Abstract
Wilson’s disease is a rare, inherited autosomal recessive disease of copper metabolism, in which the causative gene, ATP7B, results in absent or reduced function of the ATP7B transporter important for biliary excretion of copper and incorporation of copper into caeruloplasmin. Affected patients accumulate excessive copper within the liver, brain and other tissues. A disease mainly of children, adolescents and young adults; clinical features vary from the asymptomatic state to chronic liver disease, acute liver failure, and neuropsychiatric manifestations. Diagnosis requires a high index of suspicion and is based on a combination of clinical signs, biochemical tests, hepatic copper content assay and mutation analysis of the ATP7B gene; to date, there are more than 500 mutations of ATP7B in patients with Wilson’s disease. Early recognition and treatment can result in an excellent prognosis whereas untreated disease is almost always fatal. Drug therapies include chelating agents, such as penicillamine or trientine, and zinc salts. Liver transplantation is curative correcting the underlying pathophysiology and is traditionally indicated in acute liver failure or end-stage liver disease refractory to medical therapy. This review provides an overview of various aspects of Wilson’s disease including molecular basis of the disease, clinical features, diagnostic and management strategies with their current limitations.
Keywords: ATP7B, chelating agents, liver failure, liver transplantation, Wilson’s disease
Introduction
Wilson’s disease (also known as hepatolenticular degeneration) is a rare, inherited autosomal recessive disease of copper metabolism with excessive copper deposition in the body.1 First described by Kinnier Wilson2 in 1912 as ‘progressive lenticular degeneration’ and liver cirrhosis, recent progress has advanced our understanding of the pathogenesis and molecular genetics of the disease. In 1993, the molecular genetic defect was identified as ATP7B on chromosome 13,3–5 which encodes a copper-transporting P-type ATPase transmembrane protein expressed mainly in hepatocytes.
Copper has an essential role in many metabolic processes including serving as a cofactor for many important enzymes.6,7 A typical diet provides 2–5 mg/day of copper with 98% of absorbed copper excreted via the liver into bile.8 The small intestines absorb dietary copper through various metal transporters and is exported into the portal circulation by copper-transporting P-type ATPase.6,7 Copper is taken up by the liver from the portal circulation through the CTR1 transporter. In the cytoplasm, important scavenger proteins such as metallothionein and glutathione protect hepatocytes from the toxic effects of copper. Located in the trans-golgi network in hepatocytes, the ATP7B transporter facilitates copper homeostasis through incorporation of copper into cuproproteins such as caeruloplasmin under normal conditions, which is then secreted into blood. With excess copper, the protein ATP7B moves towards the canalicular part of the hepatocyte, where it promotes biliary copper excretion by taking on a predominantly excretory role.9 Functional ATP7B mutations, as seen in Wilson’s disease, results in absent or reduced function of the ATP7B transporter.
Caeruloplasmin is a serum glycoprotein synthesized and secreted by the liver. Almost all of the body copper is protein bound with free copper inevitably injurious to cells through the production of free radicals. Failure to incorporate copper into caeruloplasmin results in hypocaeruloplasminaemia found in most patients with Wilson’s disease.6,10 The resulting slow hepatic copper accumulation over years produces progressive hepatocellular damage once storage capacity of the liver is exceeded. Eventually, noncaeruloplasmin bound copper spills into the circulation and deposits in other organs with common extrahepatic sites including the brain, eyes and kidneys. Urinary copper excretion increases in those with Wilson’s disease but cannot compensate for the impaired biliary excretion of copper or the progressive serum copper accumulation.11
Wilson’s disease has been attributed to more than 500 disease causing genetic mutations and most patients are compound heterozygotes.12,13 This therefore requires mutation analysis to be used carefully diagnostically as well as for screening purposes. Recent developments in sequencing methods has increased the success in mutation detection, with the highest reported detection rate at 98% in a laboratory proven Wilson’s disease cohort.14 Other studies however, have reported a lower detection rate of 69%.15 While there remains no defined genotype–phenotype pattern in Wilson’s disease, several groups have attempted to interpret phenotype–genotype correlation data. Fulminant hepatic failure at an early age of onset was observed in those with mutant alleles for truncated ATP7B, probably as a consequence of early copper overload from complete nonfunction.16 Conversely, those with a H714Q mutation tend to have a late neurological presentation; the residual functional activity likely accounting for the delayed onset of the disease.17 The H1069Q mutation is the most frequently occurring mutation in European and North American White patients and presents with equal chance for either neurologic or hepatic presentations.18,19
Estimates suggest approximately 30 individuals are affected per million population worldwide.20 Wilson’s disease may present symptomatically at any age, although most cases are between the ages of 5–35 years. Untreated Wilson’s disease is usually fatal typically as a consequence of liver disease in the majority of cases. Medical therapy and liver transplantation has enabled prolonged survival with drug treatment compliance being the main prognostic determinant.8,21
