Abstract
Background and objectives
The optimal type of initial permanent access for hemodialysis among the elderly is controversial. Duration of central venous catheter dependence, patient comorbidities, and life expectancy are important considerations in whether to place an arteriovenous fistula or graft. We used an observational study design to compare clinical outcomes in elderly patients who initiated hemodialysis with a central venous catheter and subsequently had an arteriovenous fistula or graft placed.
Design, setting, participants, & measurements
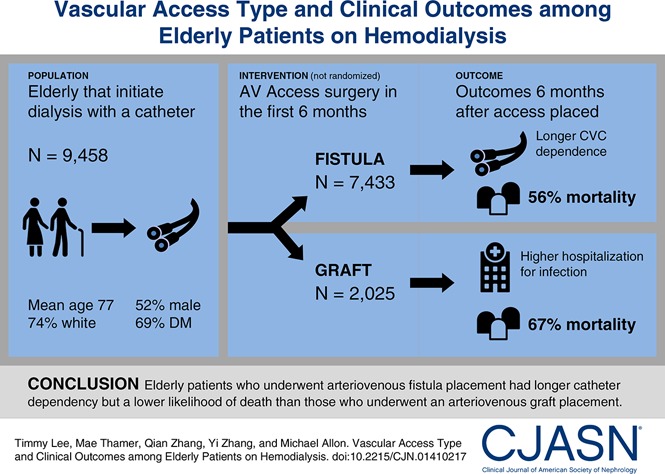
We identified 9458 United States patients ages ≥67 years old who initiated hemodialysis from July 1, 2010 to June 30, 2011 with a central venous catheter and no secondary vascular access and then received an arteriovenous fistula (n=7433) or graft (n=2025) within 6 months. We evaluated key clinical outcomes during the 6 months after vascular access placement coincident with high rates of catheter use and used a matched propensity score analysis to examine patient survival.
Results
Central venous catheter dependence was greater in every month during the 6-month period after arteriovenous fistula versus graft placement (P<0.001). However, rates of all-cause infection-related hospitalization (adjusted relative risk, 0.93; 95% confidence interval, 0.87 to 0.99; P=0.01) and bacteremia/septicemia-related hospitalization (adjusted relative risk, 0.90; 95% confidence interval, 0.82 to 0.98; P=0.02) were lower in the arteriovenous fistula versus graft group as was the adjusted risk of death (hazard ratio, 0.76; 95% confidence interval, 0.73 to 0.80; P<0.001).
Conclusions
Despite extended central venous catheter dependence, elderly patients initiating hemodialysis with a central venous catheter who underwent arteriovenous fistula placement within 6 months had fewer hospitalizations due to infections and a lower likelihood of death than those receiving an arteriovenous graft.
Keywords: dialysis access; dialysis; Aged; arteriovenous fistula; Arteriovenous Shunt, Surgical; Bacteremia; Central Venous Catheters; Confidence Intervals; hospitalization; Humans; Life Expectancy; Propensity Score; renal dialysis; Risk; Sepsis
Introduction
Of the 110,000 patients starting hemodialysis (HD) annually in the United States, over 80% initiate HD with a central venous catheter (CVC) (1). The majority of patients initiating HD with a CVC will subsequently undergo placement of a permanent vascular access, either an arteriovenous fistula (AVF) or an arteriovenous graft (AVG). The current national vascular access guidelines strongly recommend placement of an AVF in preference to an AVG (2,3). These recommendations are on the basis of the observation that, after they are used for dialysis, AVFs have superior long-term survival and require fewer interventions to maintain their long-term patency (4) compared with AVGs. However, a substantial proportion (20%–60%) of new AVFs is never useable for dialysis (AVF nonmaturation) (4,5), consigning many patients to prolonged CVC dependence, which is associated with higher mortality (6–11). Even among AVFs that do mature properly to be used for dialysis, AVF maturation often takes an average of 4 months compared with 2–4 weeks for an AVG (12). The advantages of AVFs over AVGs may be less apparent in elderly (age >65 years old) patients on HD for several reasons. First, AVF nonmaturation is higher in elderly patients compared with younger patients (13). Second, elderly patients have more comorbidities (e.g., coronary or peripheral artery disease) associated with a high rate of AVF nonmaturation (14). Third, elderly patients have shorter life expectancy, such that they may not live long enough to see the benefits of prolonged AVF survival (15).
Prolonged CVC dependence is associated with a higher frequency of bacteremia events, hospitalizations, and mortality (6,16–19). Patients on HD receiving an AVF have a substantially longer duration of CVC dependence than those with AVG placement (12), and this difference may translate to more hospitalizations or deaths. Given this rationale, we hypothesized that, in older patients on HD who initiate HD with a CVC, placement of an AVG rather than an AVF will result in (1) shorter CVC dependence time, (2) fewer infection-related hospitalizations, and (3) longer overall patient survival.
