 2002 and 2009
| Characteristic | All Patients | No AKI | AKI | AKIN 1 | AKIN 2 | AKIN 3 without Dialysis | AKIN 3 with Dialysis | P Value |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| No. of subjects, % | 239,906 (100) | 206,650 (86) | 33,256 (14) | 25,495 (10.6) | 4598 (1.9) | 2493 (1.0) | 670 (0.3) | |
| Age, mean (SD) | 63 (18) | 62 (18) | 69 (16) | 69 (17) | 69 (16) | 67 (16) | 62 (16) | <0.01 |
| Sex, men, % | 47 | 46 | 55 | 56 | 50 | 53 | 58 | <0.01 |
| Comorbid disease, % | ||||||||
| Nondermatologic malignancy | 24 | 23 | 27 | 26 | 29 | 33 | 20 | <0.01 |
| Cerebrovascular disease | 12 | 11 | 14 | 14 | 13 | 12 | 12 | <0.01 |
| Congestive heart failure | 16 | 13 | 32 | 32 | 32 | 26 | 32 | <0.01 |
| Chronic pulmonary disease | 29 | 28 | 35 | 35 | 36 | 33 | 35 | <0.01 |
| Dementia | 7 | 7 | 12 | 12 | 14 | 14 | 3 | <0.01 |
| Diabetes with complications | 6 | 6 | 15 | 15 | 16 | 16 | 20 | <0.01 |
| Diabetes without complications | 17 | 16 | 19 | 19 | 18 | 18 | 15 | <0.01 |
| Peripheral vascular disease | 8 | 8 | 13 | 13 | 12 | 10 | 17 | <0.01 |
| Peptic ulcer disease | 6 | 5 | 7 | 6 | 8 | 7 | 11 | <0.01 |
| Liver disease | 4 | 4 | 7 | 6 | 10 | 14 | 20 | <0.01 |
| Metastatic carcinoma | 8 | 7 | 9 | 8 | 11 | 15 | 6 | <0.01 |
| Myocardial infarction | 14 | 12 | 21 | 22 | 20 | 17 | 26 | <0.01 |
| Paraplegia and hemiplegia | 2 | 2 | 3 | 2 | 3 | 4 | 2 | <0.01 |
| Connective tissue disease-rheumatic disease | 4 | 4 | 4 | 4 | 5 | 4 | 6 | 0.03 |
| Mean CCI score (median, IQR) | 2.5 (2, 1–3) | 2.3 (2, 0–3) | 3.6 (3, 2–5) | 3.5 (3, 1–5) | 3.9 (3, 2–5) | 4.3 (4, 2–6) | 4.2 (4, 2–6) | <0.01 |
| Intensive care unit | 13 | 11 | 23 | 20 | 25 | 25 | 75 | <0.01 |
| Primary diagnostic code for hospitalization, % | ||||||||
| Cardiovascular | 18 | 17 | 24 | 25 | 19 | 12 | 26 | <0.01 |
| Respiratory | 7 | 7 | 10 | 10 | 11 | 9 | 8 | <0.01 |
| Gastrointestinal | 13 | 13 | 12 | 11 | 14 | 15 | 13 | <0.01 |
| Infectious disease | 2 | 1 | 3 | 2 | 6 | 7 | 10 | <0.01 |
| Cancer | 13 | 13 | 12 | 12 | 12 | 11 | 7 | <0.01 |
| Orthopedics | 10 | 10 | 5 | 5 | 4 | 2 | 3 | <0.01 |
| Hematologic | 3 | 3 | 5 | 5 | 6 | 6 | 4 | <0.01 |
| Genitourinary | 7 | 6 | 10 | 9 | 11 | 23 | 10 | <0.01 |
| Injury/poisoning | 8 | 8 | 5 | 5 | 5 | 4 | 10 | <0.01 |
| Other disease | 22 | 23 | 15 | 16 | 12 | 12 | 9 | <0.01 |
| Baseline kidney function | ||||||||
| eGFR mean (SD), ml/min per 1.73 m2 | 78 (25) | 80 (24) | 66 (27) | 65 (27) | 70 (27) | 72 (26) | 68 (29) | <0.01 |
| Microalbuminuria, % | 50 | 48 | 61 | 62 | 58 | 62 | 64 | <0.01 |
| No urine protein measurement, % | 21 | 21 | 16 | 16 | 18 | 16 | 15 |
P value is for comparing subjects with and without AKI. AKIN, Acute Kidney Injury Network; CCI, Charlson Comorbidity Index; IQR, interquartile range.
