Abstract
Background and objectives
Intradialytic hypotension (IDH) is associated with morbidity. The effect of blood volume–guided ultrafiltration biofeedback, which automatically adjusts fluid removal rate on the basis of blood volume parameters, on the reduction of IDH was tested in a randomized crossover trial.
Design, setting, participants, & measurements
We performed a 22-week, single blind, randomized crossover trial in patients receiving maintenance hemodialysis who had >30% of sessions complicated by symptomatic IDH in five centers in Calgary, Alberta, Canada. Participants underwent a 4-week run-in period to standardize dialysis prescription and dry weight on the basis of clinical examination. Those meeting inclusion criteria were randomized to best clinical practice hemodialysis (control) or best clinical practice plus blood volume–guided ultrafiltration biofeedback (intervention) for 8 weeks, followed by a 2-week washout and subsequent crossover for a second 8-week phase. The primary outcome was rate of symptomatic IDH.
Results
Thirty-five participants entered, 32 were randomized, and 26 completed the study. The rate of symptomatic IDH with biofeedback was 0.10/h (95% confidence interval, 0.06 to 0.14) and 0.07/h (95% confidence interval, 0.05 to 0.10) during control (P=0.29). There were no differences in the rate or proportion of sessions with asymptomatic IDH or symptoms alone. Results remained consistent when adjusted for randomization order and study week. There were no differences between intervention and control in the last study week in interdialytic weight gain (difference [SD], −0.02 [0.8] kg), brain natriuretic peptide (1460 [19,052] ng/L), cardiac troponins (3 [86] ng/L), extracellular water–to–intracellular water ratio (0.05 [0.33]), ultrafiltration rate (1.1 [7.0] ml/kg per hour), and dialysis recovery time (0.43 [19.25] hours).
Conclusion
The use of blood volume monitoring–guided ultrafiltration biofeedback in patients prone to IDH did not reduce the rate of symptomatic IDH events.
Keywords: hemodialysis; randomized controlled trials; intradialytic hypotension; biofeedback; Cross-Over Studies; renal dialysis; ultrafiltration; Natriuretic Peptide, Brain; Single-Blind Method; Alberta; Weight Gain; Confidence Intervals; Water; Random Allocation; Blood Volume; hypotension; Biofeedback, Psychology; Fluid Therapy; Troponin; Canada
Introduction
Patients typically remove (ultrafiltration) 1–5 L fluid per hemodialysis (HD) session to maintain euvolemia. Rapid ultrafiltration results in intradialytic hypotension (IDH) in as many as 25%–50% of patients treated with HD (1–9). IDH is a serious complication of HD associated with an increase in morbidity and mortality (1–9).
The development of symptoms and IDH might correlate with patient-specific thresholds of relative blood volume (10,11). The relative blood volume is measured by tracking changes in total protein or hemoglobin concentration at the arterial port during HD, using optical or ultrasound techniques. The relative blood volume decreases with ultrafiltration (10,12–18) and higher ultrafiltration rates result in steeper declines in the blood volume monitoring–curve (17–19).
Blood volume monitoring–guided biofeedback technology has been developed, whereby the ultrafiltration rate and/or dialysate sodium are automatically adjusted in response to the relative blood volume (16,19,20). Although this technology may be effective in reducing IDH, there are limitations and knowledge gaps in the existing literature (21–25). The majority of the studies used biofeedback that adjusts both dialysate sodium and ultrafiltration (21,22,25–29), which can lead to positive sodium balance and volume overload.
We conducted a randomized crossover trial to study whether biofeedback technology incorporating blood volume monitoring–guided ultrafiltration alone, without adjusting dialysate sodium, resulted in a reduction in the rate of symptomatic IDH episodes when compared with best clinical practice alone.
Materials and Methods
Overview
We performed a randomized, single blind, crossover trial in patients receiving maintenance HD. We accrued participants from five centers (two hospital units: Foothills Medical Centre and Peter Lougheed Centre; and three community units: Carewest Dr. Vernon Fanning HD, Sheldon M. Chumir HD, and Sunridge HD) in Calgary, Alberta, Canada. Recruitment took place between June and December of 2014; study follow-up was completed in May of 2015. The study protocol was approved by the University of Calgary Conjoint Health Research Ethics Board and was conducted in accordance with the Helsinki Declaration (registered at clinicaltrials.gov: NCT01988181).
Study Population
All people treated with HD for >3 months were screened for eligibility. Medically stable participants older than 18 years of age, undergoing HD three to four times per week for a minimum of 3 hours per session, and who had ≥30% of their HD sessions in the preceding 8 weeks complicated by symptomatic IDH were eligible. Participants with serum hemoglobin <8.0 g/dl (<80 g/L), serum sodium <133 meq/L (≤133 mmol/L), an active malignancy, a history of blood transfusion or hospitalization in the preceding 4 weeks, ongoing urine output estimated ≥250 ml per day or who routinely used diuretics for volume management, a planned change in the renal replacement modality during the study period, or who were unable to provide written informed consent were excluded.
Study Protocol
The study design has been previously described (30). Eligible participants underwent a 4-week run-in period during which a standardized clinical assessment of dry weight, antihypertensive medications, and dialysis prescription was performed. Participants who met study eligibility criteria at the end of the run-in period were randomized to one of these two sequences: best clinical practice (control) for 8 weeks (24 runs) followed by best clinical practice plus blood volume monitoring–guided ultrafiltration biofeedback (intervention) for 8 weeks; or intervention followed by control. Between the control and intervention period, participants underwent a 2-week washout. All IDH events were managed using our standard algorithm (Supplemental Figure 1). Randomization of the allocation sequence was done using computer-generated random numbers under the supervision of a statistician in the Department of Medicine, University of Calgary. The allocation sequence was inserted into sequentially numbered, opaque, sealed envelopes. Because of the nature of the intervention, only the patient was blinded.