Materials and Methods
Observational equivalent of the intention to treat principle was applied to conduct this retrospective observational study to compare the effect of AVG with the effect of AVF on clinical outcomes of elderly patients initiating HD with a CVC.
Data Sources and Study Population
The Institutional Review Board at the University of Alabama at Birmingham approved this study. We used standard analytic files derived from the US Renal Data System (USRDS) for July 1, 2010 and December 31, 2013. In addition, 2 years of pre-ESRD Medicare data were used to collect additional baseline information, including comorbidities and hospital stays before the start of HD.
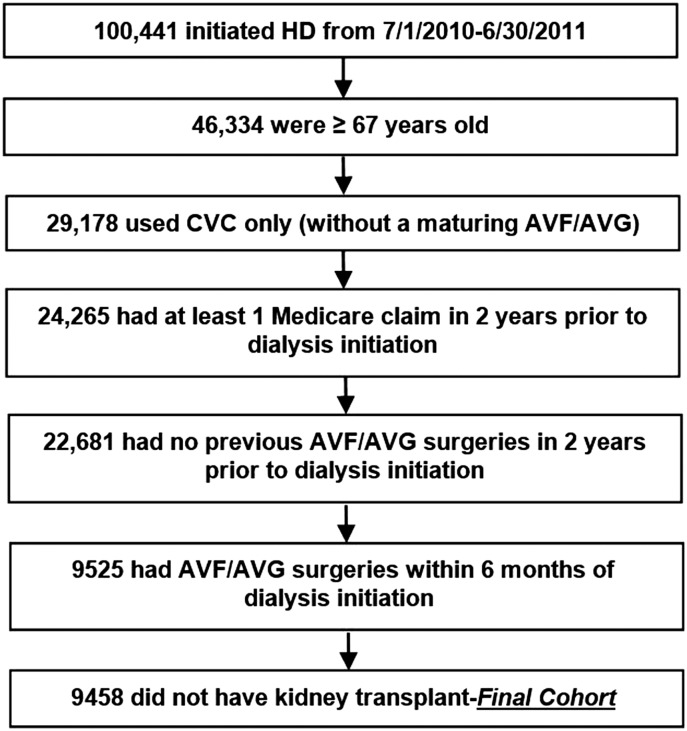
All patients on incident HD who were ≥67 years of age and had their first ESRD service in the 1-year period between July 1, 2010 and June 30, 2011 were identified as our baseline population. To ensure that CVC was the only vascular access present at the start, patients on HD were excluded from the study cohort if (1) they were using AVF or AVG or had a maturing AVF or AVG at HD initiation as reported in the 2728 Medical Evidence Form (20,21) or (2) they underwent AVF or AVG surgery in the 2-year period preceding HD initiation as assessed by Current Procedural Terminology-4 procedure codes. Figure 1 shows study inclusion and exclusion criteria with a final CVC-only study sample size of 9458—21% received an AVG, and 79% received an AVF within 6 months of dialysis initiation.
Figure 1.
Patient cascade using US Renal Data System data. AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter; HD, hemodialysis.
Variables of Interest
The main study exposure was AVG versus AVF insertion within 6 months of dialysis initiation identified using Current Procedural Terminology-4 codes for AVF/AVG insertion (Supplemental Table 1). Three outcomes of the study were ascertained.
(1) Catheter dependence defined as use of a CVC as the vascular access for dialysis in each of the first 6 months after AVF/AVG placement. Effective July of 2010, all dialysis units were required by Centers for Medicare and Medicaid Services to report monthly vascular access use for all active patients on HD using vascular access modifiers: V5 (CVC), V6 (AVG), or V7 (AVF).
(2) Hospitalization in the 6-month period after AVF/AVG insertion was identified separately for all-cause infection-related hospitalizations, vascular access–related hospitalizations, sepsis/bacteremia hospitalizations, and cardiovascular disease–related hospitalizations. The list of the relevant International Statistical Classification of Diseases-9 codes used is presented in Supplemental Table 1. The incidence rate for hospitalization for each cohort (AVG or AVF placement) was calculated as the total number of hospitalizations divided by person-years of follow-up. We used negative binomial regression to calculate an incidence ratio adjusted for age, sex, and race, because the data were overdispersed (mean was not equal to variance of the distribution).
(3) Mortality. All-cause mortality was obtained from the USRDS Patient Profile File. The follow-up period for mortality was calculated from the date of access surgery until December 31, 2013, death, or loss to follow-up (defined as missing HD or hospital information for ≥30 consecutive days), whichever was earliest.