AKI was associated with increases in length of stay in the index hospitalization (Table 2) compared with length of stay for patients without AKI by 2.5, 2.8, 4.8, and 7.6 days for AKIN 1, 2, 3 without dialysis, and 3 with dialysis, respectively. In a fully adjusted model, the incremental cost of AKI during the index hospitalization was +$2882–$14,816 CAD (Table 3). Compared with patients without AKI, adjusted health care costs of the index hospitalization were 1.31- to 2.56-fold greater and increased in a graded fashion by AKI severity (Table 3). AKIN 3 with dialysis had the highest costing ratio compared with any other covariate in the model, including intensive care unit admission (1.87; 95% CI, 1.84 to 1.90).
Table 2.
Length of stay by AKI status in adults hospitalized in Alberta between 2002 and 2009 (95% confidence interval)
| Time Perioda | No AKI | AKIN 1 | AKIN 2 | AKIN 3 without Dialysis | AKIN 3 with Dialysis |
|---|---|---|---|---|---|
| (1) Index hospitalization | |||||
| No. of subjects (%) | 206,650 (86.2) | 25,495 (10.6) | 4598 (1.9) | 2493 (1.0) | 670 (0.3) |
| LOS | 8.9 (8.9 to 9.0) | 11.4 (11.3 to 11.6) | 12.8 (12.4 to 13.1) | 13.7 (13.2 to 14.2) | 16.5 (15.3 to 17.6) |
| Incremental LOS | — | +2.5 (1.0 to 4.1) | +3.8 (2.8 to 4.9) | +4.8 (4.1 to 5.5) | +7.6 (7.4 to 7.8) |
| (2) Admission to assessment of kidney recovery or 90 db | |||||
| No. of subjects (%) | 206,501 (86.2) | 25,482 (10.6) | 4595 (1.9) | 2491 (1.0) | 670 (0.3) |
| Hospitalization days | 11.0 (10.9 to 11.0) | 13.8 (13.7 to 14.0) | 15.0 (14.6 to 15.5) | 16.0 (15.4 to 16.7) | 18.4 (17.0 to 19.7) |
| Incremental hospitalization days | — | +2.8 (1.4 to 4.3) | +4.0 (3.2 to 4.9) | +5.0 (4.5 to 5.6) | +7.4 (7.2 to 7.5) |
| No AKI | AKIN 1–3 without Dialysis | AKIN 3 with Dialysis | |||
|---|---|---|---|---|---|
| Recovered | Not Recovered | Recovered | Not Recovered | ||
| (3) Assessment of kidney recovery or 90 d to 1 yrc | |||||
| No. of subjects (%) | 191,921 (88.2) | 12,558 (5.8) | 4134 (1.9) | 181 (0.1) | 72 (0.03) |
| Hospitalization days | 4.4 (4.3 to 4.4) | 4.9 (4.7 to 5.1) | 4.8 (4.5 to 5.0) | 4.9 (2.9 to 6.9) | 5.9 (4.1 to 7.8) |
| Incremental hospitalization days | — | +0.6 (−0.8 to 1.9) | +0.4 (−0.8 to 1.6) | +0.5 (0.4 to 0.6) | +1.6 (1.5 to 1.7) |
AKIN, Acute Kidney Injury Network; LOS, length of stay; —, not applicable.
