Abstract
A systematic review was conducted to categorize the types of cancerous tissues that express orexin receptors and also to examine the effect of in vitro administration of orexin A or B to corresponding cell samples. Comprehensive literature analyses of primary experimental studies were performed. The results of the review included an increased frequency of orexin receptor expression in many colon and prostate cancer tissues and an upward trend of pro-apoptotic activity in these aggressive cell types.
Keywords: Cancer, orexin, hypocretin, apoptosis, orexin receptor, orexin 1 receptor, orexin 2 receptor, orexin A, orexin B
Background
Recently published in the Journal of Biomedical Science (March, 2017) was the review article entitled “Evaluation of the use of therapeutic peptides for cancer treatment.”1 Notably absent from this review was data from two peptides which have been regularly researched for anti-cancer effects, orexin A (OXA), and orexin B (OXB). These neurotransmitters, also known as hypocretins, have incited enthusiasm in the field of cancer research as promoters of apoptosis in various colon, prostate, and hepatic cancer cells lines. Therefore, the prospect of identifying the role of orexin peptides as a potential new treatment modality for cancer patients is appealing. Furthermore, studies that reveal orexins’ effect in cancer cell death in later-stage metastatic colon cancer encourages research models that target this patient population, which typically faces limited treatment options.2 It is important to note, however, that these endogenous peptides have a relatively short plasma half-life of approximately 27 min, along with conflicting data of the effects of orexins in certain malignant cell types. Therefore, a closer examination of the potential limitations of the use of this treatment within certain conditions is required.3 This review of current research focuses on the expression of orexin receptors and the consequences of receptor stimulation in transformed cells with the objective of providng in guidance for future orexin research and to promote a currently hypothetical, but highly specific cure for certain localized and metastatic solid tumors originating in the colon and prostate.
Orexins are produced in the hypothalamic region of the brain and act upon two specific G protein–coupled receptors found on the surface of native neurons in the central nervous system (CNS), namely, orexin 1 receptor (OX1R also known as HCRT1) and orexin 2 receptor (OX2R also known as HCRT2)4,5 Occupation of these receptors regulates several important physiological functions, including the sleep–wake cycle, energy metabolism, and feeding behaviors5 as listed in Table 1. While OXA binds to both receptors with a high affinity, OXB only binds to OX2R with a high affinity and to OX1R with a markedly reduced affinity.5
Table 1.
Summary of orexin receptor effects in the central nervous systema.
| Receptor | Proposed effect of stimulation | Proposed effect of antagonism | Binding affinity |
|
|---|---|---|---|---|
| Orexin A | Orexin B | |||
| OX1R | Reward-seeking | Anti-addiction | +++ | +/− |
| Anxiety | Anxiolytic | |||
| ↑ levels of amyloid beta (theoretically increasing risk of Alzheimer’s disease) | ↓ levels of amyloid beta (theoretically anti-Alzheimer’s disease) | |||
| Wakefulness | Narcolepsy | |||
| OX2R | Insomnia | Sleep/cataplexy | +++ | +++ |
| Increased food intake (short-term) | Anorexia (short-term) | |||
| Anti-obesity and anti-insulin-resistance effects by improving leptin sensitivity (long-term) | High-fat diet–induced obesity and insulin resistance (long-term) | |||
OX1R: orexin 1 receptor; OX2R: orexin 2 receptor.
Adapted from the study of Kodadek et al.5 and references therein.
OXA is a large, lipophilic polypeptide (MW = 3562) which effluxes from the brain to the systemic blood circulation at a rate similar to that of albumin.6 It has been shown to have an influx rate (Ki) of 2.5 ± 0.3 × 10−4 mL/g min, which is one of the fastest rates of non-saturable influx into the brain through the blood–brain barrier (BBB) of any peptide.6 Because of this, OXA is largely confined to the central nervous system (CNS). In contrast, OXB is less lipophilic, hampering its passage through the BBB following experimental intravenously administration, resulting in its rapid serum degradation prior to entry into the CNS.6 Therefore, the vast majority of OXB also resides within the CNS.
Although the main actions of both orexins are exerted on neurons in the CNS; orexin receptors have also been identified in a select few endogenous tissues in the periphery such as adrenocortical cells.7 Stimulation of orexin receptors located on the surface of these cells results in cortical secretion and cell proliferation.7 It remains controversial as to the function, if any, of orexins acting upon proposed endogenous orexin receptors found in other non-transformed peripheral cells of the intestine, pancreas, kidney, adipose tissue, and reproductive tract.5 However, if the expression of orexin receptors in the periphery is as limited as assumed, the potential adverse effects of orexin administration would then theoretically be limited as well, namely, due to a lack of receptors upon which to exert an effect.