The Medical Evidence Form was used to determine patient demographics, functional status, and laboratory values at HD initiation. Major comorbidities (e.g., diabetes, acute myocardial infarction, and coronary revascularization) were identified using one inpatient or two outpatient Medicare claims during the 2-year predialysis period. We imputed missing data by stochastic imputations (random drawings from an underlying distribution), a method suitable to impute continuous variables with nonrandom distributions (22,23). We examined the missing patterns of laboratory values before conducting imputation. Missing data were not random but rather, varied by comorbidity index, body mass index, and age. We included these variables in the regression model to impute missingness. Patients in the AVF group were more likely to have missing laboratory values. We used the same baseline for all outcomes defined as HD initiation. Start of follow-up was defined as surgery date for both patients with AVGs and patients with AVFs. We used a 6-month follow-up period for catheter dependence and hospitalization outcomes, because this was the time of greatest differential in CVC use between the two cohorts. For the mortality outcome, we used a longer (maximum 3.5 years) follow-up period. We included time between HD initiation and vascular access placement as an important potential confounder in our propensity score estimation.
Statistical Analyses
To minimize treatment allocation bias (or selection bias) on the basis of the physicians’ clinical judgement (13,14,24–30), we calculated a propensity score (i.e., the probability that a patient would undergo a new AVF or AVG creation) using logistic regression to create comparable groups of AVG and AVF primarily by matching (31). We chose propensity score matching, because it estimates the average treatment effect on the treated (i.e., the average effect if all patients with AVGs would choose AVG), which is more clinically relevant. Propensity score matching also achieved a better balance between treatment groups. Factors for propensity score matching were selected on the basis of the published literature and expert knowledge of the nephrologist authors. We included potential confounders that affect both treatment and outcomes in the model to estimate propensity scores. In the absence of unmeasured confounding factors, two subjects with the same propensity score but different exposure status can be considered as if they were randomly assigned to the exposure. Thus, matching for the propensity score can balance the distribution of the observed confounders that may arise (32). Because AVG creation was threefold less common than AVF surgery, we applied a 1:3 greedy matching algorithm using SAS, version 9.3, macro %GMATCH to increase study power (33).
We used Cox proportional hazard models stratifying on matched pairs and models with robust SEM to account for clustering to analyze time to event for mortality by vascular access choice. Kaplan–Meier plots were used to display the survivor functions. Hazard ratios and their 95% confidence intervals (95% CIs) were calculated, and statistical significance was assessed by the log rank test. We tested the proportional hazard assumption of Cox model by plotting Schoenfeld residuals against time and performed complimentary log-log analysis of cumulative hazard. All analyses were performed using SAS, version 9.3 software (www.sas.com).
We examined treatment differences (AVG versus AVF) stratified by quintile of propensity score (Supplemental Figure 1) and found that the hazard ratio is consistent across quintiles. We used the Rosenbaum method of sensitivity analysis for matched data to measure how inferences (odds or hazards ratios) might change if unmeasured confounding was present (34–36) and obtained a Γ value of 1.29, indicating that our study results were very sensitive to hidden bias; the established cutoff for the influence of hidden bias is a Γ<5. Our mortality estimates could, therefore, change with an unmeasured covariate to produce an effect as low as a 1.3 times increase in the odds of treatment assignment, which will definitely affect the ultimate inference that we make between treatment assignment and mortality.
Results
Baseline Characteristics of Elderly Patients with ESRD and AVF/AVG Surgeries within 6 Months of HD Initiation
From a cohort of 100,441 patients who initiated HD from July 1, 2010 to June 30, 2011, 46,334 (46%) were ≥67 years old. Of these, 29,178 (63%) initiated HD with a CVC only without a maturing AVF or AVG (Figure 1). Of these, 24,265 had at least one inpatient/outpatient Medicare claim in the 2 years before HD initiation, and 22,681 had no previous AVF/AVG surgeries using 2 years of pre-ESRD claims; of these, 9458 patients without a transplant during the first year of dialysis underwent AVF or AVG surgery within 6 months of HD initiation.
In summary, we identified a cohort of 9458 elderly Medicare-eligible patients who initiated HD with a CVC and no secondary vascular access between July 1, 2010 and June 30, 2011 who received an AVF (n=7433) or AVG (n=2025) during the ensuing 6 months. Table 1 summarizes the baseline demographics and comorbidities of this patient cohort. The mean patient age was 77±6 years old, 74% were white, and 52% were men; diabetes was present in 69%. Acute myocardial infarction occurred in the prior year in 11% of patients, and 11% of patients had a history of coronary revascularization. The mean comorbidity index score was 10.3±4.4. These patients had a mean of 18 hospital days in the 6-month period preceding ESRD.
Table 1.