Adjusted for age, sex, Charlson comorbidities (14 variables), hospitalization primary diagnosis (case mix group including ten variables), baseline eGFR, and albuminuria.
In total, 167 (0.1%) patients with outmigration during this period were excluded from analysis.
In total, 20,957 (8.7%) subjects died between admission and recovery of renal function, and 1406 (0.6%) subjects outmigrated and were excluded from analysis.
Table 3.
Total and incremental costs and cost ratios by AKI status in adults hospitalized in Alberta between 2002 and 2009 (95% confidence interval)
| Time Perioda | No AKI | AKIN 1 | AKIN 2 | AKIN 3 without Dialysis | AKIN 3 with Dialysis |
|---|---|---|---|---|---|
| (1) Index hospitalization | |||||
| No. of subjects (%) | 206,650 (86.2) | 25,495 (10.6) | 4598 (1.9) | 2493 (1.0) | 670 (0.3) |
| Cost, $ | 9444 (9395 to 9493) | 12,356 (12,166 to 12,486) | 14,370 (13,894 to 14,847) | 14,822 (14,177 to 15,467) | 24,260 (22,262 to 26,258) |
| Incremental cost, $b | — | +2882 (2714 to 3049) | +4926 (4447 to 5406) | +5378 (4731 to 6025) | +14,816 (12,817 to 16,815) |
| Cost ratioa | 1 | 1.31 (1.29 to 1.33) | 1.52 (1.48 to 1.57) | 1.58 (1.51 to 1.64) | 2.56 (2.37 to 2.77) |
| (2) Admission to assessment of kidney recovery or 90 dc | |||||
| No. of subjects (%) | 206,501 (86.2) | 25,482 (10.6) | 4595 (1.9) | 2491 (1.0) | 670 (0.3) |
| Cost, $ | 15,445 (15,378 to 15,512) | 19,225 (19,012 to 19,439) | 21,216 (20,569 to 21,863) | 21,673 (20,781 to 22,564) | 33,736 (31,020 to 36,454) |
| Incremental cost, $b | — | +3779 (3555 to 4004) | +5771 (5120 to 6421) | +6227 (5334 to 7121) | +18,291 (15,573 to 21,009) |
| Cost ratioa | 1 | 1.25 (1.23 to 1.26) | 1.37 (1.34 to 1.41) | 1.40 (1.35 to 1.45) | 2.19 (2.04 to 2.35) |
| (4) Admission to 1 yrd | |||||
| No. of subjects (%) | 205,396 (86.2) | 25,367 (10.6) | 4586 (1.9) | 2483 (1.0) | 668 (0.3) |
| Cost, $ | 22,000 (21,907 to 22,094) | 26,080 (25,755 to 26,404) | 27,449 (26,643 to 28,254) | 27,095 (2599 to 28,198) | 38,794 (35,902 to 41,687) |
| Incremental cost, $b | — | +4079 (3947 to 4212) | +5448 (5111 to 5786) | +5094 (4284 to 5905) | +16,794 (15,687 to 17,901) |
| Cost ratioa | 1 | 1.18 (1.17 to 1.20) | 1.25 (1.21 to 1.29) | 1.23 (1.18 to 1.29) | 1.76 (1.63 to 1.90) |
| No AKI | AKIN 1–3 without Dialysis | AKIN 3 with Dialysis | |||
|---|---|---|---|---|---|
| Recovered | Not Recovered | Recovered | Not Recovered | ||
| (3) Assessment of kidney recovery or 90 d to 1 yre | |||||
| No. of subjects (%) | 191,921 (88.2) | 12,558 (5.8) | 4134 (1.9) | 181 (0.1) | 72 (0.03) |
| Cost, $ | 6999 (6925 to 7073) | 9911(9529 to 10,292) | 13,034 (12,252 to 13,816) | 10,230 (6450 to 14,011) | 15,562 (10,862 to 20,262) |
| Incremental cost, $b | — | +2912 (2523 to 3301) | +6035 (5249 to 6820) | +3231 (−550 to 7012) | +8563 (3863 to 13,263) |
| Cost ratioa | 1 | 1.42 (1.35 to 1.48) | 1.87 (1.73 to 2.01) | 1.46 (1.03 to 2.05) | 2.27 (1.32 to 3.90) |
Adjusted for age, sex, Charlson comorbidities (14 variables), hospitalization primary diagnosis (case mix group including ten variables), baseline eGFR, and albuminuria. All costs are reported in Canadian dollars. AKIN, Acute Kidney Injury Network; —, not applicable.
