Abstract
Background and objectives
Anemia is an early complication of CKD that is associated with increased morbidity and mortality. Prior data show associations between abnormal mineral metabolism markers and decreased erythropoiesis. However, few studies have investigated elevated fibroblast growth factor 23 as a risk factor for the development of anemia in patients with CKD.
Design, setting, participants, & measurements
We conducted a prospective cohort study of 3869 individuals with mild to severe CKD enrolled in the Chronic Renal Insufficiency Cohort Study between 2003 and 2008 and followed through 2013. We hypothesized that elevated baseline fibroblast growth factor 23 levels are associated with prevalent anemia, decline in hemoglobin over time, and development of incident anemia, defined as serum hemoglobin level <13 g/dl in men, serum hemoglobin level <12 g/dl in women, or use of erythropoietin stimulating agents.
Results
In the 1872 of 3869 individuals who had prevalent anemia at baseline, mean age was 58 (11) years old, and mean eGFR was 39 (13) ml/min per 1.73 m2. Higher levels of fibroblast growth factor 23 were significantly associated with prevalent anemia (odds ratio per 1-SD increase in natural log–transformed fibroblast growth factor 23, 1.39; 95% confidence interval, 1.26 to 1.52), decline in hemoglobin over 4 years, and risk of incident anemia (hazard ratio per 1-SD increase in natural log–transformed fibroblast growth factor 23, 1.13; 95% confidence interval, 1.04 to 1.24; quartile 4 versus quartile 1: hazard ratio, 1.59; 95% confidence interval, 1.19 to 2.11) independent of demographic characteristics, cardiovascular disease risk factors, CKD-specific factors, and other mineral metabolism markers. The results of our prospective analyses remained unchanged after additional adjustment for time-varying eGFR.
Conclusions
Elevated fibroblast growth factor 23 is associated with prevalent anemia, change in hemoglobin over time, and development of anemia. Future studies are needed to elucidate the mechanisms for these associations.
Keywords: anemia; mineral metabolism; glomerular filtration rate; risk factors; Odds Ratio; erythropoietin; Confidence Intervals; Prospective Studies; Erythropoiesis; Fibroblast Growth Factors; Renal Insufficiency, Chronic; Hemoglobins; Demography; Cardiovascular Diseases; Minerals; Humans; Male; Female
Introduction
Anemia is a common and early complication of CKD (1). It is associated with poor quality of life, increased risk of cardiovascular disease, and mortality in patients with CKD (2–7). Traditional causes of anemia in CKD include iron deficiency and erythropoietin deficiency, and current treatment strategies focus on these targets (1). Cross-sectional studies suggest that there may be a link between markers of mineral metabolism and hemoglobin parameters (8–10). Whether disordered mineral metabolism contributes to the onset of anemia in early CKD remains unknown.
Prior investigations propose that factors released by osteocytes may mediate changes in the bone marrow microenvironment and thereby, influence hematopoietic cell outcomes (11,12). Fibroblast growth factor 23 (FGF23) is an osteocyte-derived hormone that exerts its primary actions in the proximal tubule of the kidney to stimulate urinary phosphate excretion and regulate production and degradation of 1,25-dihydroxyvitamin D (13). Preclinical studies of wild-type mice show that exogenous administration of FGF23 at a dose that approximates FGF23 concentrations detected in early CKD decreases erythropoiesis (14). In contrast, genetic deletion of FGF23 promotes red cell production independent of active vitamin D (14). These findings suggest that elevated levels of FGF23 may have direct effects on the hematopoietic system. Because serum FGF23 levels rise early in the course of CKD (13), elevated FGF23 may represent a novel pathway in the development of anemia in CKD. Using a large prospective cohort of patients with CKD, we tested the hypotheses that elevated baseline FGF23 is independently associated with prevalent anemia, decline in hemoglobin over time, and development of incident anemia in CKD stages 2–4.
Materials and Methods
Study Population
The Chronic Renal Insufficiency Cohort (CRIC) Study is an ongoing prospective cohort study that is investigating risk factors for cardiovascular disease and CKD progression in individuals with impaired kidney function. During phase 1 of the study, 3939 men and women ages 21–74 years old with CKD stages 2–4 defined by age-specific criteria for eGFR between 20 and 70 ml/min per 1.73 m2 were recruited from seven clinical centers across the United States between June of 2003 and September of 2008 (15). Exclusion criteria included pregnancy, cirrhosis, New York Heart Association class 3 or 4 heart failure, multiple myeloma, polycystic kidney disease, renal cancer, HIV infection, organ transplantation, previous dialysis treatment for at least 1 month, recent chemotherapy or immunosuppressive therapy, institutionalization, enrollment in other studies, or inability to provide informed consent (15). All participants provided written informed consent, and the institutional review boards at each of the participating centers approved the final study protocol.
