Abstract
Background and objectives
The natural history of kidney disease among blacks who carry the APOL1 high-risk variants varies, with only a subgroup progressing to ESRD. We aimed to determine whether the APOL1 risk variants are associated with incident proteinuria in the context of hypertension-attributed CKD, and whether subsequent kidney function decline after the onset of proteinuria differs by APOL1 risk status.
Design, setting, participants, & measurements
Using Cox models, we studied the association between APOL1 risk status and incident proteinuria (defined as a doubling of urine protein-to-creatinine ratio to a level ≥0.22 g/g creatinine) among African-American Study of Kidney Disease and Hypertension (AASK) trial participants with APOL1 genotyping and without proteinuria at baseline.
Results
Of the 480 participants in our study, 82 (17%) had the high-risk genotypes (2 alleles), and 254 (53%) developed proteinuria over a median follow-up of 6.8 years. At baseline, mean eGFR was lower in the APOL1 high-risk group compared with the low-risk group (0 or 1 allele; 49.6 versus 53.2 ml/min per 1.73 m2, respectively; P=0.02), but median proteinuria was similar (0.04 g/g creatinine for both groups; P=0.43). Individuals with the high-risk genotypes were 1.72-fold more likely to develop incident proteinuria compared with those with the low-risk genotypes (95% confidence interval, 1.27 to 2.32), independent of age, sex, ancestry, baseline eGFR, baseline systolic BP, and randomized treatment groups. Although eGFR declined faster after the onset of proteinuria, this rate did not differ significantly by APOL1 risk status.
Conclusions
Among blacks with established moderate CKD, the APOL1 high-risk variants are associated with greater risk of incident proteinuria. After proteinuria onset, kidney function declines more rapidly but does not differ by APOL1 risk status. This suggests that factors that lead to proteinuria, beyond APOL1, may additionally drive CKD progression.
Keywords: APOL1; Apo L1; CKD; AASK (African American Study of Kidney Disease and Hypertension); African Americans; Alleles; BP; creatinine; Follow-Up Studies; Genotype; GFR; Humans; hypertension; Kidney Failure, Chronic; Proportional Hazards Models; proteinuria; Renal Insufficiency, Chronic; United States
Introduction
Compared with European Americans, blacks have an increased burden of albuminuria and progressive CKD (1–4). Although much of this risk difference is explained by an increased prevalence of hypertension, diabetes mellitus, and obesity among blacks as well as racial disparities in access to care (5,6), the G1 and G2 variants in the gene encoding apolipoprotein L1 (APOL1) likely also contribute (7,8). Studies to date have demonstrated that blacks with the APOL1 high-risk genotypes (2 risk alleles) have a 2- to 20-fold higher risk for various forms of kidney disease compared with those with the low-risk genotypes (0–1 risk alleles) (1,3,7–10). In a community-based study, however, the distribution of annual eGFR decline among APOL1 high- versus low-risk individuals substantially overlapped (11). Even among blacks with established CKD, a notable proportion of individuals with the high-risk genotypes had stable kidney function over long-term follow-up (12). These observations demonstrate that not all individuals with the APOL1 high-risk genotypes will experience progressive CKD during their lifetime (1,11,13).
To inform screening and treatment strategies, the identification of individuals with the APOL1 high-risk genotypes at greatest risk for adverse renal outcomes is critical, yet it remains elusive. In a recent population-based cohort consisting of young adults, the APOL1 high-risk genotypes were shown to be associated with a nearly three-fold higher odds of incident albuminuria compared with the low-risk genotypes. Moreover, kidney function loss accelerated after the onset of albuminuria and was more pronounced in the APOL1 high-risk group. These findings suggest that albuminuria could serve as an early marker to discern which individuals with the APOL1 high-risk variants are at heightened risk for subsequent kidney function decline (2). Whether these associations also exist among individuals with established CKD is unknown.
In this study, we aimed to determine whether the APOL1 high-risk genotypes were associated with an increased risk of incident proteinuria among blacks with hypertension-attributed CKD. We also sought to evaluate whether the rate of eGFR decline differed before and after the onset of proteinuria and was modified by APOL1 risk status.