Generalized linear model cost ratios by AKI and recovery status compared with no AKI.
All incremental costs are compared with no AKI.
In total, 167 (0.1%) patients with outmigration during this period were excluded from analysis.
In total, 1406 (0.6%) outmigrated subjects during the 1-year follow-up were excluded from analysis.
In total, 20,957 (8.7%) subjects died between admission and recovery of renal function, and 1406 (0.6%) subjects outmigrated and were excluded from analysis.
Over the time from assessment of kidney recovery or 90 days to 1 year postadmission (approximately 9 months), subjects with AKI had greater cost ratios and incremental health care costs compared with those without AKI (Table 3). Nonrecovery was associated with the largest cost ratios and incremental costs for both those not requiring dialysis (+$6035 CAD; 95% CI, 5249 to 6820) and those requiring dialysis (+$8563 CAD; 95% CI, 3863 to 13,263), but this was conditional on living to the assessment of kidney recovery, with almost 10% of the study cohort dying before this, including >50% of patients with AKIN 3 with dialysis. Poisson regression models with logarithm transformation of costs showed similar results of greater incremental costs by severity of AKI, although the magnitude of incremental costs tended to be larger (Supplemental Table 2).
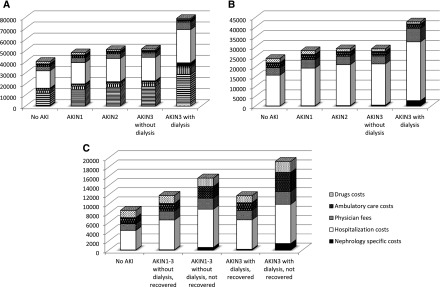
Costs associated with nephrology care (not including index admission) are shown in Figure 3, indicating large cost ratios for patients with AKIN stage 3, particularly those requiring dialysis, with cost ratio of 42.22 in the assessment of kidney recovery or the 90-day to 1-year period and per patient incremental costs of $2722 (Supplemental Table 5). A detailed summary of costs by AKI and recovery statuses showed that costs were driven predominantly by hospitalizations followed by physician fees (Figure 3, Supplemental Tables 6–9).
Figure 3.
Costs categories by AKI severity and kidney recovery for all time periods except index hospitalization. (A) Admission to assessment of kidney recovery or 90 days. (B) Admission to 1 year. (C) Assessment of kidney recovery or 90 days to 1 year. AKIN, Acute Kidney Injury Network.
A sensitivity analysis including only survivors did not change the study results (Supplemental Table 3). AKI severity and lack of kidney recovery were associated with higher incremental costs across all time periods, despite the higher mortality associated with AKIN stage and lack of kidney recovery (Supplemental Table 4).
When our data on the proportion and cost of AKI were applied to the Canadian population, including 313,553 adult patient hospitalizations in 2013–2014 (44), the annual incremental health care costs associated with AKI from admission to recovery assessment were $126 million, $35 million, $20 million, and $16 million for AKIN 1, 2, 3 without dialysis, and 3 with dialysis, respectively (Table 4).
Table 4.