We investigated associations between baseline plasma FGF23 and baseline and follow-up serum hemoglobin as well as between baseline FGF23 and prevalent and incident anemia. Our study population was composed of 3869 participants with baseline hemoglobin and FGF23 measurements available for analyses. Anemia was defined as serum hemoglobin level <13 g/dl in men and <12 g/dl in women or use of erythropoietin stimulating agents. At enrollment, 1872 (48.4%) participants had anemia. Analyses of incident anemia were restricted to the remaining 1834 participants without anemia at baseline and with at least one additional follow-up hemoglobin or data on the use of erythropoietin during follow-up.
Primary Exposure
The primary exposure was plasma C-terminal FGF23. Samples were collected fasting in 97% of participants at baseline and stored at −80°C at the CRIC Central Laboratory. FGF23 measurements were conducted after a single thaw of frozen samples in duplicate using a second generation assay (Immutopics, San Clemente, CA). The mean interassay coefficient of variation was 7.6%, the intra-assay coefficient of variation was <5%, and the lower limit of detection was 3 RU/ml (16,17).
Outcomes
Laboratory measurements for serum hemoglobin were conducted at annual CRIC Study visits using standardized assays. The primary outcome for prevalent analyses was presence of anemia at study enrollment. The primary outcomes for longitudinal analyses were change in hemoglobin over time and time to development of anemia during follow-up.
Covariates
Information on participant demographics, medication use, and clinical data was collected at the baseline and annual study visits. Self-reported history of cardiovascular disease was defined as a composite of coronary revascularization, prior heart failure, myocardial infarction, ischemic stroke, peripheral artery revascularization, or amputation (18). Resting BP was measured via standardized protocols (15). A centralized laboratory measured serum creatinine, calcium, phosphate, and urinary albumin-to-creatinine ratio via standard assays (15,19). Plasma parathyroid hormone was measured using the Scantibodies total intact assay (Santee, CA) (13). eGFR was calculated using the creatinine-based CKD Epidemiology Collaboration equation (20).
Statistical Analyses
We compared baseline characteristics and demographics of our study population according to the presence of anemia at study enrollment. For the subset of participants without prevalent anemia, we summarized baseline characteristics according to ascending quartiles of FGF23. Because FGF23 was not normally distributed, it was natural log–transformed (ln) for all analyses.
We used logistic regression models to examine the association between baseline FGF23, expressed both as a continuous variable and in ascending quartiles, and prevalent anemia.
We used linear mixed effects models to examine the association between baseline FGF23 quartiles and absolute change in hemoglobin over 4 years. For this analysis, we excluded 1872 individuals with prevalent anemia at baseline, 163 individuals who only had baseline hemoglobin, and 61 who used erythropoietin during the follow-up period. Both random intercept and slope terms were included in the model to allow individual-specific intercepts and slopes. We tested the FGF23 quartile × time interaction as the primary test for the difference between groups. We calculated the P value for trend across ascending quartiles.
We used Cox proportional hazards models to analyze time to incident anemia according to baseline FGF23 levels expressed in quartiles and as a continuous variable. For this analysis, we excluded the 1872 individuals with prevalent anemia at baseline and censored for administrative end of follow-up on December 31, 2013, death, or development of ESRD, defined as initiation of chronic dialysis or receipt of a kidney transplant. We verified that there was no violation of the proportional hazards assumption.
For all outcomes, we adjusted for demographics (age, sex, race, and ethnicity), cardiovascular risk factors (prior cardiovascular disease, systolic BP, diabetes, smoking, and C-reactive protein), CKD-specific factors (eGFR and urinary albumin-to-creatinine ratio categorized as <300 mg/dl, ≥300 mg/dl, or missing), and markers of mineral metabolism (calcium, phosphate, and parathyroid hormone). Kidney disease progression predicts onset of anemia (21), and higher levels of FGF23 are associated with loss of kidney function (22). Therefore, in our prospective analyses, we adjusted for eGFR as a time-varying covariate to account for changes in kidney function over time as a potential confounder of the relationships between baseline FGF23 and change in hemoglobin over time and incident anemia.
Iron deficiency can lead to the development of a microcytic anemia characterized by a low mean corpuscular volume (MCV), which measures the average erythrocyte volume (23). Because iron deficiency increases FGF23 production (24) and can cause anemia (23), in additional analyses of incident anemia, we individually adjusted our final incident anemia models for MCV and oral iron supplementation as imperfect but available surrogates of iron deficiency. Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) may also alter hemoglobin levels (25–27). In further analyses of incident anemia, we adjusted the final models for the use of ACE inhibitors or ARBs.