The profound effect of orexin interaction with malignant cells was first discovered as a result of an in vitro study in 2004 that demonstrated apoptosis in colon cancer cells subsequent to orexin treatment.8 It was discovered here that certain colon cancers ectopically express orexin receptors and stimulation of these receptors triggers robust apoptosis of the host cell.8 Many further studies tested the frequency of orexin receptor expression in a wide variety of cancer cells and sought to determine the effect of orexin stimulation of these receptors. One experimental model included the subcutaneous inoculation of LoVo colon cancer cells in nude mice, which lead to tumor formations.2 The mice were then treated daily for a period of 15 days with 1.12 μmol/kg intraperitoneal injections of OXA.2 The subsequent results were astonishing with an 80% average reduction in tumor size and were reproduced with similar results in HT29-derived colon tumors.2 No apparent side effects were noted in the study animals, which corroborates the theory that a lack of orexin receptor expression in the periphery may naturally limit the possible adverse effects that would result from future in vivo orexin treatment models.2
As orexin research has progressed over the years, it was discovered that not only are orexin receptors ectopically expressed in colon cancer cells but it appears that orexin receptor expression is upregulated in later-staged metastatic cells.2 Further, in hepatic metastases of colon cancer, the presence of OX1Rs were confirmed only on the surface of cancerous cells and not on surrounding hepatocytes, which is consistent with previous observations in localized colon cancer tissues versus surrounding non-transformed tissues.2 This finding is significant because current treatments for metastatic cancers are often limited by systemic toxicity, which may be potentially ameliorated with orexin treatment due to a lack of receptors in the normal tissues surrounding tumors. Given this, orexin therapy in cancer patients has the potential of becoming an unprecedented, highly-specific treatment option with limited adverse effects.
Similar to its effects in colon cancer cells, orexin also promotes apoptosis in prostate cancer cells.9 Because there are many studies involving orexin receptors in various types of cancers, it is important to analyze the presence and extent of orexin receptor expression in all cancers cell types. The only prior literature review of orexin receptors and cancer tissue types known to us is a 2011 review focused on an overview of orexin’s role in colon cancer cells.10 Therefore, the purpose of the present review is to provide a comprehensive analysis of published studies which details all cancerous tissues tested for orexin receptor expression and the effects of orexin stimulation of these receptors, if tested.
Methods
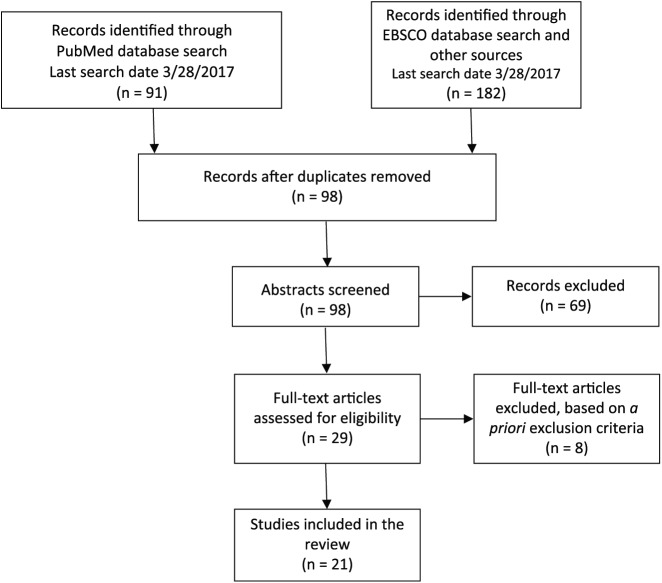
The systematic scientific literature search for this reporting included the PubMed database with the search algorithm: (orexin or hypocretin) AND cancer (last search date: 28 March 2017). A second search was performed using the EBSCO database using the same search algorithm: (orexin or hypocretin) AND cancer (last search date: 28 March 2017). All available literatures were searched, which yielded 98 unique references. These references were screened for full-text review based on a priori inclusion criteria. Inclusion parameters required primary experimental studies of in vivo cancer models or in vitro cancerous tissues that identified cells expressing native (non-transfected) orexin receptors and/or studies that tested the effect of the application of orexin to cancer tissues. Cancerous tissues included experimental cell lines, cell models, or clinical samples. References from identified studies were screened by title or abstract for inclusion based on the a priori inclusion parameters applied to the original search articles (see Figure 1). All studies were assessed for potential bias based on funding or author affiliations.
Figure 1.
Study selection*.
*Data set compiled by authors.