Dialysis Prescription
All study participants were dialyzed using the Fresenius 5008 HD machine (Fresenius Medical Care, Bad Homburg, Germany) with high-flux dialyzers. A standardized dialysate prescription was used with a dialysate sodium of 138 meq/L (138 mmol/L), dialysate calcium of 2.5 mg/dl (1.25 mmol/L), dialysate temperature of 36°C, and constant ultrafiltration rate. Dialysate potassium was individualized according to the participant’s predialysis serum potassium. Those receiving the biofeedback intervention in the randomization period had the same prescription, but had their ultrafiltration rate automatically adjusted during each dialysis session on the basis of the changes in relative blood volume. Blood volume–monitoring technique validation (12,15) and description of the ultrafiltration biofeedback algorithm (Supplemental Figure 2) has been previously published (12,15,31). A single investigator (K.C.W.L.) reviewed and adjusted the patient-specific critical relative blood volume and dry weight weekly. The most recent episode of symptomatic IDH was identified as per study definition and the critical blood volume was set equal to the blood volume recorded immediately before that episode.
Study Endpoints
The primary outcome, rate of symptomatic IDH, was calculated by dividing the number of symptomatic IDH episodes by the duration of each dialysis session in hours. We used rates because hypotension episodes can recur, and HD sessions vary in duration within and between participants. Rates allow more powerful capture of potential changes in the IDH over time. Symptomatic IDH was defined as a drop in systolic BP of ≥20 mm Hg from baseline with associated symptoms: sudden-onset headache, dizziness, loss of consciousness, thirst, dyspnea, angina, muscle cramps, or vomiting (32–34). Prespecified subgroup analyses were conducted using a larger cut-off (≥30 mm Hg) in systolic BP drop, with associated symptoms, and in participants with starting systolic BPs of <100 mm Hg. The secondary outcomes of interest were the proportion of HD sessions affected by symptomatic IDH; the rate and proportion of asymptomatic IDH; rate and proportion of dialysis symptoms; single-session Kt/V; total body water, extracellular water, intracellular water, extracellular water–to–intracellular water ratio, and extracellular water–to–total body water ratio as determined by whole-body electrical bioimpedance (Quantum IV Bioimpedance, RJL Systems, Clinton Township, Michigan); changes in B-type natriuretic peptide; changes in high-sensitivity cardiac troponin; changes in antihypertensive medications; and recovery time from the previous HD session.
Data Collection
BPs, single-session Kt/V, pre- and postdialysis weights, and dialysis symptoms were recorded (usual care) at the end of each dialysis session on the electronic run sheet. BPs were electronically captured, with drops of >20 mm Hg automatically highlighted. Whole-body electrical bioimpedance, B-type natriuretic peptide, high-sensitivity cardiac troponin, and interdialytic symptoms surveys were collected during midweek HD sessions in weeks 1, 4, 8, 12, 14, 18, and 22. The interdialytic survey, provided at the beginning of the dialysis session, asked participants to record the time to recover after the last dialysis session (35).
Sample Size Calculation
Previously published data estimated that 23% of our HD population were affected by IDH (36), with an average rate of 0.3 IDH episodes per run (i.e., seven episodes over a period of 24 HD runs). We assumed a Poisson distribution for the count of hypotensive episodes in power analyses. We estimated the power by simulation using a Poisson random intercept model (37). We estimated that a sample size of 30 participants followed for 24 runs per treatment would provide a power of 90% to demonstrate a 30% reduction in the rate of IDH using the biofeedback intervention compared with the control, with a two-sided α of 0.05. We aimed to enroll 35 participants and treat them for 24 runs per intervention.
Statistical Analyses
Baseline characteristics are presented as means and 95% confidence intervals (95% CI). We planned to analyze the data using random intercept Poisson regression and address extra Poisson variation, if present, with the corresponding negative binomial mixed-effects model. In these models, data from 24 HD sessions (expressed as IDH episodes per hour) were clustered within each participant. The presence of carry-over effect was assessed by testing the interaction between randomization order and study treatment. Secondary outcomes were compared using standard methods. Percent hydration was calculated as (overhydration)/extracellular water × 100 = (extracellular water −[0.63 × intracellular water])/extracellular water × 100. Statistical analysis was performed with Stata (www.STATA.com).