Analyses were performed using SAS, version 9.4 (Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Associations of Baseline FGF23 with Prevalent Anemia
Table 1 presents clinical and demographic characteristics of the CRIC Study participants according to anemia prevalence. Individuals with anemia at baseline were older, were more likely to be black or Hispanic, had higher BP, had greater prevalence of diabetes, and had lower eGFR. Higher plasma FGF23, expressed as a continuous variable, was associated with significantly higher likelihood of prevalent anemia in univariate and multivariable-adjusted analyses (adjusted odds ratio, 1.39 per 1-SD increase in ln FGF23; 95% confidence interval [95% CI], 1.26 to 1.52) (Table 2). Categorical analyses showed an independent, linear, and stepwise increase in anemia prevalence, with quartile 4 having 2.5-fold greater odds of prevalent anemia compared with quartile 1 (Table 2).
Table 1.
Baseline characteristics according to anemia prevalence
| Baseline Characteristics, Total N=3869 | Prevalent Anemia, N=1872 | No Prevalent Anemia, N=1997 | P Value |
|---|---|---|---|
| Age, yr | 58±11 | 57±11 | <0.001 |
| Sex, n (%) | 875 (47) | 861 (43) | 0.02 |
| Black, n (%) | 929 (50) | 689 (35) | <0.001 |
| Hispanic, n (%) | 317 (17) | 178 (9) | <0.001 |
| Current smoking, n (%) | 227 (12) | 280 (14) | 0.08 |
| BMI, kg/m2 | 32±8 | 32±7 | 0.003 |
| Systolic BP, mm Hg | 133±23 | 125±21 | <0.001 |
| Diabetes mellitus, n (%) | 1206 (64) | 668 (33) | <0.001 |
| Any CVD, n (%) | 722 (39) | 571 (29) | <0.001 |
| C-reactive protein, mg/L | 2.7 (1.0–7.4) | 2.4 (1.1–5.7) | 0.01 |
| UACR | 118 (16–827) | 27 (6–241) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 39±13 | 49±15 | <0.001 |
| Serum calcium, mg/dl | 9.1±0.5 | 9.3±0.5 | <0.001 |
| Serum phosphate, mg/dl | 3.9±0.7 | 3.6±0.6 | <0.001 |
| PTH, pg/ml | 66 (40–114) | 46 (32–72) | <0.001 |
| Hemoglobin, g/dl | 11.2±1.1 | 13.9±1.2 | <0.001 |
| FGF23, RU/ml | 179 (118–298) | 119 (83–187) | <0.001 |
Results are reported as means±SD, proportions, or medians (interquartile ranges). N, number; BMI, body mass index; CVD, cardiovascular disease; UACR, urinary albumin-to-creatinine ratio; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23.
Table 2.
Associations of baseline fibroblast growth factor 23 with prevalent anemia
| Number of Patients, Total N | Per 1 SD ln FGF23 | FGF23 Quartiles, RU/ml | P Value for Trend | |||
|---|---|---|---|---|---|---|
| Quartile 1: ≤96.0, N=969 | Quartile 2: 96.1–145.6, N=966 | Quartile 3: 145.7–239.2, N=967 | Quartile 4: ≥239.3, N=967 | |||
| Prevalence | ||||||
| 1872 | 48 | 26 | 46 | 55 | 67 | |
| 3869 | ||||||
| Unadjusted odds ratio (95% CI) | ||||||
| 1872 | 1.88 (1.75 to 2.03) | Reference | 2.37 (1.96 to 2.87) | 3.34 (2.76 to 4.04) | 5.58 (4.59 to 6.79) | <0.001 |
| 3869 | ||||||
| Plus demographic factorsa | ||||||
| 1872 | 1.85 (1.71 to 2.00) | Reference | 2.31 (1.90 to 2.82) | 3.30 (2.71 to 4.02) | 5.34 (4.36 to 6.55) | <0.001 |
| 3869 | ||||||
| Plus cardiovascular risk factorsb | ||||||
| 1871 | 1.75 (1.61 to 1.90) | Reference | 2.14 (1.74 to 2.62) | 2.77 (2.25 to 3.41) | 4.65 (3.73 to 5.79) | <0.001 |
| 3866 | ||||||
| Plus CKD-specific factorsc | ||||||
| 1871 | 1.41 (1.30 to 1.54) | Reference | 1.78 (1.44 to 2.20) | 1.85 (1.48 to 2.30) | 2.62 (2.06 to 3.34) | <0.001 |
| 3866 | ||||||
| Plus markers of mineral metabolismd | ||||||
| 1817 | 1.39 (1.26 to 1.52) | Reference | 1.76 (1.42 to 2.18) | 1.80 (1.43 to 2.26) | 2.52 (1.95 to 3.24) | <0.001 |
| 3750 | ||||||
Continuous results are reported as odds ratios per 1-SD increase in ln-transformed FGF23. N, number; ln, natural log; FGF23, fibroblast growth factor 23; 95% CI, 95% confidence interval.
Adjusts for age, sex, race, and ethnicity.
Adjusts for factors in model 1 as well as cardiovascular disease, systolic BP, diabetes, smoking, and C-reactive protein.