Baseline characteristics of patients who initiated hemodialysis with a central vein catheter between July 1, 2010 and June 30, 2011 and had an arteriovenous fistula or graft surgery within 6 months
| Variable | All, % | AVF, n=7433 | AVG, n=2025 | P Value |
|---|---|---|---|---|
| Age at dialysis initiation, yr | 77±7 | 77±6 | 78±7 | <0.001 |
| 67 to <75 | 41 | 3127 (42) | 735 (36) | <0.001 |
| 75 to <85 | 45 | 3,311 (45) | 921 (46) | |
| ≥85 | 14 | 995 (13) | 369 (18) | |
| Sex | ||||
| Men | 52 | 4102 (55) | 825 (41) | <0.001 |
| Women | 48 | 3331 (45) | 1200 (59) | |
| Race | ||||
| White | 74 | 5660 (76) | 1324 (65) | <0.001 |
| Black | 21 | 1372 (19) | 605 (30) | |
| Other/unknown | 5 | 401 (5) | 96 (5) | |
| Body mass index, kg/m2 | 28±7 | 29±7 | 28±7 | <0.001 |
| Comorbidities | ||||
| Liu comorbidity index | 10±4 | 10±4 | 11±5 | <0.001 |
| 0–7 | 21 | 1619 (22) | 394 (20) | 0.01 |
| 7–13 | 43 | 3236 (44) | 861 (43) | |
| ≥13 | 35 | 2578 (35) | 768 (38) | |
| Diabetes, yes | 69 | 5123 (69) | 1406 (69) | 0.66 |
| Cancer, yes | 21 | 1534 (21) | 461 (23) | 0.04 |
| Chronic obstructive pulmonary disease, yes | 38 | 2779 (37) | 828 (41) | 0.004 |
| Peripheral vascular disease, yes | 56 | 4119 (55) | 1191 (59) | <0.01 |
| Depression, yes | 14 | 975 (13) | 356 (18) | <0.001 |
| Dementia, yes | 11 | 745 (10) | 276 (14) | <0.001 |
| Acute myocardial infarction in prior 1 yr, yes | 11 | 827 (11) | 235 (12) | 0.55 |
| Coronary revascularization, yes | 11 | 837 (11) | 187 (9) | <0.01 |
| History of stroke, yes | 19 | 1362 (18) | 467 (23) | <0.001 |
| Amputation, yes | 3 | 211 (3) | 67 (3) | 0.27 |
| Inability to ambulate//institutionalized/functional limitations, yes | 26 | 1758 (24) | 674 (33) | <0.001 |
| Hospital days, 6 mo before ESRD | 18±18 | 17±17 | 21±21 | <0.001 |
| GFR, mean±SD, ml/min per 1.73 m2 | 13±6 | 13±6 | 14±6 | <0.001 |
| Hemoglobin, mean±SD, g/dl | 10±3 | 10±3 | 10±3 | <0.001 |
| Serum albumin, g/dl | ||||
| Mean±SD | 3±0.9 | 3±0.8 | 3±1 | <0.001 |
| <3.5 | 71 | 3889 (70) | 1107 (75) | 0.001 |
| ≥3.5 | 29 | 1649 (30) | 380 (26) | |
| Dialysis initiation to VA surgery, d | 69±45 | 71±43 | 60±50 | <0.001 |
All values are n (%) or mean±SD unless otherwise indicated. P values by Pearson chi-squared or t test. Percentages of missing: body mass index (0.7%), hemoglobin (8%), serum albumin (26%), and GFR (3%). AVF, arteriovenous fistula; AVG, arteriovenous graft; VA, vascular access.
Compared with patients receiving an AVF, the cohort with AVG placement was significantly older (78 versus 77 years old; P<0.001), had more women (59% versus 45%; P<0.001), and had more blacks (30% versus 19%; P<0.001). In addition, the AVG cohort had higher comorbidity index (11% versus 10%; P<0.001), history of stroke (23% versus 18%; P<0.001), dementia (14% versus 10%; P<0.001), and inability to ambulate (33% versus 24%; P<0.001) (Table 1). In addition, the AVG cohort had more hospital days in the 6 months before dialysis initiation (21 versus 17 days; P<0.001). Finally, the mean time from HD initiation to the vascular access surgery was about 2 weeks shorter in the AVG cohort (60 versus 71 days; P<0.001).
We generated matched propensity scores as the probability of having AVF or AVG as a function of age, body mass index, Liu comorbidity score, sex, race, inability to ambulate/transfer, history of acute myocardial infarction, history of amputation at baseline, and other variables listed in Table 2. Good balance of propensity scores was achieved as revealed by common support and standardized bias <0.10.
Table 2.
Standardized difference for each covariate before and after propensity score matching
| Variable | Unmatched Sample | Matched Sample |
|---|---|---|
| Age | 0.175 | 0.002 |
| BMI | 0.048 | 0.011 |
| Liu comorbidity score | 0.103 | 0.007 |
| Time to study entry | 0.245 | 0.005 |
| Albumin | 0.043 | 0.007 |
| Hemoglobin | 0.02 | 0.017 |
| GFR | 0.055 | 0.006 |
| Sex | 0.292 | 0.004 |
| White | 0.238 | 0.003 |
| Black | 0.269 | 0.006 |
| Other race | 0.03 | 0.005 |
| Depression | 0.124 | 0.019 |
| Dementia | 0.112 | 0.017 |
| Institutionalized/inability to ambulate/transfer | 0.215 | 0 |
| Acute myocardial Infarction | 0.015 | 0.001 |
| Stroke | 0.117 | 0.017 |
Standardized bias was calculated by difference in means divided by SD of the total sample. A standardized bias <0.10 is considered a balance of the potential confounder between arteriovenous fistula and graft groups. BMI, body mass index.