Estimated yearly incremental cost of AKI in Canada by AKI status
| Time Period | AKIN 1 | AKIN 2 | AKIN 3 without Dialysis | AKIN 3 with Dialysis |
|---|---|---|---|---|
| (2) Admission to assessment of kidney recovery or 90 d | ||||
| Total incremental cost, $ | 125,778,483 | 34,631,512 | 20,260,288 | 16,006,192 |
| AKIN 1–3 without Dialysis | AKIN 3 with Dialysis | |||
|---|---|---|---|---|
| Recovered | Not Recovered | Recovered | Not Recovered | |
| (3) Assessment of kidney recovery or 90 d to 1 yr | ||||
| Total incremental cost, $ | 52,405,344 | 35,754,453 | 838,192 | 883,619 |
On the basis of 313,553 hospitalizations in Canada (Canadian Institutes for Health Information 2012–2013). All costs are in Canadian dollars. AKIN, Acute Kidney Injury Network.
Discussion
This population-based analysis shows the graded association between AKI severity and health care costs during the index hospitalization, over the subsequent 90 days, and over 1 year. Even the mildest forms of AKI resulted in adjusted costs that were 1.2–1.3 times greater than those for patients without AKI; more severe AKI was associated with costs that were 1.8–2.5 times greater. These increases in resource utilization were present even when patients were stratified by recovery status; patients who recovered from their AKI had cost ratios of 1.4–1.5, whereas patients who did not recover experienced costs 1.9–2.3 times greater compared with those with no AKI over the time period from assessment of AKI recovery to 1 year.
Although the incremental cost per patient was much greater for those with more severe AKI, particularly in those requiring dialysis, the less severe forms of AKI resulted in greater aggregate costs from a population perspective. Over the timeframe of admission to the assessment of kidney recovery or 90 days, patients with AKIN 1 had incremental costs of approximately $3800 per patient, whereas patients requiring dialysis had costs of $18,300. However, there were over 25,000 patients with AKIN 1, but only 670 required dialysis. As such, the effect on health care resource utilization is far greater for the large number of patients with less severe forms of AKI. If this association of increased health care resource utilization with AKI is causal and if effective strategies to reduce the frequency or mitigate all severities of AKI can be efficiently deployed on a large-scale basis during hospitalization and the postdischarge period, there is a potential for significant cost savings in addition to the effect on clinical outcomes. Because costs beyond the index hospitalization were driven predominantly by hospitalizations, interventions that prevent readmission related to recurrent AKI or CKD are needed, but given the relative lack of contribution by nephrology-specific costs, comorbidities related to kidney dysfunction leading to hospitalizations may be the more appropriate interventional targets. Prospective microcosting studies are needed to allow greater granularity in the types of resources used.
Our results are congruent with other published studies of AKI with associations between AKI and increased length of stay and health care costs (Supplemental Table 10). However, previous studies are limited by their differentiated settings and populations, heterogeneous assessment and definitions of AKI and kidney recovery, a lack of detailed costing methodology, and a focus on dialysis-specific costs or only hospitalization costs, with infrequent evaluation of costs beyond the index hospitalization. Lack of recovery from AKI requiring dialysis has previously been shown to be associated with increased costs but was limited to a dialysis dependency definition of kidney recovery (and not a serum creatinine–based definition), with only an estimation of long-term costs (30). The economic implications of AKI on a system perspective have also previously been shown to be substantial in another population-based study (45), but its analysis relied on AKI-specific health care resource groups and their associated excess bed days in addition to a Markov model for long-term costs to estimate a yearly AKI cost of 1 billion pounds in the United Kingdom.
The strength of this analysis is use of a population-based cohort that allowed for accurate determination of AKI and recovery using serum creatinine measurements, although urine output data were not available. This analysis considers health care resource utilization during the index hospitalization episode, during the period that was used to observe for recovery, and up to 1 year after AKI; follow-up of patients was complete given that the population is covered under universal health care. Furthermore, the data used for costing are deemed to be of high quality (37).