Results
Orexin receptor expression in colon cancers
OX1R messenger RNA (mRNA) was expressed in all clinical colon cancer samples tested2 and in the following cell lines: HT-29,2 HT29-FU,2 HT29-D4,8 SW48,2 SW620,2 SW480,2,8 Caco-2,2,8 LoVo,2,8 Colo205,2 T84,2 and LS174T.2 OX1 mRNA was not expressed in HCT-116 cell lines.2,8 OX2R mRNA was not expressed in any of the cell lines tested.2,8
OXA and OXB induced significant apoptosis in the following cell lines: SW48,2 SW620,2 SW480,2,8 Caco-2,2,8 LoVo,2,8 Colo205,2 T84,2 and LS174T.2 OXB reduced cell production in the following cell lines: SW480,8 Caco-2,8 and LoVo.8 OXA drastically reduced tumor size in LoVo and HT29 xenograft tumors,2 while no effect was observed in HCT-116 xenograft tumors.2 OXA had a variable response in the HCT-116 cell line, which included induced autophagy (resistance to apoptosis),11 reduced cell viability,11 apoptosis promotion,11 and no significant effect.2 OXB had no significant effect on the HCT-116 cell line.2,8 OXA and OXB induced significant apoptosis2,8,9 and inhibited cell growth8,9 in HT-29 and HT29-DR cell lines. OXA and OXB induced significant apoptosis in the HT29-FU cell line.2
Orexin receptor expression and effects on gastric cancers
OX1R mRNA was expressed in the following cell lines: BGC-82312 and SGC-7901.13 OX2R mRNA was not expressed in either of these cell lines.12,13
OXA increased OX1R expression and increased cell proliferation and viability by 150% in the BGC-823 cell line.12 OXA stimulated cell proliferation and viability13 in the SGC-7901 cell line.
Orexin receptor expression in hepatic cancers
OX1R mRNA was expressed in 28 of 41 clinical hepatocellular cancer samples14 as well as in the Hep3B14 cell line. Neither the sample tissues nor the cell line tested positive for OX2R mRNA expression.14
OXA increased glucose uptake in the Hep3B cell line.14
Orexin receptor expression in prostate cancers
OX1R mRNA was expressed in all clinical prostate cancer samples tested.15 The expression of OX1R mRNA was variable in LNCaP9,15,16 and DU1459,16 cell lines. OX1R mRNA was not found in the following cell lines: PC3,16 PrEC,16 PrSmC,16 PrSc,16 or in any clinical normal and benign prostatic hyperplasia (BPH) sample tissues15 tested. OX2R mRNA was expressed in all clinical and BPH sample tissues,15 but was not expressed in LNCaP,9,16 DU145,9,16 PC3,16 PrEC,16 PrSmC,16 or PrSc16 cell lines.
OXA significantly decreased cell survival and significantly reduced androgen receptor nuclear translocation in the presence of testosterone in the LNCaP cell line.15 OXA and OXB increased cell growth in non-differentiated cells and induced significant apoptosis in differentiated cells9 in DU145 cell lines.
Orexin receptor expression in cervical cancers
OX1R and OX2R mRNA was expressed in 45 of 47 clinical cervical cancer samples.17
Orexin receptor expression in endometrial cancers
OXA and OXB resulted in no observed apoptosis effect in the following cell lines: ECC-1, Ishikawa, and MFE-280.18
Orexin receptor expression in adrenal cancers
Cancers of the adrenal cortex
OX1R and OX2R mRNA was not expressed in rat pheochromocytoma (PC), PC12.19 OX2R mRNA was expressed in all human PC clinical samples tested, but OX1R mRNA was not.20
OXA and OXB increased basal cortisol levels and proliferation in clinical samples of adenomas.7 OXA increased cortisol secretion,21,22 down-regulated OX2R mRNA,21 increased OX1R mRNA,22 and enhanced cell proliferation22 in the NCI-H295R cell line.
Cancers of the adrenal medulla
OX1R and OX2R mRNA was expressed on all clinical cortisol-secreting adenoma samples tested.7 OX1R and OX2R mRNA expression was variable for the NCI-H295R21,22 cell line.
OXA and OXB decreased tyrosine hydroxylase production in the PC12 cell line12 and increased inositol triphosphate (IP3), epinephrine, and norepinephrine release in clinical samples of PC.20
Orexin receptor expression in brain cancers
OX1R and OX2R mRNA was expressed in the rat glioma C623 cell line.
OXA significantly decreased cell viability of the rat glioma C6 cell line at a dose of 1 μM and resulted in an IC50 of 4.7 nM.23 OXB resulted in a non-statistically significant decrease in cell viability in the rat glioma C6 cell line.23
Orexin receptor expression in nerve tissue cancers
OX1R mRNA was not expressed in the SK-N-MC cell line.24
Orexin receptor expression in chronic myelogenous leukemia
OX1R and OX2R mRNA was expressed in chronic myelogenous leukemia (CML) clinical samples (Tables 2 and 3).25
Table 2.