Medical therapy is rarely effective in adults presenting with Wilson’s related acute liver failure due to the inability of medical therapy to remove sufficient excess copper during the acute phase. In this setting, apoptosis of liver cells leads to an acute surge of copper release into the circulation. The resulting consequence is haemolysis and excess copper related injury to renal tubules with reduced renal filtration of copper.8 There is a perpetuating cycle of copper release through the release of haem iron from haemolysis causing further injury to hepatocytes. A reduction in circulating copper may be achieved by plasmapheresis, haemofiltration, exchange transfusion and molecular absorbent recirculating system (MARS). However, these strategies are usually not effective in reversing critical hepatic and progenitor cell injury, to enable timely regeneration to occur. Ultimately, liver transplantation is required in almost all cases of acute liver failure due to Wilson’s disease.22 Several recent papers have reported remission by plasma exchange, chelator and other medical therapy without the need for liver transplantation in the setting of Wilson’s-related acute liver failure, albeit predominantly in the paediatric population.23–25
Clinical features
Common presentations for Wilson’s disease are with liver disease or neuropsychiatric features. Asymptomatic patients are usually found through family screening. Those presenting with neuropsychiatric manifestations tend to be older than those with liver disease who tend to be younger.6
Hepatic
Wilson’s disease may present with liver disease varying on a spectrum ranging from acute liver failure, asymptomatic with only biochemical abnormalities, to overt cirrhosis with gradual decompensation from progressive portal hypertension and its associated complications. Acute viral hepatitis-like symptoms may also occur, although there is usually evidence of chronic liver disease such as splenomegaly and thrombocytopenia.26 Some patients may have Coombs-negative (nonimmune) haemolytic anaemia with transient episodes of jaundice or low-grade haemolysis.27,28 All young patients with unexplained chronic liver disease or acute hepatitis should be screened for Wilson’s disease.8
Presentation with acute liver failure is seen in ~3–5%, predominantly in young females (female:male ratio 4:1), in the second decade of life.29 Usually it is rapidly progressive and fatal unless transplantation is an option. Cirrhosis is usually present in most cases although some might show evidence of massive necrosis with only bridging fibrosis.6,30 Diagnosis of Wilson’s disease in those with acute liver failure is often difficult, although patients may present with Coombs-negative haemolytic anaemia, evidence of underlying cirrhosis, acute kidney injury and elevated serum or urine copper levels.8,31 Serum alkaline phosphatase levels are disproportionately low, while total bilirubin is disproportionately high due to concomitant rise of indirect bilirubin from copper induced haemolysis. Transaminases may be only mildly elevated. An acute presentation may be observed in patients previously treated for Wilson’s disease but in whom medication is stopped.28
Neurological
Neurological features are presenting signs in 40–50% of patients with Wilson’s disease.32 They tend to present later than hepatic manifestations, in childhood but frequently in the third decade of life.33 They can be extremely subtle or may develop rapidly with complete disability apparent within a few months. Neurological manifestations can be classified as: (1) a dystonic syndrome,34 (2) akinetic-rigid syndrome akin to Parkinson’s disease, (3) pseudosclerosis dominated by tremor, and (4) ataxia.6 Other neurological features include dysarthria, drooling, dysphagia (pseudobulbar palsy with a risk of aspiration), a proximal wing-beating tremor, dystonia leading to severe contractures, migraines and insomnia, spasticity and lack of coordination.35 Untreated patients continue to deteriorate eventually becoming bedbound and dependent in activities of daily living. In patients with advanced liver disease, neurological manifestations can be misdiagnosed as hepatic encephalopathy.31
Psychiatric
Behavioural and psychiatric features may precede neurological and hepatic manifestations in about one-third of patients.31 There is often a delay in diagnosing Wilson’s disease in patients with neuropsychiatric features.36 These can be subtle including academic decline, changes in behaviour with loss of inhibitions, hypersexuality and mild cognitive impairment.37,38 Other psychiatric manifestations include depression, anxiety and even overt psychosis often misdiagnosed as a primary mental health disorder.
Other clinical changes
Infrequent presentations include osteoporosis, osteoarthritis, chondrocalcinosis,39 hypercalciuria and nephrocalcinosis,40 cardiomyopathy and arrhythmias,41 gigantism, hypoparathyroidism,42 infertility and repeated miscarriages.43,44
Kayser–Fleischer ring
Kayser–Fleischer rings are caused by deposition of copper in Desçemet’s membrane on the inner surface of the cornea.6 They are present in 44–62% of patients with mainly hepatic disease at the time of diagnosis.8 However, they are almost universal in patients with neuropsychiatric presentations. Kayser–Fleischer rings have a golden-brown appearance when visible on direct inspection. In most patients, slit-lamp examination is necessary to confirm their presence or absence, and bedside nonspecialist examination is not generally adequate.