Results
Cohort Formation
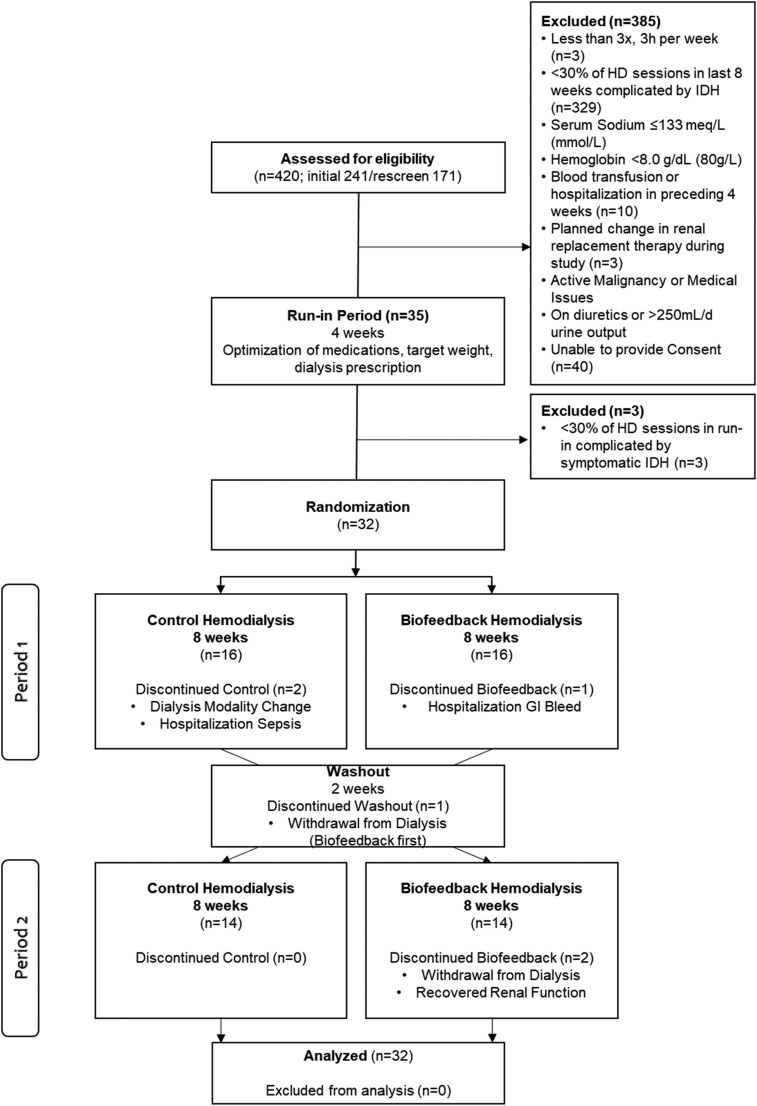
A total of 420 people were screened, and 385 people excluded (Figure 1). Thirty-five participants meeting inclusion criteria entered the 4-week run-in period. These participants had an average of 38% HD sessions affected by IDH in the preceding 8 weeks. By the end of the run-in period, three participants were excluded because <30% of their dialysis sessions were complicated by symptomatic IDH. A total of 32 participants were randomized, 16 to each randomization order, and analyzed in an intent-to-treat analysis. In the first randomization order (biofeedback followed by control), two participants dropped out: one during the intervention period due to hospitalization from gastrointestinal bleed and the other during the washout period due to voluntary withdrawal from dialysis. In the second randomization order (control followed by biofeedback), four participants dropped out: two during the control period (one for sepsis requiring hospitalization and the other changed dialysis modality), and two during the interventional period (one with improvement of kidney function, and the other withdrew from dialysis).
Figure 1.
Participant flow diagram. GI, gastrointestinal; HD, hemodialysis; IDH, intradialytic hypotension.
Cohort Characteristics
Baseline characteristics are described in Table 1. Our cohort had an average age (SD) of 67 (13) years; 13 (41%) of these participants were above the age of 70; 17 (53%) were women. The majority (65%) of participants were overweight (body mass index >25 kg/m2) and >30% had a body mass index >30 kg/m2. All participants were treated with standard, thrice-weekly dialysis using high-flux dialyzers.
Table 1.
Baseline patient characteristics
| Patient Characteristic (n=32) | Value |
|---|---|
| Mean age (SD), yr | 67 (13) |
| Women, n (%) | 17 (53) |
| Mean dialysis vintage (SD), yr | 3.65 (3.50) |
| Mean weight (SD), kg | 78 (21) |
| Mean BMI (SD), kg/m2 | 30 (7) |
| Race, n (%) | |
| White | 16 (50) |
| Asian | 10 (31) |
| Other | 6 (19) |
| Cause of ESRD, n (%) | |
| Diabetes and hypertension | 23 (72) |
| GN | 3 (9) |
| Obstructive | 3 (9) |
| Other | 3 (9) |
| Comorbidities, n (%) | |
| Coronary artery disease | 12 (38) |
| Systolic heart failure | 7 (22) |
| Atrial fibrillation | 4 (13) |
| Diabetes | 24 (75) |
| Diabetes with insulin use | 15 (63) |
| Peripheral vascular disease | 9 (28) |
| Stroke | 4 (13) |
| Hypertension | 28 (88) |
| Lower limb amputation | 3 (9) |
| Vascular access, n (%) | |
| Central venous catheter | 20 (63) |
| Arteriovenous fistula | 12 (38) |
| Dialysis prescription | |
| HD duration of <4 h, n (%) | 4 (12) |
| HD duration of 4 h, n (%) | 28 (88) |
| Mean dialysate sodium (SD), meq/L (mmol/L) | 138 (2) |
| ≥140 mmol/L, n (%) | 11 (34) |
| Dialysate calcium ≥3 mg/dl (1.5 mmol/L), n (%) | 5 (16) |
| Sodium profile, n (%) | 5 (16) |
| Ultrafiltration profile, n (%) | 6 (19) |
| Blood temperature monitoring enabled, n (%) | 28 (88) |
BMI, body mass index; HD, hemodialysis.
Run-In Period
During the 4-week run-in period, the dialysis prescription was standardized and dry weights were optimized (Table 2). The dry weights, interdialytic weight gain, ultrafiltration rate, and number of antihypertensive medications were constant throughout the run-in period (1.6 medications per patient).
Table 2.