Adjusts for factors in model 2 as well as eGFR and urinary albumin-to-creatinine ratio.
Full multivariable model: adjusts for factors in model 3 as well as calcium, phosphate, and parathyroid hormone.
Associations of Baseline FGF23 with Change in Hemoglobin over Time
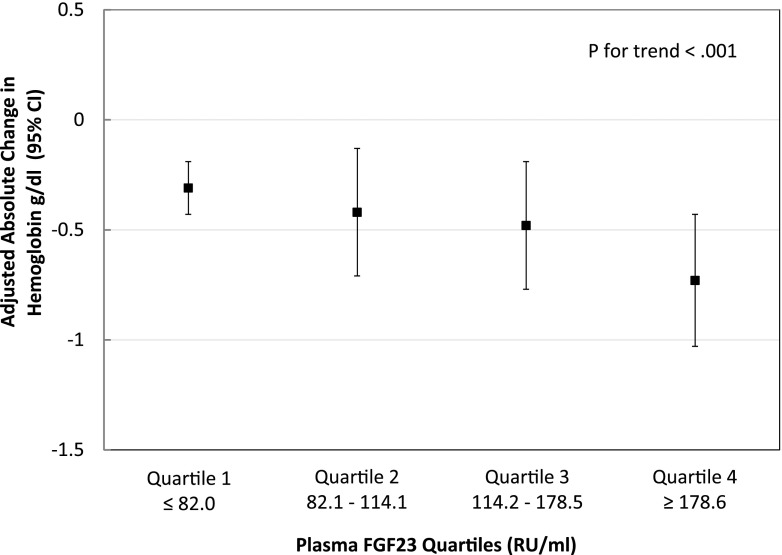
Our total population included 1773 individuals. Adjusted absolute changes in hemoglobin over 4 years according to FGF23 quartiles are summarized in Figure 1. Mean hemoglobin levels decreased during follow-up in the overall population and declined stepwise with ascending FGF23 quartiles (P value for FGF23 quartile × time interaction in the fully adjusted model <0.001). The adjusted absolute decline in hemoglobin over 4 years was more than double in those with FGF23 levels in quartile 4 compared with those with levels in quartile 1 (Figure 1) (quartile 4: −0.73 g/dl; 95% CI, −1.03 to −0.43 g/dl versus quartile 1: −0.31 g/dl; 95% CI, −0.43 to −0.19 g/dl).
Figure 1.
In longitudinal analyses, ascending fibroblast growth factor 23 (FGF23) quartiles were associated with greater decline of hemoglobin over 4 years. Adjusted by age, sex, race, ethnicity, cardiovascular disease, systolic BP, diabetes, smoking, C-reactive protein, eGFR, urinary albumin-to-creatinine ratio, calcium, phosphate, and parathyroid hormone from baseline. 95% CI, 95% confidence interval.
Associations of Baseline FGF23 with Incident Anemia
Among the 1834 remaining participants at risk for the development of anemia, mean baseline serum hemoglobin was 13.9 g/dl (SD=1.2), mean eGFR was 49 (SD=15) ml/min per 1.73 m2, and median FGF23 was 117 RU/ml (interquartile range, 83–183 RU/ml). Clinical and demographic characteristics of participants at risk for incident anemia according to FGF23 quartiles in this population are presented in Table 3. During a mean follow-up of 3.1 years (SD=1.2 years), 654 individuals developed anemia (11.4 per 100 person-years). Of these, 648 met criteria for incident anemia by hemoglobin values, and six met criteria for incident anemia by the use of erythropoietin. The incidence rate increased across ascending FGF23 quartiles (Table 4) (P for trend <0.001). In unadjusted and adjusted analyses, higher baseline FGF23, expressed as a continuous variable, was associated with increased risk of incident anemia (adjusted hazard ratio [HR], 1.13 per 1-SD increase in ln FGF23; 95% CI, 1.04 to 1.24) (Table 4). Categorical analyses showed that quartile 4 was associated with almost a 1.6-fold increased risk versus reference quartile 1 (Table 4).
Table 3.