There are acknowledged limitations to this analysis. Although a comprehensive adjustment for potential confounders was conducted, many factors that may associate with both the increased risk of developing AKI and health care resource utilization (e.g., severity of underlying acute and chronic illness) were not available. Furthermore, it is not clear that all health care resource use is causal to AKI, and AKI itself may only be a marker of severity of illness: randomized, controlled trials of strategies to reduce AKI or its consequences should be performed that carefully capture clinical and health care resource outcomes. A sensitivity analysis in subjects surviving all time periods did not change our results, but they are dependent on the epidemiology of AKI and kidney recovery in our cohort, which may not be generalizable to other settings. This analysis was unable to capture complete medication utilization for all ages in the outpatient setting, but this likely underestimates further excess costs between the nonrecovery and recovery groups, especially in advanced CKD or dialysis. The study cohort is also relatively dated, but a sensitivity analysis (Supplemental Table 11) showed no evidence of increasing AKI health care costs over time. The focus on early or community-acquired AKI during the first 14 days of hospitalization may have resulted in late AKI occurring after 14 days of hospitalization being misclassified as no AKI, but this would likely underestimate any costs attributable to AKI, and a sensitivity analysis limiting the study cohort to hospitalizations ≤30 days did not change our results (Supplemental Table 12). Finally, although we attempted to identify costs specific to nephrology care after the index hospitalization, the administrative codes used have not been thoroughly validated, and it is likely that these costs are underestimated compared with previous studies (17).
In conclusion, this population-based cohort shows that the severity of AKI, the need for dialysis, and the lack of kidney recovery are all associated with significant increases in health care resource utilization and costs in patients who are hospitalized. Furthermore, these costs persist well beyond the index admission and are evident for up to 1 year. Although milder forms of AKI are associated with numerically lower incremental costs per patient than more severe forms, given the very large number of patients with less severe AKI, they have the greatest total effect on health care resource utilization. Strategies to prevent AKI and mitigate its severity as well as facilitate recovery posthospital discharge are needed. Investment in candidate strategies can be informed by this analysis, and randomized, controlled trials that test these interventions should capture health care resource use as an outcome to determine cost-effectiveness.
Disclosures
None.
Supplementary Material
Acknowledgments
This project was supported by a Canadian Institutes of Health Research grant. S.K. is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta.
This study is, in part, on the basis of data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. The Government of Alberta, Alberta Health, and Alberta Health Services did not express any opinion in relation to this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00950117/-/DCSupplemental.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaño F, Pascual J; Madrid Acute Renal Failure Study Group : Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu VC, Wu PC, Wu CH, Huang TM, Chang CH, Tsai PR, Ko WJ, Chen L, Wang CY, Chu TS, Wu KD; National Taiwan University Study Group on Acute Renal Failure (NSARF) Group : The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 3: e000933, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ahlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM; VA/NIH Acute Renal Failure Trial Network : Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study. Clin J Am Soc Nephrol 5: 1366–1372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morsch C, Thomé FS, Balbinotto A, Guimarães JF, Barros EG: Health-related quality of life and dialysis dependence in critically ill patient survivors of acute kidney injury. Ren Fail 33: 949–956, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Nisula S, Vaara ST, Kaukonen KM, Reinikainen M, Koivisto SP, Inkinen O, Poukkanen M, Tiainen P, Pettilä V, Korhonen AM; FINNAKI-QOL Study Group : Six-month survival and quality of life of intensive care patients with acute kidney injury. Crit Care 17: R250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR: The prognostic importance of a small acute decrement in kidney function in hospitalized patients: A systematic review and meta-analysis. Am J Kidney Dis 50: 712–720, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 