Average weights, interdialytic weight gain, percentage reaching dry weight, and ultrafiltration rate
| Start | End | P Value | |
|---|---|---|---|
| Run-In Period | |||
| Weight, kg | 79.1 (71.8 to 86.5) | 79.3 (71.8 to 86.8) | 0.60 |
| Interdialytic weight gain, kg | 1.7 (1.5 to 1.9) | 2 (1.8 to 2.2) | 0.36 |
| Ultrafiltration rate, ml/kg per h | 7.4 (6.4 to 8.5) | 6.0 (5.0 to 7.0) | 0.06 |
| B-type natriuretic peptide, ng/L | 5916 (2941 to 8891) | 6441 (4803 to 10,081) | 0.76 |
| High-sensitivity cardiac troponin, ng/L | 84 (63 to 105) | 74 (57 to 91) | 0.46 |
| Recovery time, h | 8.3 (4.9 to 11.7) | 9.3 (5.8 to 12.8) | 0.66 |
| Biofeedback Period | |||
| Weight, kg | 79.5 (72.9 to 86.0) | 80.2 (73.4 to 86.9) | 0.16 |
| Interdialytic weight gain, kg | 1.7 (1.4 to 2.1) | 1.9 (1.6 to 2.1) | 0.83 |
| Ultrafiltration rate, ml/kg per h | 7.3 (6.0 to 8.6) | 5.9 (4.1 to 7.7) | 0.17 |
| B-type natriuretic peptide, ng/L | 6719 (3284 to 10,154) | 7793 (2803 to 12,783) | 0.72 |
| High-sensitivity cardiac troponin, ng/L | 79 (57 to 101) | 73 (55 to 91) | 0.62 |
| Recovery time, h | 7.4 (4.3 to 10.5) | 9.1 (4.3 to 13.9) | 0.54 |
| Control Period | |||
| Weight, kg | 81.1 (74.0 to 88.3) | 81.4 (74.2 to 88.6) | 0.29 |
| Interdialytic weight gain, kg | 1.9 (1.6 to 2.2) | 1.9 (1.8 to 2.0) | 0.94 |
| Ultrafiltration rate, ml/kg per h | 7.0 (5.5 to 8.6) | 4.8 (3.1 to 6.5) | 0.05 |
| B-type natriuretic peptide, ng/L | 7505 (2095 to 12,915) | 6333 (2210 to 10,455) | 0.73 |
| High-sensitivity cardiac troponin, ng/L | 69 (52 to 86) | 70 (46 to 93) | 0.97 |
| Recovery time, h | 9.5 (5.3 to 13.7) | 8.7 (4.2 to 13.1) | 0.78 |
| Mean Difference (Biofeedback Minus Control) | |||
| Weight, kg | 1.6 (0.3 to 2.9) | 1.2 (−0.1 to 2.5) | 0.56 |
| Interdialytic weight gain, kg | 0.1 (−0.3 to 0.4) | 0.0 (−0.2 to 0.4) | 0.32 |
| Ultrafiltration rate, ml/kg per h | −0.3 (−1.6 to 1.0) | −1.1 (−2.4 to 0.2) | 0.48 |
| B-type natriuretic peptide, ng/L | 787 (−5622 to 7195) | −1460 (−7604 to 4683) | 0.49 |
| High-sensitivity cardiac troponin, ng/L | −9 (−25 to 28) | −3 (−27 to 29) | 0.65 |
| Recovery time, h | 2.1 (−0.2 to 3.4) | −0.4 (−0.7 to 3.1) | 0.39 |
Values are expressed as mean (95% confidence interval) unless otherwise indicated.
Primary Outcome
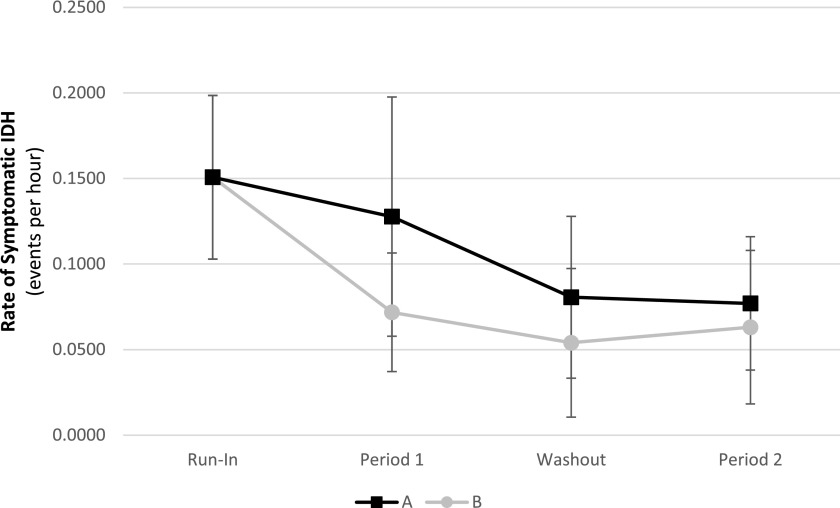
The rate of symptomatic IDH by study period and randomization order is shown in Figure 2. There were no interactions between the randomization order and study treatment. The primary outcome by treatment received is shown in Figure 3 and Table 3. Although there was a significant reduction in the rate of symptomatic IDH from run-in to the eighth week in the biofeedback period (P=0.01), there were no differences between the two treatments at the eighth week (P=0.41). Furthermore, there were no differences in the degree of change from either the run-in to the eighth week (P=0.48) or from the first week to the eighth week between the two treatments (P=0.14). When all 8 weeks of each treatment period were combined together, the rate of symptomatic IDH did not differ between biofeedback and control period (P=0.29). Although the rate of symptomatic IDH in the control period was lower than the run-in period (50.8% decline, P=0.01), there were no differences in the degree of change in the rate of IDH from either the run-in to the control or the run-in to the intervention period (P=0.55).
Figure 2.
The rate of symptomatic IDH did not differ in each period between the two randomization orders. In randomization order A, biofeedback in period 1 followed by control in period 2. In randomization order B, control in period 1 followed by biofeedback in period 2. IDH, intradialytic hypotension.
Figure 3.
Although there was a significant reduction in the rate of symptomatic IDH from run-in to the eighth week in the biofeedback period, there were no differences between the two treatments at the eighth week. IDH, intradialytic hypotension.
Table 3.