Orexin receptor mRNA expression in cancer cellsa.
| System | Organ | Cell type | OX1R | OX2R |
|---|---|---|---|---|
| Digestive system | Colon | 38 of 38 clinical colon cancer samples2 | + | |
| 10 of 10 clinical liver metastases of colon cancer samples2 | + | |||
| HT-292 | + | − | ||
| HT29-FU2 | + | − | ||
| HT29-D48 | + | − | ||
| SW482 | + | − | ||
| SW6202 | + | − | ||
| SW4802,8 | + | − | ||
| Caco-22,8 | + | − | ||
| LoVo2,8 | + | − | ||
| Colo2052 | + | − | ||
| T842 | + | − | ||
| LS174T2 | + | − | ||
| HCT-1162,8 | − | − | ||
| Stomach | BGC-82312 | + | − | |
| SGC-790113 | + | − | ||
| Liver | Hep3B14 | + | − | |
| 28 of 41 clinical hepatocellular cancer samples14 | + | − | ||
| Endocrine/reproductive system | Prostate | LNCaP9,15,16 | +/− | − |
| DU1459,16 | +/− | − | ||
| PC316 | − | − | ||
| PrEC16 | − | − | ||
| PrSmC16 | − | − | ||
| PrSc16 | − | − | ||
| 15 of 15 clinical prostate cancer samples15 | + | |||
| 30 of 30 clinical normal and BPH samples26 | − | + | ||
| Cervix | 45 of 47 clinical cervical cancer samples17 | + | + | |
| Adrenal gland (cortex) | 10 of 10 clinical samples of cortisol-secreting adenomas7 | + | + | |
| NCI-H295R21,22 | +/− | +/− | ||
| Adrenal gland (medullary) | Rat PC, PC1219 | − | − | |
| 10 of 10 clinical samples of human PCs20 | − | + | ||
| Central nervous system | Brain | Rat glioma C623 | + | + |
| Peripheral nervous system | Nerve tissues | SK-N-MC24 | − | |
| Stem cells | CD34+ | Clinical samples of CML25 | + | + |
OX1R: orexin 1 receptor; OX2R: orexin 2 receptor; PC: pheochromocytoma; CML: chronic myelogenous leukemia; BPH: benign prostatic hyperplasia.
Originally compiled summary of data from references cited within table.
Table 3.
Orexin effects on cancer cell survivala.
| System | Organ | Cell type | OXA | OXB |
|---|---|---|---|---|
| Digestive system | Colon | HCT-116 | Induced autophagy (resistance to apoptosis), reduced cell viability, and promoted apoptosis;11 no significant effect2 | No significant effect2,8 |
| HT-29 | Induced significant apoptosis2,9 and inhibited cell growth9 | Induced significant apoptosis2,9 and inhibited cell growth9 | ||
| HT29-FU | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| HT29-DR | Suppression of cell growth and induction of apoptosis8 | Suppression of cell growth and induction of apoptosis8 | ||
| SW48 | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| SW620 | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| SW480 | Induced significant apoptosis2 | Reduced cell production8
Induced significant apoptosis2,8 |
||
| Caco-2 | Induced significant apoptosis2 | Reduced cell production8
Induced significant apoptosis2,8 |
||
| LoVo | Induced significant apoptosis2 | Reduced cell production8
Induced significant apoptosis2,8 |
||
| Colo205 | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| T84 | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| LS174T | Induced significant apoptosis2 | Induced significant apoptosis2 | ||
| Xenograft LoVo tumor | Drastic reduction in tumor size2 | |||
| Xenograft HT29 tumor | Drastic reduction in tumor size2 | |||
| Xenograft HCT-116 tumor | No effect2 | |||
| Stomach | BGC-823 | Increased OX1R expression; 1.5-fold increase in cell proliferation and viability12 | ||
| SGC-7901 | Stimulated cell proliferation and viability13 | |||
| Liver | Hep3B | Increased glucose uptake14 | ||
| Endocrine/reproductive system | Prostate | LNCaP | Significantly decreased cell survival;15 significantly reduced androgen receptor nuclear translocation in the presence of testosterone15 | |
| DU145 | Increased cell growth in non-differentiated cells;9 induced significant apoptosis in differentiated cells9 | Increased cell growth in non-differentiated cells;9 induced significant apoptosis in differentiated cells9 | ||
| Endometrium | ECC-1 | No apoptosis effect18 | No apoptosis effect18 | |
| Ishikawa | No apoptosis effect18 | No apoptosis effect18 | ||
| MFE-280 | No apoptosis effect18 | No apoptosis effect18 | ||
| Adrenal gland (cortex) | Clinical samples of adenomas | Increased basal cortisol levels; increased proliferation7 | No effect on basal cortisol levels; increased proliferation7 | |
| NCI-H295R | Increased cortisol secretion;21,22 down-regulated OX2R mRNA;21 increased OX1R mRNA;22 enhanced cell proliferation22 | |||
| Adrenal gland (medullary) | PC12 | Decreased tyrosine hydroxylase19 | Decreased tyrosine hydroxylase19 | |
| Clinical samples of PC | Increased IP3, epinephrine and norepinephrine release20 | Increased IP3, epinephrine, and norepinephrine release20 | ||
| Central nervous system | Brain | Rat glioma C6 | Significantly decreased cell viability at a dose of 1 μM; IC50 of 4.7 nM23 | Non-statistically significant decreased cell viability23 |
OXA: orexin A; OXB: orexin B; PC: pheochromocytoma.