17.Manns B, Doig CJ, Lee H, Dean S, Tonelli M, Johnson D, Donaldson C: Cost of acute renal failure requiring dialysis in the intensive care unit: Clinical and resource implications of renal recovery. Crit Care Med 31: 449–455, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA; BEST Kidney Investigators : Cost of acute renal replacement therapy in the intensive care unit: Results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care 14: R46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A: Cost and mortality associated with postoperative acute kidney injury. Ann Surg 261: 1207–1214, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT; The Multicenter Study of Perioperative Ischemia Research Group : Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med 128: 194–203, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Callahan M, Battleman DS, Christos P, Efimba M, Whitelaw G: Economic consequences of renal dysfunction among cardiopulmonary bypass surgery patients: A hospital-based perspective. Value Health 6: 137–143, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA: Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23: 1970–1974, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Huber M, Ozrazgat-Baslanti T, Thottakkara P, Efron PA, Feezor R, Hobson C, Bihorac A: Mortality and cost of acute and chronic kidney disease after vascular surgery. Ann Vasc Surg 30: 72–81.e2, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candrilli S, Bell T, Irish W, Morris E, Goldman S, Cairo MS: A comparison of inpatient length of stay and costs among patients with hematologic malignancies (excluding hodgkin disease) associated with and without acute renal failure. Clin Lymphoma Myeloma 8: 44–51, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Lahoti A, Nates JL, Wakefield CD, Price KJ, Salahudeen AK: Costs and outcomes of acute kidney injury in critically ill patients with cancer. J Support Oncol 9: 149–155, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Dasbach EJ, Platt R: Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis 32: 686–693, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Joo EJ, Peck KR, Ha YE, Kim YS, Song YG, Lee SS, Ryu SY, Moon C, Lee CS, Park KH: Impact of acute kidney injury on mortality and medical costs in patients with meticillin-resistant Staphylococcus aureus bacteraemia: A retrospective, multicentre observational study. J Hosp Infect 83: 300–306, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Pannu N, James M, Hemmelgarn BR, Dong J, Tonelli M, Klarenbach S; Alberta Kidney Disease Network : Modification of outcomes after acute kidney injury by the presence of CKD. Am J Kidney Dis 58: 206–213, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 31.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd , Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 37.McKillop I, Pink G, Johnson L: The Financial Management of Acute Care in Canada: A Review of Funding Performance Monitoring, and Reporting Practices, Ottawa, ON, Canada, Canadian Institute for Health Information, 2001 [Google Scholar]
- 38.Canadian Institute for Health Information: Case Mix Groups+ Methodology. Available at: https://www.cihi.ca/en/data-and-standards/standards/case-mix/cmg. Accessed January 13, 2017
- 39.Canadian Institute for Health Information: National Ambulatory Care Reporting System (NACRS) Metadata Web Site. Available at: https://www.cihi.ca/en/types-of-care/hospital-care/emergency-and-ambulatory-care/nacrs-metadata. Accessed January 13, 2017
- 40.Carpenter J, Bithell J: Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat Med 19: 1141–1164, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Afifi AA, Kotlerman JB, Ettner SL, Cowan M: Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health 28: 95–111, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Mahendra G: Using “recycled predicitons” for computing marginal effects, statistics and data analysis. SAS Glob Forum 272: 1–10, 2010 [Google Scholar]
- 43.Hwang YJ, Shariff SZ, Gandhi S, Wald R, Clark E, Fleet JL, Garg AX: Validity of the international classification of diseases, tenth revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open 2: e001821, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canadian Institute For Health Information: Inpatient Hospitalizations: Volumes, Length of Stay, and Standardized Rates Web Site, 2016. Available at: https://apps.cihi.ca/mstrapp/asp/Main.aspx?Server=apmstrextprd_i&project=Quick%20Stats&uid=pce_pub_en&pwd=&evt=2048001&visualizationMode=0&documentID=C6F8B4144B03958E3AE3CAB5DD440EA7. Accessed January 13, 2017
- 45.Kerr M, Bedford M, Matthews B, O’Donoghue D: The economic impact of acute kidney injury in England. Nephrol Dial Transplant 29: 1362–1368, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.