Materials and Methods
Study Population and Design
This study utilized data from the African-American Study of Kidney Disease and Hypertension (AASK). AASK originated as a multicenter, randomized, 3×2 factorial trial that was designed to investigate the effects of intensive BP control and different BP medications on kidney disease progression. From February of 1995 to September of 1998, 1094 blacks with hypertension-attributed CKD were randomized to one of two BP goals (mean arterial BP≤92 mm Hg or 102–107 mm Hg) nd one of three BP medications (metoprolol, ramipril, or amlodipine) (14–16). When the trial phase ended at the prespecified endpoint of September of 2001, participants who had not yet developed ESRD were invited to continue on with the cohort phase of the study, which spanned from April of 2002 to June of 2007. During this second phase of AASK, all participants were assigned a common BP goal (<140/90 mm Hg; then <130/80 mm Hg in 2004 due to changes in national guidelines in BP control) and drug (ramipril) (15,16). Details regarding the recruitment and flow of AASK trial and cohort participants have previously been published (14,15). For both the trial and cohort phases, institutional review boards from each participating center approved the study protocols (15).
Of the 1094 trial participants, 836 consented to genetic research, and 693 were successfully genotyped for the APOL1 G1 and G2 risk variants. Comparisons of these 693 individuals to those who either failed genotyping (n=143) or did not provide DNA/genetic consent or developed ESRD before DNA/genetic consent (n=258) have previously been published. Mean eGFR was lower and median proteinuria was higher among participants who did not provide DNA/genetic consent compared with participants who were successfully genotyped (P<0.001 for each) (1). Our study population consisted of 480 individuals with APOL1 genotyping and without proteinuria at baseline. Compared with the 213 excluded participants with APOL1 genotyping available (211 with prevalent proteinuria and two with missing baseline proteinuria), our study population was older; had lower systolic BP, body mass index, and 24-hour urine sodium levels; and had higher eGFR and serum albumin levels at baseline. Compared with those included in our study, excluded participants had a 16-fold higher median baseline proteinuria (0.04 versus 0.64 g/g Cr, respectively; P<0.01) and were more likely to carry an APOL1 high-risk genotype (17% versus 37%, respectively; P<0.01; Supplemental Table 1). Consistent with prior studies in AASK, proteinuria was defined as a urine protein-to-creatinine ratio >0.22 g/g Cr on 24-hour urine collection (15).
Outcomes and Predictors
The primary outcome of this study was incident proteinuria, which we defined as a doubling of urine protein-to-creatinine ratio to a level ≥0.22 g/g Cr. Proteinuria was measured from 24-hour urine collections that were collected at baseline, every 6 months during the trial phase, and annually during the cohort phase (15–17). All urine specimens were processed centrally at the AASK Central Laboratory at the Cleveland Clinic, using the pyrogallol red method for measurement of urine total protein and the modified Jaffé reaction (by autoanalyzer) for measurement of urine creatinine (17,18). We also explored annual change in eGFR by APOL1 risk status. eGFR was estimated using the following validated AASK trial equation: eGFR = 329 × (Scr)−1.096 × (age)−0.294 × (0.736 if female) (19,20). During the trial and cohort phases, eGFR was measured at baseline and semiannually thereafter (16,17). Dietary sodium intake (grams per day) was estimated from baseline 24-hour urine collections that were also processed at the AASK Central Laboratory (17).
The primary exposure was APOL1 risk status. Participants were previously genotyped for the APOL1 risk alleles G1 (rs73885319 or rs60910145; both missense mutations) and G2 (rs71785313; a six base pair deletion) using ABI Taqman (Applied Biosystems, Foster City, California) (1). Consistent with prior studies on the APOL1 risk variants, we used a recessive genetic model in which the high-risk genotypes were defined as having two APOL1 risk alleles and the low-risk genotypes were defined as having one or no risk allele (1,9,10). Percentage of European ancestry was estimated using 140 ancestry informative markers and the software ANCESTRYMAP (1,21).