Originally compiled summary of data from references cited within table.
Discussion
Orexin receptor expression in colon cancers
Colorectal cancer samples comprise the most common study material for orexin treatment. It has been consistently demonstrated that both orexins A and B significantly induce apoptosis and inhibit growth of human colorectal adenocarcinoma (HT-29 and HT29-D4) cell lines.8,9 In mRNA and immunohistochemistry (IHC) studies, OX1R was observed in many colon cancer cell lines, namely HT-29, SW48, SW620, SW480, Caco-2, LoVo, Colo205, T84, LS174T, and in 38 clinical colon cancer samples.2 Surprisingly, OX1R mRNA was discovered in difficult-to-treat, 5-fluorouracil-resistant HT-29 cell samples (HT29-FU) as well as in 10 liver metastases of primary colon cancer samples.2 In addition, non-transformed, adjacent cells to the malignant clinical samples and metastases tested with quantitative real-time polymerase chain reaction (qRT-PCR) and IHC were negative for OX1R mRNA and staining.2,8 Robust apoptosis was observed in HT-29, SW48, SW620, SW480, Caco-2, LoVo, Colo205, T84, LS174T, HCT-116, 38 clinical colon cancer samples, and 10 clinical liver metastases of colon cancer samples treated with OXA.2 Apoptosis results were also observed in a separate study for Caco-2, LoVo, and Colo205 cell lines.8 The only colon cancer cell line which was found to not express OX1R and did not undergo apoptosis upon treatment with OXA was HCT-116.2,8 Finally, the only known in vivo orexin cancer study to date included the inoculation of three types of colon tumors xenografts derived from HT-29, LoVo, and HCT-116 cell lines in nude mice, which were thereafter treated with OXA intraperitoneal injection for up to 30 days.2 Dramatic reductions in tumor sizes were observed in both the HT-29 and LoVo-derived tumors at effective doses of 0.112, 1.12, and 11.2 μmol/kg, but were not observed in the HCT-116-derived tumor.2
In contrast to the previously negative HCT-116 findings, Wen et al.11 found that OXA reduced cell viability and promoted apoptosis in HCT-116 colon cancer cells. Additionally, autophagy, which is a cellular mechanism to resist cell death, was purportedly activated via the extracellular-signal-regulated kinase (ERK) pathway in HCT-116 cells after treatment with OXA.11 These results reflect an overall anti-cancer effect mediated by OX1R.
Orexin receptor expression and effects on gastric cancers
OXA treatment resulted in a protective effect in the gastric cancer cell line, BGC-823, including decreased caspase-3 apoptotic activity and improved cell proliferation and survival via the protein kinase B (AKT) signaling pathway.12 In this cell type, the presence of OXA appeared to lead to the overexpression of OX1R.12 Similar results were observed in the gastric cancer cell line, SGC-7901, which resulted in increased cell proliferation and viability and apoptosis protection via activation of the ERK1/2 pathway upon OXA treatment.13 OX1R, and not OX2R, was detected in both the BGC-823 and SGC-7901 cell lines tested.12,13 Therefore, in primary gastric cancers, orexin treatment may result in a counter-productive effect.
Orexin receptor expression in hepatic cancers
IHC and western blot analyses in the human hepatocellular carcinoma cell line, Hep3B, showed the presence of OX1R mRNA, but not OX2R.14 This finding was consistent in further studies of clinical human hepatocellular samples tested by IHC, which showed that 28 of 41 tumor samples and 9 of 14 non-tumor samples tested positive for OX1R, but were negative for OX2R.14 In addition, Hep3B cells incubated with OXA resulted in a concentration-dependent increased glucose uptake into the Hep3B cells, possibly promoting pyruvate shunting into the tricarboxylic acid (TCA) cycle and oxidative phosphorylation, and thereby reducing glycolysis.14 Further testing is required to determine whether this effect either promotes or decreases viability in this cell type.