Baseline characteristics of individuals at risk for development of anemia according to fibroblast growth factor 23 quartiles
| Baseline Characteristics Total, N=1834 | FGF23 Quartile | P Value | |||
|---|---|---|---|---|---|
| Quartile 1: ≤82.6, N=458 | Quartile 2: 82.7–116.2, N=457 | Quartile 3: 116.3–182.1, N=457 | Quartile 4: ≥182.2, N=462 | ||
| Age, yr | 55±11 | 56±11 | 58±11 | 58±11 | <0.001 |
| Women, n (%) | 166 (36) | 150 (33) | 219 (48) | 256 (55) | <0.001 |
| Black, n (%) | 161 (35) | 150 (33) | 147 (32) | 164 (36) | 0.64 |
| Hispanic, n (%) | 23 (5) | 40 (9) | 50 (11) | 35 (8) | 0.01 |
| Current smoking, n (%) | 32 (7) | 46 (10) | 63 (14) | 107 (23) | <0.001 |
| BMI, kg/m2 | 30±6 | 31±6 | 32±7 | 34±8 | <0.001 |
| Systolic BP, mm Hg | 122±18 | 123±19 | 125±22 | 127±21 | <0.001 |
| Diabetes mellitus, n (%) | 100 (22) | 122 (27) | 161 (35) | 205 (44) | <0.001 |
| Any CVD, n (%) | 86 (19) | 97 (21) | 142 (31) | 174 (38) | <0.001 |
| C-reactive protein, mg/L | 1.8 (0.9–4.1) | 1.9 (0.9–4.4) | 2.4 (1.2–5.6) | 3.8 (1.5–8.1) | <0.001 |
| UACR | 10 (4–69) | 18 (5–133) | 30 (6–244) | 81 (16–578) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 58±14 | 52±13 | 47±12 | 39±13 | <0.001 |
| Serum calcium, mg/dl | 9.2±0.4 | 9.3±0.4 | 9.3±0.5 | 9.3±0.5 | 0.04 |
| Serum phosphate, mg/dl | 3.3±0.5 | 3.5±0.5 | 3.6±0.6 | 3.8±0.7 | <0.001 |
| PTH, pg/ml | 37 (28–53) | 41 (31–62) | 49 (33–73) | 61 (39–98) | <0.001 |
| Hemoglobin, g/dl | 14.2±1.2 | 14.1±1.2 | 13.8±1.2 | 13.6±1.2 | <0.001 |
| FGF23, RU/ml | 65 (53–74) | 97 (90–106) | 143 (129–160) | 264 (213–364) | <0.001 |
Results are reported as means±SD, proportions, or medians (interquartile ranges). N, number; FGF23, fibroblast growth factor 23; BMI, body mass index; CVD, cardiovascular disease; UACR, urinary albumin-to-creatinine ratio; PTH, parathyroid hormone.
Table 4.
Association of baseline fibroblast growth factor 23 concentrations with incident anemia
| Events, Total n | Per 1 SD ln FGF23 | FGF23 Quartiles, RU/ml | P Value for Trend | |||
|---|---|---|---|---|---|---|
| Quartile 1: ≤82.6 | Quartile 2: 82.7–116.2 | Quartile 3: 116.3–182.1 | Quartile 4: ≥182.2 | |||
| Incidence rate, events per 1000 person-yr follow-up (95% CI) | ||||||
| 654 | 114.3 (105.7 to 123.4) | 60.0 (48.6 to 73.2) | 89.4 (74.9 to 105.9) | 136.5 (117.7 to 157.5) | 191.2 (167.5 to 217.3) | <0.001 |
| 1834 | ||||||
| Unadjusted hazard ratio | ||||||
| 654 | 1.51 (1.41 to 1.61) | Reference | 1.52 (1.17 to 1.97) | 2.35 (1.84 to 3.00) | 3.38 (2.67 to 4.28) | <0.001 |
| 1834 | ||||||
| Plus demographic factorsa | ||||||
| 654 | 1.43 (1.32 to 1.53) | Reference | 1.45 (1.11 to 1.88) | 2.18 (1.70 to 2.80) | 2.94 (2.30 to 3.77) | <0.001 |
| 1834 | ||||||
| Plus cardiovascular risk factorsb | ||||||
| 654 | 1.37 (1.26 to 1.48) | Reference | 1.45 (1.11 to 1.88) | 2.06 (1.60 to 2.65) | 2.67 (2.07 to 3.44) | <0.001 |
| 1833 | ||||||
| Plus CKD-specific factorsc | ||||||
| 654 | 1.20 (1.10 to 1.31) | Reference | 1.28 (0.98 to 1.67) | 1.64 (1.27 to 2.12) | 1.80 (1.37 to 2.37) | <0.001 |
| 1833 | ||||||
| Plus markers of mineral metabolismd | ||||||
| 626 | 1.13 (1.04 to 1.24) | Reference | 1.23 (0.94 to 1.62) | 1.54 (1.18 to 2.01) | 1.59 (1.19 to 2.11) | <0.001 |
| 1774 | ||||||
| Sensitivity analyses | ||||||
| Final model plus MCV | ||||||
| 626 | 1.13 (1.04 to 1.24) | Reference | 1.23 (0.94 to 1.62) | 1.54 (1.18 to 2.01) | 1.59 (1.19 to 2.11) | <0.001 |
| 1774 | ||||||
| Final modele | ||||||
| 495 | 1.20 (1.08 to 1.34) | Reference | 1.17 (0.88 to 1.57) | 1.40 (1.04 to 1.87) | 1.59 (1.17 to 2.18) | 0.002 |
| 1448 | ||||||
| Final model plus iron supplementation | ||||||
| 495 | 1.20 (1.08 to 1.34) | Reference | 1.17 (0.87 to 1.56) | 1.40 (1.05 to 1.88) | 1.59 (1.16 to 2.17) | 0.002 |
| 1448 | ||||||
| Final modelf | ||||||
| 621 | 1.13 (1.03 to 1.23) | Reference | 1.24 (0.95 to 1.63) | 1.52 (1.16 to 1.98) | 1.57 (1.18 to 2.09) | 0.001 |
| 1761 | ||||||
| Final model plus ACE/ARB use | ||||||
| 621 | 1.13 (1.03 to 1.23) | Reference | 1.24 (0.95 to 1.63) | 1.50 (1.15 to 1.96) | 1.56 (1.18 to 2.08) | 0.001 |
| 1761 | ||||||
Continuous results are reported as hazard ratios per 1-SD increase in plasma ln-transformed FGF23. n, number; ln, natural log; FGF23, fibroblast growth factor 23; 95% CI, 95% confidence interval; MCV, mean corpuscular volume; ACE/ARB, angiotensin-converting enzyme/angiotensin receptor blocker.