Statistical Analyses
We compared the baseline characteristics of participants by APOL1 risk status using t tests and Wilcoxon rank sum tests for continuous variables and Fisher exact tests for categorical variables. Cox proportional hazard models were then constructed to assess the association between APOL1 risk status and time to incident proteinuria. We defined baseline as date of randomization into the AASK trial. Participants were censored at onset of ESRD, loss to follow-up, death, or administrative censoring. We adjusted for age, sex, European ancestry, baseline eGFR, baseline systolic BP, randomized BP goal, and randomized BP drug. These covariates were selected a priori with the exception of systolic BP, which was additionally included because the APOL1 high-risk group was noted to have a significantly lower mean systolic BP at baseline compared with the low-risk group (P<0.001). The proportionality hazards assumption was confirmed using Schoenfeld residuals and log-log plots. To assess for effect modification by age, sex, baseline systolic BP, body mass index, and dietary sodium intake, we constructed additional models that included each candidate modifier (treated as a continuous variable) and an interaction term with APOL1 risk status. In sensitivity analyses, we estimated the difference in annual change in proteinuria (from baseline until the onset of proteinuria) as a continuous variable, by APOL1 risk status. We first constructed a linear regression model of follow-up time in years on log2 (proteinuria) and used the slope to estimate each participant’s annual change in proteinuria (22). A second linear regression model was then used to assess the association of APOL1 risk status with proteinuria slope, adjusting for age, sex, European ancestry, baseline systolic BP, baseline eGFR, baseline log2 (proteinuria), randomized BP goal, and randomized BP drug. For ease of interpretation, the exponent of the β in the log2 scale was calculated to yield the percent difference in the annual change in proteinuria, by APOL1 risk status (i.e., percent difference = 2β-1).
To explore whether annual eGFR change before and after the onset of proteinuria differed by APOL1 risk status, we constructed linear mixed-effects models with random intercepts and slopes using longitudinal eGFR as the outcome. Predictors included APOL1 risk status, incident proteinuria, and time of eGFR measure. We adjusted for age, sex, European ancestry, baseline systolic BP, log-transformed baseline proteinuria, randomized BP goal, and randomized BP drug. Stratified analyses using two separate models (before versus after the onset of proteinuria) were performed, and the interaction between time and APOL1 risk status was evaluated to determine whether eGFR declined faster in the APOL1 high- versus low-risk group. To confirm that the interaction term between time and APOL1 risk status was appropriate in assessing the difference in annual eGFR change by APOL1 risk status, we tested for the interaction term between the square of time and APOL1 risk status and found that this second order interaction term was not statistically significant (P>0.10). This result suggested that the difference in annual eGFR change between APOL1 high- versus low-risk groups was relatively stable over time, thus supporting the use of a linear mixed-effects model to assess the difference in annual eGFR change by APOL1 risk status.
We performed power analyses for detecting differences in incident proteinuria and annual eGFR change by APOL1 risk status. The power analysis for the difference in annual eGFR change was conducted using bootstrapping. The same model for evaluating the difference in annual eGFR change by APOL1 risk status was tested in each iteration of the sampled data, and power was estimated as the number of iterations with a P value <0.05 for the interaction term between time and APOL1 risk status. This approach has the advantage of preserving the correlation structure of all variables in the data without making potentially inappropriate assumptions.
To further investigate whether annual eGFR change differed before and after the onset of proteinuria, we again constructed linear mixed-effects models with random intercepts and slopes using longitudinal eGFR as the outcome and included only eGFR observations from participants who had experienced incident proteinuria. Each eGFR observation had an indicator variable denoting whether the observation occurred before or after the onset of proteinuria. The covariates were the same as the model described above (age, sex, European ancestry, baseline systolic BP, log-transformed baseline proteinuria, randomized BP goal, and randomized BP drug). The interaction term between follow-up time and the indicator variable for the onset of proteinuria was evaluated to determine whether eGFR declined faster after incident proteinuria.
Data were analyzed using Stata (Version 12, 2011; College Station, TX) and R (Version 3.21) statistical software with P values <0.05 considered to be statistically significant.
Results
Baseline Characteristics
Among the 1094 AASK trial participants, we excluded 358 individuals with prevalent proteinuria (defined as a 24-hour urine protein-to-creatinine ratio ≥0.22 g/g Cr at baseline) and four individuals with missing data on proteinuria at baseline. Of the remaining 732 participants, 480 individuals had APOL1 genotyping available for the G1 and G2 risk alleles and were included in our study population. Among these 480 participants, 82 (17%) carried APOL1 high-risk genotypes.
At baseline, APOL1 high-risk participants had a lower mean eGFR (50 versus 53 ml/min per 1.73 m2; P=0.02) and mean systolic BP (139 versus 150 mm Hg; P<0.01) compared with low-risk participants. Otherwise, the two risk groups were similar at baseline (Table 1).