Orexin receptor expression in prostate cancers
The results of mRNA testing for orexin receptor expression in prostate cancer cells are contradictory. Szyszka et al.16 reported negative polymerase chain reaction (PCR) mRNA results for both OX1R and OX2R in normal prostate cell lines (PrEC, PrSc, PrSmC) and for prostatic carcinoma cell lines (DU145, LNCaP, and PC3). Alexandre et al.9 also observed undetectable OX1R mRNA in LNCaP cells. In contrast, Valiante et al.15 reported positive results for OX1R mRNA in the LNCaP cell line and that, upon treatment with OXA, LNCaP expression of OX1R increased while cell survival decreased.15 Noteworthy was the finding that OXA has an antagonistic effect in androgen receptor translocation in LNCaP cells in the presence of exogenous testosterone.15 Since androgen receptor translocation can promote the progression of this aggressive form of cancer, blocking this process could potentially compliment current cancer therapy.15 While Szyszka et al.16 found an absence of mRNA for OX1R expression in DU145 cells, Alexandre et al.9 showed that androgen-independent DU145 cells that had undergone neuroendocrine differentiation not only expressed OX1R but also increased as the grade of prostate cancer increased.9 Also, while non-transformed DU145 cells treated with OXA or OXB resulted in increased cell growth, cells that had undergone neuroendocrine transformation treated with OXA underwent significant apoptosis.8 This may point to the conclusion that while potentially harmful in early-staged cancers, orexin may provide therapeutic benefit in certain late-staged cancers of the prostate, but further research is required to confirm this role.
A study of clinical prostate cancer tissues showed that 15 of 15 samples tested positive for OX1R IHC and western blot analysis.15 Another study of clinical samples demonstrated, through IHC staining, the expression of both orexin receptors in 27 of 30 (90%) prostate cancer samples, in 16 of 30 (53.3%) BPH samples, and in 8 of 30 (26.7%) chronic prostatitis samples.26 This may reflect an increased orexin receptor expression in malignant cells as a part of the transformational process of prostate cancer.
Orexin receptor expression in cervical cancers
IHC staining in clinical cervical cancer and cervicitis specimens were positive for expression of both OX1R and OX2R, but OX2R was found to be upregulated in 95.7% of cancer samples as opposed to 50.0% of cervicitis samples.17 No tests were completed to investigate the effect of orexin treatment in these cell types.
Orexin receptor expression in endometrial cancers
IHC testing results of clinical samples of endometrial endometrioid carcinomas (EEC) were negative for OX2R, while normal tissues tested positive for OX2R.18 This may be due to epigenetic silencing involving hypermethylation during the progression into cancer.18 OXA treatment in human endometrial cancer cell lines, ECC-1, Ishikawa, and MFE-280 did not result in apoptosis.18
Orexin receptor expression in adrenal cancers
In normal tissues, the adrenal cortex (glomerulosa, fasciculate, and reticular zones) expresses OX1R, but not OX2R, while the adrenal medulla (epinephrine and norepinephrine cells) expresses only OX2R, but not OX1R.27
Cancers of the adrenal cortex
Consistent with the normal distribution of orexin receptors in native tissues, Blanco et al.27 demonstrated by IHC that 4 clinical samples of adrenocortical adenomas tested positive for OX1R, while OX2R expression was absent. In another study of clinical samples, Spinazzi, et al.7 utilized qRT-PCR and western blot analysis methods to test 10 clinical adrenocortical adenoma tissues, which were all positive for the expression of both OX1R and OX2R mRNAs. In addition, it appeared that both receptors were overexpressed in adenomas as compared to normal adrenocortical tissue samples.7 When treated with orexins, OXA, alone, increased cortisol secretion, while both OXA and OXB treatment resulted in proliferative effects.7 This appears to reflect an upregulation of OX2R in maligancies versus normal tissues.
Studies of adrenal cortical cancer cell lines were also inconsistent. Wenzel et al.21 conducted a qRT-PCR analysis of National Cancer Institute (NCI) H295R human adrenocortical cells and reported high expression of OX2R. After treatment with OXA, the H295R cells resulted in significantly increased cortisol levels.21 It was also observed that OX2R receptor mRNA was down-regulated in response to OXA treatment. Although OX1R mRNA was present, it was found in very low levels.21 In contrast to the Wenzel et al.21 study, Chang et al.22 found that mRNA for OX1R, and not OX2R, was expressed in NCI-H295R cells, expression of which was increased significantly subsequent to treatment of OXA, in a dose-dependent manner. Similar to Wenzel et al.,21 Chang et al.22 found that OXA induced cortisol secretion from NCI-H295R cells, but additionally observed that cell proliferation was also enhanced. These inconsistent results demonstrate the complexity of the regulation of orexin receptors in the adrenal gland, especially in malignancies.
Cancers of the adrenal medulla
Studies of clinical samples of pheochromocytomas consistently report the presence of OX2R and an absence of OX1R, which is in line with endogenous tissue expression in these regions.20,27 Furthermore, OXA and OXB treatment of samples resulted in a 3-fold increase in IP3 production and a dose-related increase in both epinephrine and norepinephrine release.20
In the Nanmoku et al.’s19 qRT-PCR study of the rat pheochromocytoma cancer cell line, PC12, an absence of both OX1R and OX2R expressions was observed. However, when the PC12 cells were treated with OXA and OXB, a dose-related reduction in tyrosine hydroxylase (the rate-limiting enzyme in catecholamine synthesis) was noted.19 Binding assays reflected that both OXA and OXB, in equal affinities, bound to the cells, which may indicate orexin interaction with a non-orexin receptor.19 This further adds to the complexity of orexin interaction with the adrenal glands.