Rates of symptomatic IDH, asymptomatic IDH, and symptoms alone by intervention
| Variable | Symptomatic IDH Rate, Events/h | Asymptomatic IDH Rate, Events/h | Symptoms Alone Rate, Events/h |
|---|---|---|---|
| Run-in period | 0.15 (0.10 to 0.20) | 0.31 (0.25 to 0.37) | 0.07 (0.04 to 0.10) |
| Biofeedback period | 0.10 (0.06 to 0.14) | 0.33 (0.24 to 0.41) | 0.05 (0.03 to 0.06) |
| Start of period | 0.15 (0.06 to 0.24) | 0.36 (0.25 to 0.47) | |
| End of period | 0.07 (0.03 to 0.10) | 0.26 (0.16 to 0.36) | |
| Control period | 0.07 (0.05 to 0.10) | 0.30 (0.23 to 0.37) | 0.06 (0.04 to 0.08) |
| Start of period | 0.09 (0.04 to 0.13) | 0.32 (0.25 to 0.40) | |
| End of period | 0.11 (0.02 to 0.19) | 0.33 (0.20 to 0.47) |
Values are expressed as mean (95% confidence interval) unless otherwise indicated. IDH, intradialytic hypotension
Sensitivity Analysis
In the prespecified sensitivity analysis, using an IDH definition of ≥30 mm Hg decline in the presence of symptoms, our results were consistent with the main analysis. The number of participants starting HD with a systolic BP of <100 mm Hg were too low for a meaningful analysis, with a mean event rate between zero and 0.01.
Secondary Outcomes
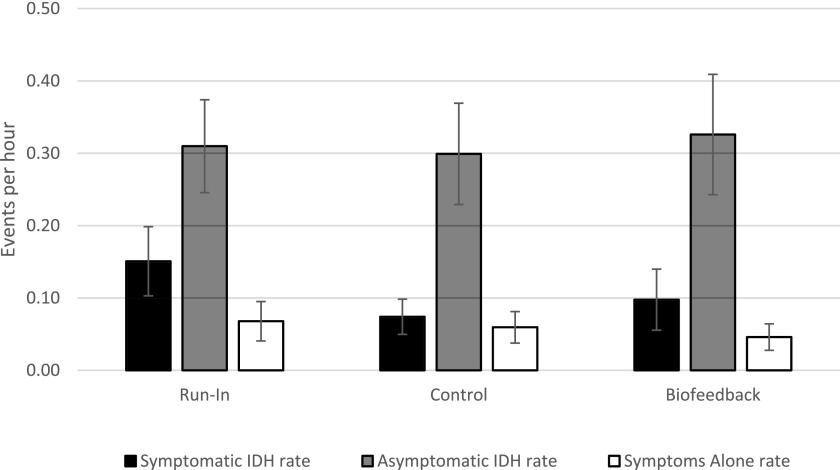
The results of the analysis for rates of asymptomatic IDH (P=0.64) and symptoms alone (P=0.37; Figure 4, Table 3), as well as for proportion of HD sessions with symptomatic IDH (P=0.52), asymptomatic IDH (P=0.67), and symptoms alone (P=0.96), remained consistent with the primary analysis.
Figure 4.
The results of the analysis for rates of symptomatic IDH, asymptomatic IDH, and symptoms alone remained consistent with the primary analysis. Symptomatic IDH rate did not differ between the run-in and the biofeedback period (P=0.12), but decreased between the run-in and the control period (P=0.01). There was no difference between the biofeedback and the control period (P=0.29). Asymptomatic IDH rate did not differ between the run-in and the biofeedback period (P=0.77) or the run-in and the control period (P=0.83); there were no differences between the biofeedback and the control period (P=0.64). Symptoms alone rate did not differ between the run-in and the biofeedback period (P=0.20) or the run-in and the control period (P=0.64); there were no differences between the biofeedback and the control period (P=0.37). IDH, intradialytic hypotension.
The remainder of the secondary outcomes are presented in Tables 2 and 4. There were no differences in the prespecified secondary outcome over the study period or between the two treatments (P>0.10). Our cohort demonstrated evidence of excess extracellular water on the basis of an elevated extracellular water–to–intracellular water ratio, extracellular water–to–total body water ratio, and percent hydration throughout the study.
Table 4.
Average fluid content, fluid ratios, brain natriuretic peptide, troponin, postdialysis recovery time, and change in standing heart rate
| Variable | Start of Run-In Period | End of Run-In Period | P Value | Biofeedback Period | Control Period | P Value |
|---|---|---|---|---|---|---|
| Extracellular water, L | 16.7 (15.0 to 18.3) | 16.7 (14.9 to 18.5) | 0.99 | 17.0 (15.2 to 18.7) | 17.3 (15.5 to 19.2) | 0.80 |
| Intracellular water, L | 19.4 (17.6 to 21.1) | 19.4 (17.5 to 21.3) | 0.99 | 19.1 (17.4 to 20.8) | 20.5 (18.6 to 22.4) | 0.29 |
| Total body water, L | 35.0 (33.1 to 39.0) | 36.1 (32.7 to 39.4) | 0.70 | 36.1 (33.1 to 39.1) | 37.8 (34.5 to 41.1) | 0.45 |
| Extracellular water–to–intracellular water ratio | 0.86 (0.78 to 0.94) | 0.86 (0.79 to 0.94) | 0.99 | 0.91 (0.82 to 0.99) | 0.86 (0.78 to 0.94) | 0.39 |
| Extracellular water–to–total body water ratio | 0.46 (0.44 to 0.48) | 0.46 (0.44 to 0.48) | 0.99 | 0.47 (0.45 to 0.49) | 0.46 (0.44 to 0.48) | 0.49 |
| Hydration, % | 25.1 (18.4 to 31.9) | 25.2 (19.0 to 31.5) | 0.98 | 27.0 (20.1 to 34.0) | 23.5 (16.9 to 30.0) | 0.47 |
| B-type natriuretic peptide, ng/L | 5916 (2941 to 8891) | 6441 (4803 to 10,081) | 0.83 | 7793 (2803 to 12,783) | 6333 (2210 to 10,455) | 0.66 |
| High-sensitivity cardiac troponin, ng/L | 84 (63 to 105) | 74 (57 to 91) | 0.47 | 73 (55 to 91) | 70 (46 to 93) | 0.85 |
| Kt/V | 1.25 (1.15 to 1.35) | 1.30 (1.21 to 1.39) | 0.47 | 1.30 (1.21 to 1.38) | 1.27 (1.21 to 1.33) | 0.56 |
| Recovery time, h | 8.27 (4.89 to 11.66) | 9.31 (5.80 to 12.83) | 0.68 | 9.07 (4.26 to 13.88) | 8.65 (4.23 to 13.08) | 0.91 |
| Change in standing heart rate (post−pre) | −1.93 (−5.15 to 1.28) | −0.79 (−3.77 to 2.19) | 0.51 | −1.71 (−5.09 to 1.67) | −1.14 (−5.33 to 3.04) | 0.84 |
Values are expressed as mean (95% confidence interval) unless otherwise indicated. Post−pre, post-hemodialysis heart rate minus pre-hemodialysis heart rate.