Table 1.
Baseline characteristics of African-American Study of Kidney Disease and Hypertension participants without baseline proteinuria, by APOL1 risk allele status
| Characteristic | All (n=480) | APOL1 Low-Risk (n=398) | APOL1 High-Risk (n=82) | P Value |
|---|---|---|---|---|
| Age at randomization, yr | 56±10 | 56±10 | 55±10 | 0.56 |
| Female | 194 (40) | 162 (41) | 32 (39) | 0.81 |
| European ancestry, % | 16.6±13.7 | 17.1±14.1 | 14.2±11.4 | 0.05 |
| Current smoker | 131 (27) | 108 (27) | 23 (28%) | 0.89 |
| Systolic BP, mm Hg | 148±24 | 150±25 | 139±19 | <0.01 |
| Body mass index, kg/m2 | 30.7±6.6 | 30.6±6.4 | 31.1±7.4 | 0.62 |
| Baseline eGFR, ml/min per 1.73 m2 | 53±13 | 53±14 | 50±12 | 0.02 |
| Total cholesterol, mg/dl | 211±43 | 211±44 | 211±37 | 0.91 |
| HDL, mg/dl | 48±16 | 49±16 | 48±13 | 0.70 |
| Serum albumin, g/dl | 4.3±0.3 | 4.3±0.3 | 4.4±0.3 | 0.09 |
| Median urine protein, g/g Cr | 0.04 (0.02–0.08) | 0.04 (0.02–0.08) | 0.04 (0.02–0.09) | 0.43 |
| Mean urine protein, g/g Cr | 0.06±0.05 | 0.06±0.05 | 0.06±0.05 | 0.55 |
| Urine sodium, g/d | 3.4 (2.2–4.5) | 3.4 (2.2–4.6) | 3.1 (2.1–4.4) | 0.72 |
| Randomized BP goal | 0.72 | |||
| Low | 243 (51) | 203 (51) | 40 (49) | |
| Usual | 237 (49) | 195 (49) | 42 (51) | |
| Randomized BP drug | 0.67 | |||
| Metoprolol | 191 (40) | 158 (40) | 33 (40) | |
| Ramipril | 198 (41) | 167 (42) | 31 (38) | |
| Amlodipine | 91 (19) | 73 (18) | 18 (22) |
Values presented as mean±SD, median (interquartile range), or n (%). APOL1 high-risk defined as having 2 risk alleles and low-risk defined as having 0–1 risk alleles.
Association between APOL1 Risk Status and Incident Proteinuria
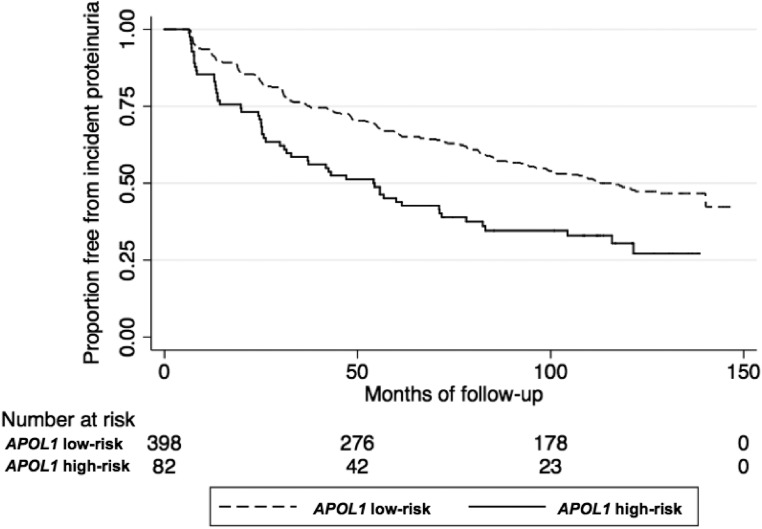
Over a median follow-up of 6.8 years, 254 (53%) individuals developed incident proteinuria, 56 (22%) of whom carried APOL1 high-risk genotypes. Censoring for ESRD and death, individuals with the APOL1 high-risk genotypes were 82% more likely to develop incident proteinuria compared with those with the low-risk genotypes in unadjusted analyses (hazard ratio, 1.82; 95% confidence interval [95% CI], 1.35 to 2.46; P<0.01; Figure 1; Table 2). This association remained robust after adjustment for age, sex, European ancestry, baseline eGFR, baseline systolic BP, and randomized treatment interventions (adjusted hazard ratio, 1.72; 95% CI, 1.27 to 2.32; P<0.01; Table 2). In exploratory analyses, the association between the APOL1 risk variants and incident proteinuria was not modified by age (P-interaction=0.84), sex (P-interaction=0.55), baseline systolic BP (P-interaction=0.69), body mass index (P-interaction=0.20), or dietary sodium intake (P-interaction=0.40). From baseline to the onset of proteinuria, the APOL1 high-risk group had a 35% faster increase in proteinuria annually compared with the low-risk group (95% CI, 12% to 61%; P<0.01). These results were consistent with our primary analyses in which the APOL1 high-risk group was at increased risk of developing incident proteinuria compared with the low-risk group.