Adjusts for age, sex, race, and ethnicity.
Adjusts for factors in model 1 as well as cardiovascular disease, systolic BP, diabetes, smoking, and C-reactive protein.
Adjusts for factors in model 2 as well as eGFR and urinary albumin-to-creatinine ratio.
Full multivariable model adjusts for factors in model 3 as well as calcium, phosphate, and parathyroid hormone.
Full multivariable model but includes only those individuals with iron supplementation data available.
Full multivariable model but includes only those individuals with ACE/ARB use data available.
Prospective Analyses Adjusted for Time-Varying eGFR
Declining kidney function may influence the development of anemia and change in hemoglobin over time, and elevated FGF23 levels are associated with CKD progression (21,22). To account for this potential confounder, we adjusted our linear mixed effects models investigating the associations of baseline FGF23 with change in hemoglobin over time for eGFR as a time-varying covariate. Our results remained qualitatively the same (quartile 4: −0.63 g/dl; 95% CI, −0.92 to −0.34 g/dl versus quartile 1: −0.25 g/dl; 95% CI, −0.37 to −0.14 g/dl). Results from the incident anemia models were also consistent. When we included eGFR as a time-varying covariate in the multivariable-adjusted Cox model, baseline FGF23 remains associated with development of anemia when expressed as a continuous variable (adjusted HR, 1.13 per 1-SD increase in ln FGF23; 95% CI, 1.04 to 1.23) or in quartiles, with quartile 4 having 1.5-fold greater risk of incident anemia compared with quartile 1 (quartile 4: HR, 1.55; 95% CI, 1.18 to 2.04 versus reference quartile 1).
Additional Analyses for Incident Anemia
Because iron deficiency can cause anemia in CKD, we adjusted our final models for MCV and oral iron supplementation (1). FGF23 remained significantly and independently associated with incident anemia after adjustment of our final model for MCV (Table 4). Among the total population of 1834 individuals, data on the use of iron supplementation were missing in 343 (19%) individuals. In those with iron supplementation data, our results remained significant with little attenuation after adjustment for the use of oral iron supplementation. In a similar approach, because ACE inhibitors can alter serum hemoglobin levels (25–27), we adjusted our final models for ACE/ARB use. In the population with data on ACE/ARB use available, further adjustment yielded results that were qualitatively similar to our primary analyses (Table 4).
Discussion
In a large, diverse cohort of patients with moderate to severe CKD, we showed that higher levels of baseline FGF23 were strongly and monotonically associated with prevalent anemia. In prospective analyses, ascending quartiles of baseline FGF23 were also independently associated with greater decrease in hemoglobin over time and the development of anemia. These findings further advance ongoing work that has uncovered systemic effects of elevated FGF23 levels in CKD.
Although FGF23 regulates phosphate and vitamin D levels, its actions extend beyond maintaining mineral metabolism homeostasis. Elevated FGF23 can induce left ventricular hypertrophy by activating FGF receptor 4 on cardiac myocytes (16,28). FGF23 signaling is involved in immune regulation through impairment of macrophages and neutrophils (29). Preclinical data suggest a novel role of elevated FGF23 in hematopoiesis through impairment of erythropoiesis in bone marrow cells (14). Deletion of FGF23 in wild-type mice resulted in increased erythropoiesis in bone marrow independent of vitamin D, and exogenous FGF23 administration resulted in a rapid decrease in erythropoiesis (14). FGF23 levels in the latter studies were parallel to levels detected in murine models with early CKD, supporting the hypothesis that moderate elevations of FGF23 observed in early CKD may contribute to the development of anemia.