Orexin receptor expression in brain cancers
In an experimental model of the aggressive brain cancer, glioblastoma multiforme (GBM), a qRT-PCR analysis was conducted with the rat C6 cell line, which confirmed the presence of both types of orexin receptors, similar to native tissue expression.23 Surprisingly, treatment of these cells with OXA, and not OXB, at a dose of 1 μM resulted in a significantly decreased rate of cell viability.23 The IC50 was calculated at 4.7 nM for OXA.23 However, lower (physiological level) doses of OXA did not compromise cell viability as compared to controls.23 Given this, orexin may provide a potential treament option for this currently terminal condition.
Orexin receptor expression in nerve tissue cancers
Bader et al.24 completed a receptor study of the SK-N-MC neuroepithelioma cell line (controversially also regarded as a neuroblastoma cell line as it was originally derived from a metastatic neuroblastoma) and revealed an absence of OX1R at the mRNA level. In contrast, Rouet-Benzineb et al.8 did not report mRNA findings for orexin receptor expression, but did complete a test-by-treatment study in the same cell line and showed that both OXA and OXB significantly inhibited cell growth (IC50 of 5 nM for both orexins) and induced apoptosis in SK-N-MC cells. Further testing is required to fully evaluate the orexin treatment potential in nerve tissue cancers.
Orexin receptor expression in CML
In a gene expression study of CD34+ hematopoietic stem and progenitor cells from newly diagnosed and untreated CML patients, a 1.8- to 2.2-fold upregulation of OX1R and OX2R was observed.25 Testing of this cell type with orexins to determine the effect of receptor stimulation may aid in the future analysis of orexin’s role in CML treatment, if any
Study limitations
The authors note several study limitations. First, orexins are relatively recently discovered neurotransmitters, inherently limiting the volume of reviewable data available. In addition, the discovery of orexin-based cancer research began only in 2004, reducing the available period of time for completion of in vitro and in vivo studies.
Conclusion
Ectopic OXR1 expression is common in many colon cancer cell lines (HT-29, HT29-D4, HT29-FU, SW48, SW620, SW480, Caco-2, LoVo, Colo205, T84, LS174T) and in clinical samples of localized and metastases of colon cancers.Significant induction of apoptosis or inhibited cell growth was observed in HT-29 and HT29-D4 cell lines upon treatment with orexins. In contrast, gastric cancer cell lines (BGC-823 and SGC-7901), while expressing OX1R, result in increased cell proliferation when treated with OXA. OX1R expression was shown in hepatic cancers (clinical hepatocellular tumor samples and Hep3B), but was not studied with orexin treatment for cell viability. While the results of prostate cancer cells were inconsistent, it appears that OX1R expression may be directly correlated to cancer progression. Furthermore, OX1R-positive prostate cancer samples treated with orexins results in reduced cell survival. It also appears that OX2R is upregulated in cervical cancer samples and down-regulated in endometrial cancer samples. The most common observations in cancers of the adrenal glands (clinical samples, NCI-H295R, PC12) show consistent orexin receptor expression that corresponds to non-transformed tissues in the region. Orexin treatment here resulted in changes in catecholamine production and, in some cases, increased cell proliferation. In rat C6 glioma cells (GBM model), high doses of OXA (1 μM) result in decreased cell viability (IC50 of 4.7 nM). In the neuroepithelioma cell line, SK-N-MC, one study showed a lack of orexin receptors, but another showed significantly inhibited cell growth (IC50 of 5 nM) upon treatment with OXA and OXB. Finally, clinical CD34+ CML samples resulted in a 1.8- to 2.2-fold up-regulation of OX1R and OX2R as compared to normal hematopoietic stem and progenitor cells. In whole, these findings demonstrate the complex, but intriguing role of orexin in the progression and treatment of various cancers.
Acknowledgments
N.L.G. wrote the paper. All authors read and approved the final manuscript.