CVC Dependence in AVF and AVG Cohorts during the 6 Months after Vascular Access Surgery
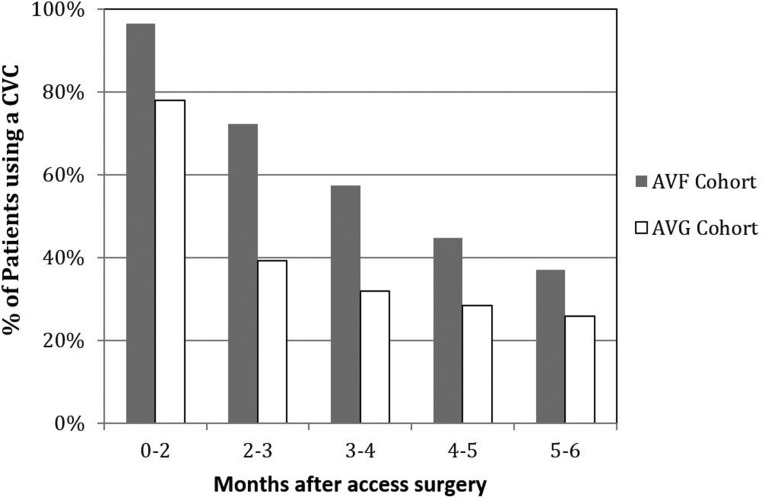
We assessed the effect of the initial type of vascular access placed on the likelihood of CVC dependence during the ensuing 6 months. The patients with AVF placement had longer CVC dependence in each month during the first 6 months after surgery compared with those with AVG placement (P<0.001 across the entire 6-month period in each month) (Figure 2). After 6 months, CVC dependence was not significantly different between the two cohorts (data not shown).
Figure 2.
Central venous catheter (CVC) dependence in arteriovenous fistula (AVF) and arteriovenous graft (AVG) cohorts by months after surgery among a cohort of elderly patients on dialysis who initiated dialysis with a CVC and had a vascular access surgery within 6 months of dialysis initiation (P<0.001 across the entire 6-month period comparing AVG with AVF cohort use of CVC).
Hospitalization during the 6 Months after Vascular Access Surgery
In the 6-month period after access surgery, compared with patients in the AVG cohort, those receiving an AVF had a significantly lower all-cause infection–related hospitalization rate (adjusted relative risk [RR], 0.93; 95% CI, 0.87 to 0.99; P=0.01) and lower bacteremia/septicemia-related hospitalization (RR, 0.90; 95% CI, 0.82 to 0.98; P=0.02) but similar rates of vascular access–related hospitalization (RR, 0.96; 95% CI, 0.80 to 1.14; P=0.61) and cardiovascular disease–related hospitalization (RR, 0.95; 95% CI, 0.89 to 1.02; P=0.17) (Table 3). Supplemental Table 2 provides crude incidence rates and numbers of events for each outcome and absolute difference in risk of these outcomes.
Table 3.
Association of arteriovenous fistula versus arteriovenous graft with risk of hospitalization
| Hospitalization | Crude RR (AVG Cohort Reference) with 95% CI | P Value | Adjusted RR (AVG Cohort Reference) with 95% CI | P Value |
|---|---|---|---|---|
| All cause infection | 0.93 (0.88 to 0.99) | 0.02 | 0.93 (0.87 to 0.99) | 0.01 |
| Vascular access related | 0.95 (0.80 to 1.13) | 0.57 | 0.96 (0.80 to 1.14) | 0.61 |
| Bacteremia/septicemia | 0.90 (0.82 to 0.98) | 0.02 | 0.90 (0.82 to 0.98) | 0.02 |
| Cardiovascular disease related | 0.96 (0.90 to 1.02) | 0.22 | 0.95 (0.89 to 1.02) | 0.17 |
Adjusted by age, sex, and race by using a negative binomial regression. RR, (incidence) rate ratio; AVG, arteriovenous graft; 95% CI, 95% confidence interval.