Human studies of FGF23 and red cell parameters are confirmatory (8). FGF23 was associated with red cell distribution width in a cross-sectional study of 52 patients with CKD stages 2–4 (8). Similarly, data from a cross-sectional study of 53 patients with CKD stages 3 and 4 showed significant and independent correlations of higher FGF23 levels and lower hemoglobin (9). Although these findings are limited by sample size and cross-sectional design, they suggest a relationship between markers of mineral metabolism and hematopoiesis. Our longitudinal data on the independent associations of baseline FGF23 with change in hemoglobin over time and risk of incident anemia offer new evidence to support a possible role of elevated FGF23 levels in the pathogenesis of anemia.
There are several possible explanations for our findings. First, higher levels of FGF23 at baseline may be a marker of declining kidney function and chronic inflammation, both of which could cause anemia (30,31). Although we cannot rule out residual confounding, our results remained consistent when we adjusted for changes in eGFR as a time-varying covariate and C-reactive protein. Second, the effects of elevated FGF23 on hemoglobin may be mediated by FGF23-induced changes in active vitamin D. FGF23 regulates vitamin D by reducing production of 1,25-dihydroxyvitamin D and increasing its degradation, and low vitamin D levels are associated with anemia (10,32). Vitamin D suppresses the iron regulatory protein, hepcidin, allowing iron absorption and release from storage sites, and it separately can increase erythroid cell proliferation (33,34). Third, elevated FGF23 could be directly harmful to erythropoiesis. We could not elaborate on mechanisms in this study, but on the basis of the findings in animals (35), we speculate that elevated FGF23 may directly reduce erythropoiesis and contribute to anemia development in CKD.
The emerging link between iron deficiency and FGF23 regulation offers yet another possible explanation pathway for the relationship between elevated FGF23 and anemia that we observed. Murine models show that iron deficiency increases bone expression of FGF23 mRNA and FGF23 protein (24). In clinical studies, iron deficiency is associated with elevation in FGF23 levels, and iron supplementation decreases FGF23 levels (36–38). Given the high prevalence of iron deficiency in CKD and its causal relationship with anemia, iron deficiency may, in part, account for the association between elevated FGF23 and incident anemia that we observed. Although iron markers were not available in the CRIC Study, our results remained significant after adjustment for surrogate markers of iron deficiency, such as use of iron supplementation and MCV. Further studies are needed to determine to what extent iron deficiency–induced FGF23 elevation is responsible for our findings.
We acknowledge additional limitations in our study. The results of our analyses cannot show causality. Collectively, our cross-sectional, longitudinal analyses of change in hemoglobin over time and incident anemia analyses support the conclusion that FGF23 is associated with development of anemia. To account for indirect FGF23 effects through inflammation, we adjusted our models for C-reactive protein, which did not diminish our results. However, despite the wealth of covariate data available for our multivariable analyses, we were not able to adjust for vitamin D, hepcidin, and erythropoietin levels (39,40). We speculate that, even if our results were attenuated after adjustment for vitamin D levels, given the known effects of FGF23 on vitamin D regulation (41), vitamin D deficiency may be on the causal pathway between elevated FGF23 and incident anemia. The CRIC Study does not have measurements of bioactive intact FGF23. However, intact FGF23 strongly correlates with C-terminal FGF23 in late CKD (42). Further clinical studies are needed to better understand the role of iron deficiency, vitamin D, and erythropoietin in intact and C-terminal FGF23 levels and how they contribute to the development of anemia in individuals with CKD.
Anemia leads to decreased quality of life, higher rates of hospitalizations, and increased risks of cardiovascular events and mortality in affected individuals (2–7). It is critical to continue investigations that help elucidate risk factors involved in the development of anemia in CKD, because current therapies of iron and erythropoietin are encumbered with safety concerns (43,44). This requires understanding of the complex relationships between traditional and nontraditional risk factors. Our findings suggest that an elevated FGF23 may contribute to the development of anemia in patients with CKD stages 2–4. Further investigations are needed to elucidate the mechanisms for our findings and support measurements of hemoglobin in studies investigating FGF23-lowering treatments.
Disclosures
M.W. has received research support, honoraria, or consultant fees from Amgen, Ardelyx, DiaSorin, Keryx, Lilly, Pfizer, Shire, and Ultragenyx. T.I. has received honoraria from Kyowa Hakko Kirin Co., Ltd and grant support from Shire.
Supplementary Material
Acknowledgments
This study was supported by a National Kidney Foundation of Illinois Young Investigator Grant (to R.M.); National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grants K23DK094829 (to A.R.), R01DK081374 (to M.W.), K24DK093723 (to M.W.), and R01DK102438 (to T.I.); American Heart Association grant 13FTF15920005 (to M.D.); and a Strategically Focused Research Network Center Grant from the American Heart Association (to M.W.). Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the NIDDK (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1TR-000424, University of Maryland General Clinical Research Center grant M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and the NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Awards grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco–Clinical and Translational Science Institute grant UL1 RR-024131.