Footnotes
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Consent for publication: All of the authors have read and approved the paper for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci 2017; 24: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voisin T, El Firar A, Fasseu M, et al. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res 2011; 71: 3341–3351. [DOI] [PubMed] [Google Scholar]
- 3. Ehrström M, Näslund E, Levin F, et al. Pharmacokinetic profile of orexin A and effects on plasma insulin and glucagon in the rat. Regul Peptides 2004; 119: 209–212. [DOI] [PubMed] [Google Scholar]
- 4. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92: 573–585. [DOI] [PubMed] [Google Scholar]
- 5. Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst 2010; 6: 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 1999; 289: 219–223. [PubMed] [Google Scholar]
- 7. Spinazzi R, Rucinski M, Neri G, et al. Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab 2005; 90: 3544–3549. [DOI] [PubMed] [Google Scholar]
- 8. Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, et al. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem 2004; 279: 45875–45886. [DOI] [PubMed] [Google Scholar]
- 9. Alexandre D, Hautot C, Mehio M, et al. The orexin type 1 receptor is overexpressed in advanced prostate cancer with a neuroendocrine differentiation, and mediates apoptosis. Eur J Cancer 2014; 50: 2126–2133. [DOI] [PubMed] [Google Scholar]
- 10. Laburthe M, Voisin T. The orexin receptor OX1R in colon cancer: a promising therapeutic target and a new paradigm in G protein-coupled receptor signalling through ITIMs. Br J Pharmacol 2012; 165: 1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen J, Zhao Y, Guo L. Orexin A induces autophagy in HCT-116 human colon cancer cells through the ERK signaling pathway. Int J Mol Med 2016; 37: 126–132. [DOI] [PubMed] [Google Scholar]
- 12. Wen J, Zhao Y, Shen Y, et al. Effect of orexin A on apoptosis in BGC-823 gastric cancer cells via OX1R through the AKT signaling pathway. Mol Med Rep 2015; 11: 3439–3444. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Zhao Y, Ju S, et al. Orexin A upregulates the protein expression of OX1R and enhances the proliferation of SGC-7901 gastric cancer cells through the ERK signaling pathway. Int J Mol Med 2015; 35: 539–545. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Zhao Y, Guo L. Effects of orexin A on glucose metabolism in human hepatocellular carcinoma in vitro via PI3K/Akt/mTOR-dependent and -independent mechanism. Mol Cell Endocrinol 2016; 420: 208–216. [DOI] [PubMed] [Google Scholar]
- 15. Valiante S, Liguori G, Tafuri S, et al. Expression and potential role of the peptide orexin-A in prostate cancer. Biochem Biophys Res Commun 2015; 464: 1290–1296. [DOI] [PubMed] [Google Scholar]
- 16. Szyszka M, Paschke L, Tyczewska M, et al. Lack of expression of preproorexin and orexin receptors genes in human normal and prostate cancer cell lines. Folia Histochem Cytobiol 2015; 53: 333–341. [DOI] [PubMed] [Google Scholar]
- 17. Taximaimaiti R, Abuliken X, Maihemuti M, et al. Elevated expression of Ox2R in cervical cancers and placentas of Uyghur women in Xinjiang, China. Asian Pac J Cancer Prev 2016; 17: 4959–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dehan P, Canon C, Trooskens G, et al. Expression of type 2 orexin receptor in human endometrium and its epigenetic silencing in endometrial cancer. J Clin Endocrinol Metab 2013; 98: 1549–1557. [DOI] [PubMed] [Google Scholar]
- 19. Nanmoku T, Isobe K, Sakurai T, et al. Orexins suppress catecholamine synthesis and secretion in cultured PC12 cells. Biochem Biophys Res Commun 2000; 274: 310–315. [DOI] [PubMed] [Google Scholar]
- 20. Mazzocchi G, Malendowicz LK, Aragona F, et al. Human pheochromocytomas express orexin receptor type 2 gene and display an in vitro secretory response to orexins A and B. J Clin Endocrinol Metab 2001; 86: 4818–4821. [DOI] [PubMed] [Google Scholar]
- 21. Wenzel J, Grabinski N, Knopp CA, et al. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1601–R1609. [DOI] [PubMed] [Google Scholar]
- 22. Chang X, Zhao Y, Ju S, et al. Orexin-A stimulates 3β-hydroxysteroid dehydrogenase expression and cortisol production in H295R human adrenocortical cells through the AKT pathway. Int J Mol Med 2014; 34: 1523–1528. [DOI] [PubMed] [Google Scholar]
- 23. Biegańska K, Sokołowska P, Jöhren O, et al. Orexin A suppresses the growth of rat C6 glioma cells via a caspase-dependent mechanism. J Mol Neurosci 2012; 48: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bader JE, Deckert CM, Koglin N, et al. From transcription profile to expression: the signaling repertoire of the SK-N-MC neuroepithelioma cell-line. J Recept Signal Transduct Res 2004; 24: 257–282. [DOI] [PubMed] [Google Scholar]
- 25. Kronenwett R, Butterweck U, Steidl U, et al. Distinct molecular phenotype of malignant CD34(+) hematopoietic stem and progenitor cells in chronic myelogenous leukemia. Oncogene 2005; 24: 5313–5324. [DOI] [PubMed] [Google Scholar]
- 26. Başar MM, Han Ü, Çakan M, et al. Orexin expression in different prostate histopathologic examinations: can it be a marker for prostate cancer? A preliminary result. Turk J Urol 2013; 39: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanco M, García-Caballero T, Fraga M, et al. Cellular localization of orexin receptors in human adrenal gland, adrenocortical adenomas and pheochromocytomas. Regul Pept 2002; 104: 161–165. [DOI] [PubMed] [Google Scholar]