Figure 1.
Kaplan–Meier curves for incident proteinuria by APOL1 risk status in the African-American Study of Kidney Disease and Hypertension.
Table 2.
Comparison of incident proteinuria by APOL1 risk allele status in the African-American Study of Kidney Disease and Hypertension
| Model | Hazard Ratio for APOL1 High-Risk versus Low-Risk | 95% CI | P Value |
|---|---|---|---|
| Unadjusted | 1.82 | (1.35 to 2.46) | <0.01 |
| Model 1: adjusted for sociodemographic factors | 1.80 | (1.33 to 2.43) | <0.01 |
| Model 2: model 1+clinical factors | 1.68 | (1.24 to 2.27) | <0.01 |
| Model 3: model 2+randomized treatment groups | 1.72 | (1.27 to 2.32) | <0.01 |
Sociodemographic factors include age, sex, and percentage of European ancestry. Clinical factors include baseline eGFR and baseline systolic BP. Randomized treatment groups include BP drug and goal. APOL1 high-risk defined as having 2 risk alleles and low-risk defined as having 0–1 risk alleles. 95% CI, 95% confidence interval.
Annual eGFR Decline Before and After Incident Proteinuria, and by APOL1 Risk Status
The overall annual decline in eGFR was significantly faster among individuals with the high-risk genotypes compared with those with the low-risk genotypes (−1.65 versus −1.11 ml/min per 1.73 m2 per year; difference of −0.54 ml/min per 1.73 m2 per year; 95% CI, −0.95 to −0.13; Table 3). In analyses stratified by onset of proteinuria, however, annual rates of eGFR decline did not differ significantly between APOL1 high- versus low-risk individuals (Table 3). Power analyses using bootstrapping showed that we had 80% power to detect minimum annual differences of −0.54 ml/min per 1.73 m2 for the overall period, −0.55 ml/min per 1.73 m2 before the onset of proteinuria with a sample size of 480, and −1.00 ml/min per 1.73 m2 after the onset of proteinuria with a sample size of 254. Among those who developed proteinuria (n=254), the overall annual change in eGFR before the development of proteinuria was −1.43 ml/min per 1.73 m2 (95% CI, −1.76 to −1.11) and increased significantly faster to −2.20 ml/min per 1.73 m2 (95% CI, −2.52 to −1.87) after the onset of proteinuria (P for interaction between follow-up time and incident proteinuria <0.01).
Table 3.
Annual eGFR decline by APOL1 risk status, overall and stratified by onset of proteinuria, in the African-American Study of Kidney Disease and Hypertension
| Model | Annual eGFR Decline | Difference in Annual eGFR Decline for APOL1 High-Risk versus Low-Risk | P-interactiona | |
|---|---|---|---|---|
| APOL1 Low-Risk (95% Confidence Interval) | APOL1 High-Risk (95% Confidence Interval) | |||
| Overall (n=480) | −1.11 (−1.32 to −0.90) | −1.65 (−2.05 to −1.25) | −0.54 (−0.95 to −0.13) | 0.01 |
| Before incident proteinuria (n=480) | −0.47 (−0.68 to −0.26) | −0.52 (−0.96 to −0.09) | −0.06 (−0.50 to 0.38) | 0.80 |
| After incident proteinuria (n=254) | −2.44 (−2.89 to −1.98) | −2.67 (−3.47 to −1.91) | −0.25 (−1.08 to 0.58) | 0.54 |
Models adjusted age at randomization, sex, percentage of European ancestry, baseline systolic BP, log-transformed baseline proteinuria, and randomized treatment groups. eGFR in ml/min per 1.73 m2.