The CRIC Study Investigators include Lawrence J. Appel, Jiang He, James P. Lash, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03950417/-/DCSupplemental.
References
- 1.Babitt JL, Lin HY: Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ: Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 38: 955–962, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Xu D, Murakoshi N, Sairenchi T, Irie F, Igarashi M, Nogami A, Tomizawa T, Yamaguchi I, Yamagishi K, Iso H, Ota H, Aonuma K: Anemia and reduced kidney function as risk factors for new onset of atrial fibrillation (from the Ibaraki prefectural health study). Am J Cardiol 115: 328–333, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 28: 53–61, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Eriksson D, Goldsmith D, Teitsson S, Jackson J, van Nooten F: Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol 17: 97, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander M, Kewalramani R, Agodoa I, Globe D: Association of anemia correction with health related quality of life in patients not on dialysis. Curr Med Res Opin 23: 2997–3008, 2007 [DOI] [PubMed] [Google Scholar]
- 8.van Breda F, Emans ME, van der Putten K, Braam B, van Ittersum FJ, Kraaijenhagen RJ, de Borst MH, Vervloet M, Gaillard CA: Relation between red cell distribution width and fibroblast growth factor 23 cleaving in patients with chronic kidney disease and heart failure. PLoS One 10: e0128994, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai MH, Leu JG, Fang YW, Liou HH: High fibroblast growth factor 23 levels associated with low hemoglobin levels in patients with chronic kidney sisease stages 3 and 4. Medicine (Baltimore) 95: e3049, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel NM, Gutiérrez OM, Andress DL, Coyne DW, Levin A, Wolf M: Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int 77: 715–720, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO: Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res 27: 1451–1461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz MC, Fretz JA: Sclerostin: A new mediator of crosstalk between the skeletal and immune systems. J Bone Miner Res 27: 1448–1450, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D: FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem 289: 9795–9810, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd , Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P; MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TK, Estrella MM, Astor BC, Greene T, Wang X, Grams ME, Appel LJ: Longitudinal changes in hematocrit in hypertensive chronic kidney disease: Results from the African-American Study of Kidney Disease and Hypertension (AASK). Nephrol Dial Transplant 30: 1329–1335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camaschella C: Iron-deficiency anemia. N Engl J Med 372: 1832–1843, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Wolf M, White KE: Coupling fibroblast growth factor 23 production and cleavage: Iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 23: 411–419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leshem-Rubinow E, Steinvil A, Zeltser D, Berliner S, Rogowski O, Raz R, Chodick G, Shalev V: Association of angiotensin-converting enzyme inhibitor therapy initiation with a reduction in hemoglobin levels in patients without renal failure. Mayo Clin Proc 87: 1189–1195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue A, Babazono T, Iwamoto Y: Effects of the renin-angiotensin system blockade on hemoglobin levels in type 2 diabetic patients with chronic kidney disease. Am J Hypertens 21: 317–322, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Gossmann J, Thürmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W, Scheuermann EH: Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int 50: 973–978, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C: Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22: 1020–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstädt HJ, Meersch M, Unruh M, Zarbock A: FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126: 962–974, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David V, Francis C, Babitt JL: Ironing out the cross talk between FGF23 and inflammation. Am J Physiol Renal Physiol 312: F1–F8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss G, Goodnough LT: Anemia of chronic disease. N Engl J Med 352: 1011–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, Nayak A, Wesseling-Perry K, Westerman M, Hollis BW, Salusky IB, Hewison M: Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol 25: 564–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, Nathan I: Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol 30: 403–409, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Zucker S, Friedman S, Lysik RM: Bone marrow erythropoiesis in the anemia of infection, inflammation, and malignancy. J Clin Invest 53: 1132–1138, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iguchi A, Kazama JJ, Yamamoto S, Yoshita K, Watanabe Y, Iino N, Narita I: Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron 131: 161–166, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Braithwaite V, Prentice AM, Doherty C, Prentice A: FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: A supplementation study. Int J Pediatr Endocrinol 2012: 27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita K, Mizuiri S, Nishizawa Y, Kenichiro S, Doi S, Masaki T: Oral iron supplementation with sodium ferrous citrate reduces the serum intact and c-terminal FGF23 levels of maintenance hemodialysis patients [published online ahead of print August 25, 2016]. Nephrology (Carlton) doi 10.1111/nep.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M: Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 89: 135–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf M, Koch TA, Bregman DB: Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28: 1793–1803, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith ER, Cai MM, McMahon LP, Holt SG: Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97: 3357–3365, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Fellner SK, Lang RM, Neumann A, Korcarz C, Borow KM: Cardiovascular consequences of correction of the anemia of renal failure with erythropoietin. Kidney Int 44: 1309–1315, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Hörl WH: Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol 9: 291–301, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.