Discussion
We found that the use of blood volume monitoring–guided ultrafiltration biofeedback did not reduce the rate of symptomatic IDH, asymptomatic IDH, or symptoms alone. Our study findings differ from prior blood volume–monitoring studies. One large randomized study by Reddan et al. (38) demonstrated increased mortality in participants randomized to blood volume–monitoring technology. This study differs from ours in that the blood volume monitoring was not integrated with computerized biofeedback, but it required the bedside nurse to act on the blood volume information in a timely manner. In addition, a large proportion of the deaths in the study were attributed to infectious causes, reducing the plausibility that the monitoring technology was responsible. Contemporary studies show a reduction in IDH with the use of blood volume monitoring–guided biofeedback (21–25). However, our study has important differences that should be highlighted. First and most importantly, our study only adjusted the ultrafiltration rate, whereas the majority of biofeedback studies adjusted both dialysate sodium and ultrafiltration rate (22,26–29). The adjustment or increase of dialysate sodium to prevent hemodynamic instability allows for a relatively rapid increase in intravascular refilling from the extracellular space, thus preventing volume-related BP drops or intradialytic symptoms (39–43). The biofeedback system in our study only adjusted the ultrafiltration rate, thus potentially reducing its effectiveness, and may explain the difference in observed results. Second, most studies that employed dialysate sodium and ultrafiltration biofeedback interventions had event reductions over a shorter period of time (2–4 weeks) as compared with our study. The consequences of elevated dialysate sodium start to appear after approximately 2 weeks and it is unclear when they would peak (44–46). Thus, if the duration of these studies were extended, we may see a gradual rise in event rates nullifying the early reduction in the intervention period as a result of sodium loading and hypervolemia. In an uncontrolled study by Colì et al. (47,48), sodium neutral profiling significantly reduced dialysis hypotension episodes without increasing dry weight, interdialytic weight gain, or predialysis sodium. These promising effects have yet to be tested in a randomized controlled trial. Lastly, the study population and measured outcomes in the other biofeedback studies differ from ours. Three of the studies included participants not prone to some form of symptomatic IDH (22,26,27), three had unclear exclusion criteria (23,28,29), and the outcomes in five studies (22,23,26–28) were either not restricted to one of the few recognized definitions of symptomatic IDH (32,49) or were combined with asymptomatic IDH. These differences make interpretation of the conflicting results difficult.
Only one other study has assessed the effect of a blood volume monitoring–guided ultrafiltration biofeedback alone, without the adjustment of dialysate sodium, and found a significant reduction of 8% in each of dialysis symptoms and hypotension (a 20% relative reduction) (23). Their results differ, likely due to a few reasons. The first is that our study used a standardized and specific definition of IDH, requiring an abrupt drop in BP in the presence of symptoms. Whereas, in the study by Gabrielli et al. (23), the primary outcome was intradialytic morbid event, defined as any symptom during dialysis with or without hypotension. This allowed any symptom to be recorded as an event. Similarly, their secondary outcome of hypotension did not specify the degree or abruptness of the BP decline. Secondly, the absence of a run-in period in their study raises concerns about proper dry-weight optimization. If the dry weight or ultrafiltration goals are inappropriately set, it could result in hemodynamic instability and symptoms. This is evident in our study where 9% of participants were excluded during the run-in period due to a lack of symptomatic IDH after the dry weight was appropriately adjusted. Considering the relatively high IDH rates in our study, in spite of our low ultrafiltration rates and small interdialytic weight gains, other factors may have played a role, including neuro-hormonal factors, decreased vascular compliance and reserve, and poor cardiac function. These factors, potentially relevant in other populations, may have minimized the effect of the intervention.