Mortality by Vascular Access Inserted within 6 Months of HD Initiation
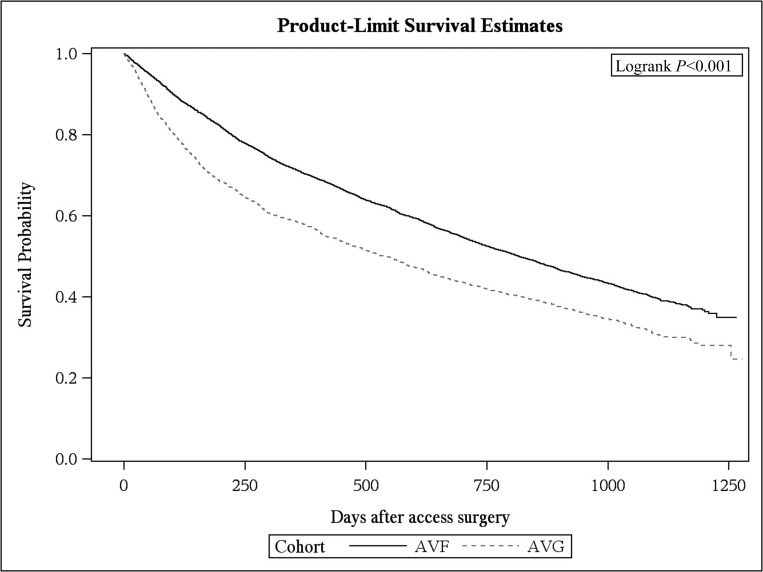
Nearly two thirds (59%) of the cohort participants died, including 4202 in the AVF group and 1353 in the AVG group. The propensity score–matched survival of patients age ≥67 years old with AVF or AVG placement within 6 months of HD initiation was significantly greater in the AVF group compared with those patients with AVG placement (P<0.001) (Figure 3). Using both a Cox hazards proportional model (hazard ratio, 0.72; 95% CI, 0.68 to 0.77; P<0.001) and a model stratified on matched pairs, patients with an AVF had a lower risk of death versus those with an AVG (hazard ratio, 0.76; 95% CI, 0.73 to 0.80; P<0.001).
Figure 3.
Propensity score–matched survival curve of patients ages ≥67 years old with arteriovenous fistula (AVF) or arteriovenous graft (AVG) placement within 6 months of dialysis initiation was significantly greater in the AVF group compared with those patients with AVG placement (P<0.001).
Discussion
This study compared key clinical outcomes in elderly patients initiating HD with a CVC who subsequently underwent placement of an AVF or AVG. As expected, patients who received an AVF were substantially more likely to remain CVC dependent during the ensuing 6 months than those receiving an AVG. However, despite the longer CVC dependence, the AVF group had a lower frequency of hospitalizations related to all-cause infection and septicemia. Moreover, the AVF group had a lower adjusted hazard of death. Taken together, these observations are not consistent with our hypothesis that longer CVC dependence in patients on dialysis receiving an AVF translates to more hospitalizations and early death.
Several previous studies have assessed the association of type of access used at initiation of HD with patient survival (6–11). They have consistently observed that patients initiating HD with a CVC have inferior adjusted survival compared with those initiating with an AVF. The survival of patients initiating HD with an AVG was intermediate between the other two groups (6–11). These studies focused on patients whose vascular access was created and matured before initiating HD, representing patients who were never CVC dependent. Three subsequent studies evaluated the effect of change in vascular access from CVC to permanent access on subsequent clinical outcomes in established patients on HD. Two of these studies observed that, compared with patients who continued to dialyze with a CVC, those who switched from a CVC to an AVF or AVG had a 30%–60% lower adjusted risk of death (6,19). A third study measured laboratory parameters before and after the switch from a CVC to an AVF or AVG (37). Compared with patients who continued to dialyze with a CVC, those who switched to a permanent access had increases in serum albumin, protein catabolic rate, and hemoglobin and a decrease in peripheral white count. These changes were interpreted as showing that elimination of a CVC alleviated malnutrition, inflammation, and anemia (37). The duration of CVC dependence is substantially longer in patients who receive an AVF versus an AVG after initiating HD (38), leading to our original hypothesis that this would place patients with AVFs at greater risk for infection-related hospitalization and death.
There are two potential interpretations of the observation that CVC use is associated with worse clinical outcomes. The first is that CVCs directly contribute to patient morbidity, thereby resulting in more hospitalizations and shortened patient survival. The second is that patients dialyzing with a CVC have a higher comorbidity burden than those dialyzing with an AVF or AVG and that their higher rate of hospitalization and premature death arises from their comorbidities rather than from CVC-related complications. It is evident from Table 1 that patients receiving an AVG had more comorbidities than those getting an AVF. Our study implemented the most sophisticated statistical techniques available to minimize the potential selection bias present in observational studies. A matched propensity score adjustment was created to ensure comparable groups of patients with AVFs and patients with AVGs. The matching on the basis of propensity score attempted to balance the distribution of observed confounders using 16 baseline covariates evaluated by the standard difference (Table 2) and showed excellent balancing of all covariates with a standard bias <0.10. However, as this study suggests, even using the most sophisticated statistical techniques to adjust for selection bias may not adequately control for unmeasured confounders. Our sensitivity analysis (discussed in Materials and Methods) provides further proof of the unmeasured confounding in this analysis.