P value for interaction term between time (in years) and APOL1 risk status (high- versus low-risk).
Discussion
In this study of blacks with hypertension-attributed CKD followed for up to 12 years, those with the APOL1 high-risk genotypes had 72% higher risk of developing incident proteinuria compared with those with the low-risk genotypes. Among blacks who developed proteinuria, kidney function decline accelerated after the onset of proteinuria with no significant difference by APOL1 risk status. These results suggest that APOL1 risk variants play a role in the development of proteinuria among blacks with established CKD. Moreover, our study suggests that, at least among this population, the onset of proteinuria in both APOL1 high- and low-risk individuals heralds accelerated kidney function decline.
Our findings extend prior work on albuminuria among healthy young adults within the Coronary Artery Risk Development in Young Adults (CARDIA) study. Peralta et al. (2) previously demonstrated that the APOL1 high-risk genotypes are associated with a 2.9-fold greater odds of incident albuminuria compared with the low-risk genotypes. We observed a similar association between the APOL1 risk variants and incident proteinuria in the context of preexisting moderate CKD, even after accounting for relevant sociodemographic and clinical factors (e.g., baseline eGFR and systolic BP). Both studies’ findings highlight that proteinuria itself, regardless of APOL1 risk status, is a substantial risk factor for progression to ESRD (23–25).
We also assessed annual eGFR change before and after incident proteinuria and found that although eGFR declined faster after the onset of proteinuria, the rate of eGFR decline did not differ significantly between APOL1 high- versus low-risk individuals. These findings contrast those among a cohort of young adults with normal kidney function. In this study by Peralta et al. (2), blacks with APOL1 high-risk genotypes experienced a steeper decline in mean eGFR after the onset of albuminuria compared with those with low-risk genotypes. Several differences in study design and methodology may underlie the discrepant findings of the two studies. First, the participants included in AASK had much lower baseline eGFRs compared with CARDIA participants. At the time of incident proteinuria, 25% of our study population already had an eGFR<35 ml/min per 1.73 m2, thus perhaps limiting our ability to detect differences in eGFR decline by APOL1 risk status. Second, we used proteinuria instead of albuminuria; our definitions for incidence (doubling of urine protein-to-creatinine ratio to a level ≥0.22 g/g Cr versus urine albumin-to-creatinine ratio ≥30 mg/g, respectively) may not be directly comparable. Third, we examined annual absolute change in serum creatinine-based eGFR whereas Peralta et al. (2) studied annualized percentage change in log-transformed serum cystatin-based eGFR. The latter outcome may be less variable and hence easier to detect a difference in eGFR slope by APOL1 risk variants after the onset of proteinuria or albuminuria. Finally, follow-up time after the onset of proteinuria or albuminuria was relatively shorter in AASK (median 4.8 years) compared with CARDIA (mean approximately 9 years). Thus, we may not have had sufficient length of follow-up and therefore adequate power to detect a difference in eGFR decline between the APOL1 risk groups in AASK. Our results are consistent with a prior investigation by Grams et al. (26) in AASK, which demonstrated that among participants with a baseline eGFR of 30 ml/min per 1.73 m2, the APOL1 high-risk genotypes were not associated with a higher risk for ESRD compared with the low-risk genotypes. Still, our results highlight the importance of continued monitoring for proteinuria in patients with hypertension-attributed CKD, regardless of their APOL1 risk status.
Our study has several strengths and limitations. First, our study population consisted of a well characterized cohort of blacks with hypertension-attributed CKD. Importantly, the data were collected prospectively with frequent data collection. Second, follow-up was long, up to 12 years, which enabled us to better capture cases of incident proteinuria. Limitations include the use of total proteinuria rather than albuminuria, because the latter was not available in AASK. Moreover, the study population consisted of a group of highly selected individuals. APOL1 high-risk individuals with more advanced kidney disease were likely excluded from our study, thus biasing our risk estimates toward the null. Although our findings may not be generalizable to other patient populations, our study population still represents a high-risk group for CKD progression that warrants investigation, particularly because there is heterogeneity in ESRD risk among APOL1 high-risk individuals. Finally, our results on annual eGFR change before and after the onset proteinuria should be interpreted with caution. We had limited statistical power to detect differences in annual eGFR change by APOL1 risk status when stratified by proteinuria onset.