We initially assumed a Poisson model in our study design and power analyses, but found extra-Poisson variation in the data that we addressed by means of mixed-effects negative binomial regression. The two approaches provided similar results (incidence rate ratio, 1.19; 95% CI, 0.96 to 1.47 [Poisson model] versus incidence rate ratio, 1.16; 95% CI, 0.90 to 1.49 [negative binomial regression]). Another study limitation was the strict inclusion and exclusion criteria in which <10% of those screened were eligible; this led to smaller study numbers than expected. Although we did not achieve the expected sample size, on the basis of our simulations 24 participants completing the study still provided >80% power (two-sided P 0.05) to detect a 30% reduction in the primary outcome. The dedicated run-in period and the long washout period reduced the potential of a carryover effect. Because of the dynamic touch-screen display and user interface of the HD machine we used, only the participants were blinded. All attempts were made to ensure nursing protocols for the management and documentation of IDH were consistent. In addition, the blood volume–monitoring technology can be affected by changes other than ultrafiltration, such as body position, activity, oral intake, or infusion of medications (50). Although we used a standardized definition for IDH, not every BP reading was analyzed. Given the large number of BP readings over the study period, only the BPs at the start and end of the HD session, and those deemed to meet our study definition for symptomatic and asymptomatic IDH or symptoms alone, were entered for analysis. Finally, the critical relative blood volume and dry weights were only reassessed on a weekly basis. However, our experience suggests that there was only minute variation between these values and weights throughout the study period.
Blood volume monitoring–guided biofeedback technology has a strong theoretic rationale, but we were unable to demonstrate an effect on important clinical parameters. There are unresolved issues about how best to implement this technology, such as the method of selection of critical relative blood volume or the selection of patients most likely to benefit, that need to be better understood in order to definitively determine its clinical usefulness, if any.
Disclosures
None.
Supplementary Material
Acknowledgments
We like to acknowledge Dr. Tyler Williamson, University of Calgary, for his assistance in verifying the statistical approach used in our study.
The authors thank the Roy and Vi Baay Foundation through the University of Calgary for financial support for the research.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Feedback Control in Hemodialysis—Much Ado about Nothing?” on pages 1730–1732.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01030117/-/DCSupplemental.
References
- 1.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Yoshimitsu T, Hirakata H, Fujii K, Kanai H, Hirakata E, Higashi H, Kubo M, Tanaka H, Shinozaki M, Katafuchi R, Yokomizo Y, Oh Y, Tomooka S, Fujimi S, Fujishima M: Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin Nephrol 53: 445–451, 2000 [PubMed] [Google Scholar]
- 4.Ishida I, Hirakata H, Sugimori H, Omae T, Hirakata E, Ibayashi S, Kubo M, Fujishima M: Hemodialysis causes severe orthostatic reduction in cerebral blood flow velocity in diabetic patients. Am J Kidney Dis 34: 1096–1104, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies HJ, McIntyre CW: Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clin J Am Soc Nephrol 7: 1285–1292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selby NM, McIntyre CW: The acute cardiac effects of dialysis. Semin Dial 20: 220–228, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Burton JO, Korsheed S, Grundy BJ, McIntyre CW: Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 30: 701–709, 2008 [DOI] [PubMed] [Google Scholar]
- 10.de Vries JP, Kouw PM, van der Meer NJ, Olthof CG, Oe LP, Donker AJ, de Vries PM: Non-invasive monitoring of blood volume during hemodialysis: Its relation with post-dialytic dry weight. Kidney Int 44: 851–854, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Steuer RR, Leypoldt JK, Cheung AK, Senekjian HO, Conis JM: Reducing symptoms during hemodialysis by continuously monitoring the hematocrit. Am J Kidney Dis 27: 525–532, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Johner C, Chamney PW, Schneditz D, Krämer M: Evaluation of an ultrasonic blood volume monitor. Nephrol Dial Transplant 13: 2098–2103, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Mann H, Stiller S, Schallenberg U, Thömmes A: Optimizing dialysis by variation of ultrafiltration rate and sodium concentration controlled by continuous measurement of circulating blood volume. Contrib Nephrol 74: 182–190, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Mancini E, Santoro A, Spongano M, Paolini F, Rossi M, Zucchelli P: Continuous on-line optical absorbance recording of blood volume changes during hemodialysis. Artif Organs 17: 691–694, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Dasselaar JJ, Huisman RM, DE Jong PE, Franssen CF: Relative blood volume measurements during hemodialysis: Comparisons between three noninvasive devices. Hemodial Int 11: 448–455, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Booth J, Pinney J, Davenport A: Do changes in relative blood volume monitoring correlate to hemodialysis-associated hypotension? Nephron Clin Pract 117: c179–c183, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Kelley K, Light RP: Diagnostic utility of blood volume monitoring in hemodialysis patients. Am J Kidney Dis 51: 242–254, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Sinha AD, Light RP, Agarwal R: Relative plasma volume monitoring during hemodialysis AIDS the assessment of dry weight. Hypertension 55: 305–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krepel HP, Nette RW, Akçahüseyin E, Weimar W, Zietse R: Variability of relative blood volume during haemodialysis. Nephrol Dial Transplant 15: 673–679, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Andrulli S, Colzani S, Mascia F, Lucchi L, Stipo L, Bigi MC, Crepaldi M, Redaelli B, Albertazzi A, Locatelli F: The role of blood volume reduction in the genesis of intradialytic hypotension. Am J Kidney Dis 40: 1244–1254, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ronco C, Brendolan A, Milan M, Rodeghiero MP, Zanella M, La Greca G: Impact of biofeedback-induced cardiovascular stability on hemodialysis tolerance and efficiency. Kidney Int 58: 800–808, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Santoro A, Mancini E, Basile C, Amoroso L, Di Giulio S, Usberti M, Colasanti G, Verzetti G, Rocco A, Imbasciati E, Panzetta G, Bolzani R, Grandi F, Polacchini M: Blood volume controlled hemodialysis in hypotension-prone patients: A randomized, multicenter controlled trial. Kidney Int 62: 1034–1045, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Gabrielli D, Krystal B, Katzarski K, Youssef M, Hachache T, Lopot F, Lasseur C, Gunne T, Draganov B, Wojke R, Gauly A: Improved intradialytic stability during haemodialysis with blood volume-controlled ultrafiltration. J Nephrol 22: 232–240, 2009 [PubMed] [Google Scholar]