Administrative databases capture the presence or absence of a medical condition but do not capture its severity. Thus, for example, two patients may be categorized as having heart failure; however, one has mild heart failure, and the second has severe heart failure. Clearly, the second patient has a higher likelihood of hospitalization or death, but this propensity is not captured by the diagnosis itself. The details of such comorbidities are likely considered by the surgeon planning the type of access surgery but cannot be deduced accurately from retrospective analysis of their comorbidities.
Two recent publications further highlight the inadequacy of adjusting for comorbidities using administrative data in predicting mortality related to access type of patients on HD. Brown et al. (39) and Quinn et al. (40) observed that, among patients initiating HD with a CVC, those who underwent AVF placement before HD initiation had superior survival to those without predialysis access surgery, despite having a very similar profile of comorbidities. The improved survival of the former group was evident even if the AVF failed to mature and was never used for HD, and thus, it could not be attributed to a shorter duration of CVC dependence. This finding suggests that the decision to place an AVF in a patient itself reflects a healthier patient in ways that are not captured by compiling a list of known comorbidities. Prospective analysis of clinical outcomes by Quinn et al. (40) found that only 2.3% of deaths in CVC-dependent patients on HD could be attributed to CVC complications. Taken together, these observations suggest that the difference in mortality between HD groups with different types of vascular access is due to the underlying characteristics that lead the patients to receive a certain vascular access type rather than complications related to the vascular access itself.
Our study has several important strengths. First, the large sample size is representative of the entire incident United States elderly HD population. Second, our vascular access data were captured using V codes reported monthly by every HD unit. Our study also has some limitations. First, it includes only elderly patients (≥67 years old) and may not generalize to younger patients on dialysis. However, >50% of United States patients on incident HD are >65 years of age (1). Second, this study is observational; thus, there is likely residual confounding due to patient characteristics (e.g., HD adherence) not available in the datasets used for this analysis. In this context, we have performed a propensity-adjusted analysis to best account for unmeasured confounding at baseline. Third, an AVG (a foreign body) may directly produce metabolic and immunologic effects that negatively affect cardiovascular health, hospitalizations, and mortality (41–43). Fourth, greater access blood flows in AVGs versus AVFs may cause heart failure. However, we found similar adjusted cardiovascular hospitalization rates in the AVG and AVF cohorts during the 6 months after vascular access placement. Fifth, we recognize that differences may exist between the average treatment effects found in this large database analysis and the best approach for individual patients given local surgical expertise and patient-specific clinical and demographic factors. In conclusion, among elderly patients who initiate HD with a CVC and have a permanent access placed within 6 months of dialysis initiation, placement of an AVF rather than an AVG was associated with better overall survival, despite longer CVC dependence. Moreover, patients with AVF placement had lower rates of hospitalization due to all-cause infection and septicemia/bacteremia. Our study highlights the challenge in any observational study of distinguishing whether vascular access type directly affects clinical outcomes or whether it is simply a surrogate marker for the severity of comorbidities that themselves affect clinical outcomes. A definitive answer would require a randomized clinical trial evaluating patients initiating dialysis with a CVC and subsequently having AVF or AVG placement.
Disclosures
T.L. is a consultant for Proteon Therapeutics and Merck. M.T. is a consultant for Proteon Therapeutics. Q.Z. and Y.Z. have no financial disclosures to report. M.A. is a consultant for CorMedix.
Supplementary Material
Acknowledgments
T.L. is supported by an American Society of Nephrology Carl W. Gottschalk Scholar grant; a University of Alabama at Birmingham Nephrology Research Center Anderson Innovation award; University of Alabama at Birmingham Center for Clinical and Translational Science Multidisciplinary Pilot award 1UL1TR001417-01; grant 1R43DK109789-01 from National Institutes of Diabetes, Digestive and Kidney Diseases (NIDDK); and grant 1I01BX003387-01A1 from a Veterans Affairs Merit Award. M.T. is supported by grant 1R21DK104248-01A1 from the NIDDK and grant R01-HS-021229 from the Agency for Healthcare Research and Quality (AHRQ). Q.Z. and Y.Z. are supported by grant 1R21DK104248-01A1 from the NIDDK and grant R03-HS-022931 from the AHRQ. M.A. is supported by grant 1R21DK104248-01A1 from the NIDDK.
Portions of this manuscript were presented in abstract form at the 2016 American Society of Nephrology Kidney Week in Chicago, Illinois, November 16, 2016.