In conclusion, APOL1 high-risk variants are associated with an increased risk for incident proteinuria among blacks with established CKD. Kidney function declines faster after the onset of proteinuria but does not differ significantly by APOL1 risk status. The results of our study suggest that individuals with established CKD attributed to hypertension should be monitored for proteinuria, because it is associated with faster kidney function decline.
Disclosures
T.K.C. previously owned stock in Pfizer Pharmaceuticals. C.A.P. owns stock in and is a consultant for Cricket Health, Inc. and previously was a consultant for Vital Labs, Inc. The other authors have nothing to declare.
Supplementary Material
Acknowledgments
T.K.C. is supported by the Norman S. Coplon Extramural Grant Program by Satellite Healthcare, a not-for-profit renal care provider. A.T. is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants 1R01DK108803 and 1R21DK112087. L.J.A. is supported by NIH/NIDDK grant 1R01DK108803. M.M.E. is supported by NIH/NIDDK grant 1R01DK103574. The African-American Study of Kidney Disease and Hypertension trial and cohort were supported by institutional grants from the NIH/NIDDK (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, DK 2818-02, DK057867, and DK048689) and the following pharmaceutical companies—King Pharmaceuticals, Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn. This project has been funded in part with federal funds from the National Cancer Institute, NIH, under contract HHSN26120080001E. This Research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Portions of this work were presented at the 2015 American Society of Nephrology Kidney Week in San Diego, California (November 3–8, 2015).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “APOL1 and Proteinuria in the AASK: Unraveling the Pathobiology of APOL1,” on pages 1723–1725.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01180117/-/DCSupplemental.
References
- 1.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peralta CA, Bibbins-Domingo K, Vittinghoff E, Lin F, Fornage M, Kopp JB, Winkler CA: APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol 27: 887–893, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR: Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55: 992–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB, AASK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J: Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tin A, Grams ME, Estrella M, Lipkowitz M, Greene TH, Kao WH, Li L, Appel LJ: Patterns of kidney function decline associated with APOL1 genotypes: Results from AASK. Clin J Am Soc Nephrol 11: 1353–1359, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen TK, Choi MJ, Kao WH, Astor BC, Scialla JJ, Appel LJ, Li L, Lipkowitz MS, Wolf M, Parekh RS, Winkler CA, Estrella MM, Crews DC: Examination of potential modifiers of the association of APOL1 alleles with CKD progression. Clin J Am Soc Nephrol 10: 2128–2135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel LJ, Middleton J, Miller ER 3rd , Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, Douglas J, Jamerson K, Lewis J, Kutner M, Randall OS, Wang SR: Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toto RD, Greene T, Hebert LA, Hiremath L, Lea JP, Lewis JB, Pogue V, Sika M, Wang X: Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: Results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis 56: 896–906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O’Connor D, Ojo A, Phillips R, Sika M, Wright J Jr. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, Greene T, Appel L, Contreras G, Douglas J, Lash J, Toto R, Van Lente F, Wang X, Wright JT Jr. A comparison of iothalamate-GFR and serum creatinine-based outcomes: Acceleration in the rate of GFR decline in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol 15: 3175–3183, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Patterson N, Hattangadi N, Lane B, Lohmueller KE, Hafler DA, Oksenberg JR, Hauser SL, Smith MW, O’Brien SJ, Altshuler D, Daly MJ, Reich D: Methods for high-density admixture mapping of disease genes. Am J Hum Genet 74: 979–1000, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie D, Joffe MM, Brunelli SM, Beck G, Chertow GM, Fink JC, Greene T, Hsu CY, Kusek JW, Landis R, Lash J, Levey AS, O’Conner A, Ojo A, Rahman M, Townsend RR, Wang H, Feldman HI: A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol 3: 1332–1338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Grams ME, Li L, Greene TH, Tin A, Sang Y, Kao WH, Lipkowitz MS, Wright JT, Chang AR, Astor BC, Appel LJ: Estimating time to ESRD using kidney failure risk equations: Results from the African American Study of Kidney Disease and Hypertension (AASK). Am J Kidney Dis 65: 394–402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.