- 24.Nesrallah GE, Suri RS, Guyatt G, Mustafa RA, Walter SD, Lindsay RM, Akl EA: Biofeedback dialysis for hypotension and hypervolemia: A systematic review and meta-analysis. Nephrol Dial Transplant 28: 182–191, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Gil H-W, Bang K, Lee SY, Han BG, Kim JK, Kim YO, Song HC, Kwon YJ, Kim Y-S: Efficacy of hemocontrol biofeedback system in intradialytic hypotension-prone hemodialysis patients. J Korean Med Sci 29: 805–810, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesrallah GE, Suri RS, Thiessen-Philbrook H, Heidenheim P, Lindsay RM: Can extracellular fluid volume expansion in hemodialysis patients be safely reduced using the hemocontrol biofeedback algorithm? A randomized trial. ASAIO J 54: 270–274, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Bégin V, Déziel C, Madore F: Biofeedback regulation of ultrafiltration and dialysate conductivity for the prevention of hypotension during hemodialysis. ASAIO J 48: 312–315, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Déziel C, Bouchard J, Zellweger M, Madore F: Impact of hemocontrol on hypertension, nursing interventions, and quality of life: A randomized, controlled trial. Clin J Am Soc Nephrol 2: 661–668, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Leung KC, Quinn RR, Ravani P, MacRae JM: Ultrafiltration biofeedback guided by blood volume monitoring to reduce intradialytic hypotensive episodes in hemodialysis: Study protocol for a randomized controlled trial. Trials 15: 483, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapdelaine I, Déziel C, Madore F: Automated blood volume regulation during hemodialysis. In: Progress in Hemodialysis - From Emergent Biotechnology to Clinical Practice, edited by Carpi A, Donadio C, Tramonti G, Rijeka, Croatia, InTechOpen, 2011, pp 27–46 [Google Scholar]
- 32.Kooman J, Basci A, Pizzarelli F, Canaud B, Haage P, Fouque D, Konner K, Martin-Malo A, Pedrini L, Tattersall J, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG guideline on haemodynamic instability. Nephrol Dial Transplant 22[Suppl 2]: ii22–ii44, 2007 [DOI] [PubMed] [Google Scholar]
- 33.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 34.Schreiber MJ, Jr: Setting the stage. Am J Kidney Dis 38[Suppl 4]: S1–S10, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R; Daily Hemodialysis Study Group London Health Sciences Centre : Minutes to recovery after a hemodialysis session: A simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol 1: 952–959, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Tai DJ, Conley J, Ravani P, Hemmelgarn BR, MacRae JM: Hemodialysis prescription education decreases intradialytic hypotension. J Nephrol 26: 315–322, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Feiveson A: Calculating power by simulation, 2002. Available at: www.stata.com/support/faqs/statistics/power-by-simulation/. Accessed May 30, 2017
- 38.Reddan DN, Szczech LA, Hasselblad V, Lowrie EG, Lindsay RM, Himmelfarb J, Toto RD, Stivelman J, Winchester JF, Zillman LA, Califf RM, Owen WF Jr: Intradialytic blood volume monitoring in ambulatory hemodialysis patients: A randomized trial. J Am Soc Nephrol 16: 2162–2169, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Penne EL, Sergeyeva O: Sodium gradient: A tool to individualize dialysate sodium prescription in chronic hemodialysis patients? Blood Purif 31: 86–91, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Moissl U, Kotanko P, Levin NW, Säemann MD, Kalantar-Zadeh K, Port FK, Wabel P: Significance of interdialytic weight gain versus chronic volume overload: Consensus opinion. Am J Nephrol 38: 78–90, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Cybulsky AV, Matni A, Hollomby DJ: Effects of high sodium dialysate during maintenance hemodialysis. Nephron 41: 57–61, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM: Sodium ramping in hemodialysis: A study of beneficial and adverse effects. Am J Kidney Dis 29: 669–677, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Mc Causland FR, Waikar SS: Optimal dialysate sodium-what is the evidence? Semin Dial 27: 128–134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moret K, Aalten J, van den Wall Bake W, Gerlag P, Beerenhout C, van der Sande F, Leunissen K, Kooman J: The effect of sodium profiling and feedback technologies on plasma conductivity and ionic mass balance: A study in hypotension-prone dialysis patients. Nephrol Dial Transplant 21: 138–144, 2006 [DOI] [PubMed] [Google Scholar]
- 45.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJM, Santos SFF: Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66: 1232–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Keen ML, Gotch FA: The association of the sodium “setpoint” to interdialytic weight gain and blood pressure in hemodialysis patients. Int J Artif Organs 30: 971–979, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Colì L, La Manna G, Comai G, Ursino M, Ricci D, Piccari M, Locatelli F, Di Filippo S, Cristinelli L, Bacchi M, Balducci A, Aucella F, Panichi V, Ferrandello FP, Tarchini R, Lambertini D, Mura C, Marinangeli G, Di Loreto E, Quarello F, Forneris G, Tancredi M, Morosetti M, Palombo G, Di Luca M, Martello M, Emiliani G, Bellazzi R, Stefoni S: Automatic adaptive system dialysis for hemodialysis-associated hypotension and intolerance: A noncontrolled multicenter trial. Am J Kidney Dis 58: 93–100, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Colì L, Ursino M, Donati G, Cianciolo G, Soverini ML, Baraldi O, La Manna G, Feliciangeli G, Scolari MP, Stefoni S: Clinical application of sodium profiling in the treatment of intradialytic hypotension. Int J Artif Organs 26: 715–722, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM: Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 26: 724–734, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasselaar JJ, Huisman RM, de Jong PE, Franssen CFM: Measurement of relative blood volume changes during haemodialysis: Merits and limitations. Nephrol Dial Transplant 20: 2043–2049, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.