The data reported herein have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be construed as the official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01410217/-/DCSupplemental.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA: US Renal Data System 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67: S1–S305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.End Stage Renal Disease (ESRD) National Coordinating Center (NCC): Fistula First Catheter Last Initiative. Available at: http://esrdncc.org/ffcl/. Accessed December 9, 2016
- 3.Access V: 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ: Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 47: 469–477, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK: Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 60: 1443–1451, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Lacson E Jr, Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Pastan S, Soucie JM, McClellan WM: Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620–626, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol 15: 477–486, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, Chen K, Piera L, Saito A, Young EW: Timing of first cannulation and vascular access failure in haemodialysis: An analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant 19: 2334–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Richardson AI 2nd, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, Glickman MH: Should fistulas really be first in the elderly patient? J Vasc Access 10: 199–202, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 15.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M: Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int 61: 1136–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Poole CV, Carlton D, Bimbo L, Allon M: Treatment of catheter-related bacteraemia with an antibiotic lock protocol: Effect of bacterial pathogen. Nephrol Dial Transplant 19: 1237–1244, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Saad TF: Bacteremia associated with tunneled, cuffed hemodialysis catheters. Am J Kidney Dis 34: 1114–1124, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lacson E Jr, Wang W, Lazarus JM, Hakim RM: Change in vascular access and hospitalization risk in long-term hemodialysis patients. Clin J Am Soc Nephrol 5: 1996–2003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishani A: Vascular Access Utilization in the Dialysis Population. United States Renal Database System Presentation, 2012. Available at https://www.usrds.org/presentations2012.aspx. Accessed February 16, 2017
- 21.Solid CA, Collins AJ, Ebben JP, Chen SC, Faravardeh A, Foley RN, Ishani A: Agreement of reported vascular access on the medical evidence report and on medicare claims at hemodialysis initiation. BMC Nephrol 15: 30, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musil CM, Warner CB, Yobas PK, Jones SL: A comparison of imputation techniques for handling missing data. West J Nurs Res 24: 815–829, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Heeringa SG, Berglund P: Multiple Imputation of Missing Data Using SAS, Cary, NC, SAS Institute, 2014 [Google Scholar]
- 24.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Lok CE, Allon M, Moist L: Predicting successful arteriovenous fistula creation. Am J Kidney Dis 60: 498, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Ishaque B, Zayed MA, Miller J, Nguyen D, Kaji AH, Lee JT, O'Connell J, de Virgilio C: Ethnic differences in arm vein diameter and arteriovenous fistula creation rates in men undergoing hemodialysis access. J Vasc Surg 56: 424–431, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Wasse H, Hopson SD, McClellan W: Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol 32: 234–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kats M, Hawxby AM, Barker J, Allon M: Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int 71: 39–43, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, Canaud BJ, Pisoni RL: Vascular access use and outcomes: An international perspective from the Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant 23: 3219–3226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judkins DR, Morganstein D, Zador P, Piesse A, Barrett B, Mukhopadhyay P: Variable selection and raking in propensity scoring. Stat Med 26: 1022–1033, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosanke J, Bergstralh E: Mayo Clinic Biomedical Statistics and Informatics, 2003. Available from http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros. Accessed January 26, 2017
- 34.Love TE: Spreadsheet-Based Sensitivity Analysis Calculations for Matched Samples, Cleveland, OH, Center for Health Care Research & Policy, Case Western Reserve University, 2008 [Google Scholar]
- 35.Keele L: An Overview of rbounds: An R package for Rosenbaum Bounds Sensitivity Analysis with Matched Data, 2010. Available at: https://urldefense.proofpoint.com/v2/url?u=http-3A__www.personal.psu.edu_ljk20_rbounds-2520vignette.pdf&d=DwMFaQ&c=o3PTkfaYAd6-No7SurnLtwPssd47t-De9Do23lQNz7U&r=9KT4eM1KKU4A8iXxvzm-Rg&m=p1I-kJUi9bMwXlGibQbEE0tj120zLU9YVR2Z-i6_pu0&s=Fqs48DP9dFCLDrKGbfQm_aRC-SlVGMnFnnABw2r8juU&e=. Accessed February 2, 2017
- 36.Rosenbaum PR: Observational Studies, New York, Springer, 2002 [Google Scholar]
- 37.Wystrychowski G, Kitzler TM, Thijssen S, Usvyat L, Kotanko P, Levin NW: Impact of switch of vascular access type on key clinical and laboratory parameters in chronic haemodialysis patients. Nephrol Dial Transplant 24: 2194–2200, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Yuo TH, Chaer RA, Dillavou ED, Leers SA, Makaroun MS: Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg 62: 1590–1597.e2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS: The survival benefit of “Fistula First, Catheter Last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 28: 645–652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn B, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasse H, Cardarelli F, De Staercke C, Hooper WC, Long Q: Accumulation of retained nonfunctional arteriovenous grafts correlates with severity of inflammation in asymptomatic ESRD patients. Nephrol Dial Transplant 28: 991–997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dukkipati R, Molnar MZ, Park J, Jing J, Kovesdy CP, Kajani R, Kalantar-Zadeh K: Association of vascular access type with inflammatory marker levels in maintenance hemodialysis patients. Semin Dial 27: 415–423, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Grubbs V, Wasse H, Vittinghoff E, Grimes BA, Johansen KL: Health status as a potential mediator of the association between hemodialysis vascular access and mortality. Nephrol Dial Transplant 29: 892–898, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.