Other rarer ophthalmological changes include sunflower cataracts which represent deposits of copper in the lens without visual impairment.45 Both Kayser–Fleischer rings and sunflower cataracts are reversible with medical therapy or after liver transplantation.46
Diagnosis
Wilson’s disease remains a diagnostic challenge as the symptoms are often nonspecific with multiorgan involvement.35 Currently, the diagnosis of Wilson’s disease is based on clinical features and a range of laboratory tests. The presence of neurological symptoms, Kayser–Fleischer rings and a low caeruloplasmin level is usually sufficient to confirm a diagnosis.6,31 The absence of Kayser–Fleischer rings does not exclude Wilson’s disease (as in the case of hepatic manifestation of Wilson’s disease) but in those with predominantly neurological disease, Kayser–Fleischer rings are present in 90.4–100% of patients.47,48 Genetic analysis is diagnostic of Wilson’s disease but impractical as there are over 500 mutations described for the disease making it laborious and expensive. However, automated technological advances to extract, sequence and analyse DNA makes this feasible, practical and financially accessible as costs fall over time. Genetic testing is particularly useful in screening relatives following confirmation of the mutation in the index patient.
A diagnostic score for diagnosing Wilson’s disease was proposed by the working party at the 8th International Meeting on Wilson’s disease, Leipzig 2001 (Table 1).49 The scoring system includes the following diagnostic elements: (1) serum caeruloplasmin, (2) 24-h urinary copper excretion, (3) the presence of nonimmune (Coombs-negative) haemolytic anaemia, (4) hepatic copper, (5) the presence of Kayser–Fleischer rings on slit-lamp examination, (6) neurologic or neuroimaging features, and (7) mutation analysis. A score of 4 or more provides good accuracy to diagnose Wilson’s disease.50 More recently, Roberts and Schilsky provided current diagnostic approaches in their guidelines prepared for the American Association for the Study of Liver Diseases.8 Table 2 summarizes the routine tests performed for diagnosis of Wilson’s disease.
Table 1.
Scoring system developed at the 8th International Meeting on Wilson’s disease, Leipzig 2001.49
| Clinical signs and symptoms | Other tests | ||
|---|---|---|---|
| KF rings | Liver copper in the absence of cholestasis | ||
| • Present | 2 | • >5 × upper limit of normal (>4 μmol/g) | 2 |
| • Absent | 0 | • 0.8–4 μmol/g | 1 |
| • Normal (<0.8 μmol/g) | −1 | ||
| • Rhodanine-positive granules (if no quantitative liver copper available) | 1 | ||
| Neurologic symptoms or typical imaging at brain MRI | Urinary copper in the absence of acute hepatitis | ||
| • Severe | 2 | • Normal | 0 |
| • Mild | 1 | • 1–2 × upper limit of normal | 1 |
| • Absent | 0 | • >2 upper limit of normal | 2 |
| • Normal, but >5 × upper limit of normal after Penicillamine | 2 | ||
| Serum caeruloplasmin | Mutation analysis | ||
| • Normal (>0.2 g/l) | 0 | • On both chromosome detected | 4 |
| • 0.1–0.2 g/l | 1 | • On one chromosome detected | 1 |
| • <0.1 g/l | 2 | • No mutations detected | 0 |
| Coombs-negative haemolytic anaemia | Total score | Evaluation | |
| • Present | 1 | • 4 or more | • Diagnosis established |
| • Absent | 0 | • 3 | • Diagnosis possible, more tests needed |
| • 2 or less | • Diagnosis very unlikely | ||
KF, Kayser–Fleischer; MRI, magnetic resonance imaging.
Table 2.
| Investigation | Features and findings |
|---|---|
| Caeruloplasmin | • Combination of Kayser–Fleischer rings and caeruloplasmin <0.2 g/l is diagnostic |
| • Overestimation by immunologic assay. Elevated in inflammation and hyperoestrogenaemic states |
|
| • A normal level does not exclude Wilson’s disease | |
| Urinary copper excretion (24-h) | • Helpful for diagnosing Wilson’s disease and for monitoring treatment |
| • >1.6 μmol (>100 μg) is considered diagnostic in adults (>0.6 μmol in children) in those symptomatic | |
| Hepatic copper concentration | • ⩾250 μg/g dry weight (considered diagnostic) |
| • <50 μg/g almost always excludes diagnosis | |
| Kayser–Fleischer ring on slit-lamp examination | • Usually present, absence does not exclude diagnosis |
| • Absent in up to 50% of patients with hepatic Wilson’s disease |
|
| • Almost universal in patients with neuropsychiatric presentations 90.4–100% prevalence) |
Liver biochemistry
Serum aminotransferase activity is usually abnormal in this disease except at a very early age. The degree of elevated aminotransferase activity may be mild for many individuals and does not reflect the severity of the liver disease.6
Caeruloplasmin
Synthesized mainly in the liver, this protein is the major carrier of copper in the circulation carrying six copper atoms per molecule of caeruloplasmin. A caeruloplasmin level of lower than 0.2 g/l has been considered consistent with Wilson’s disease and diagnostic in association with Kayser–Fleischer rings.6,8 Caeruloplasmin levels within normal range does not exclude it. A prospective study demonstrated a subnormal caeruloplasmin had a positive predictive value of only 6% when used as the sole screening test for Wilson’s disease.51 Caeruloplasmin may be measured enzymatically or, more commonly in clinical practice, by antibody-dependant assays.52 Serum caeruloplasmin as a diagnostic criterion may be difficult to interpret as immunologic assays may overestimate concentrations, levels may be elevated during acute inflammation given it is an acute phase reactant (including acute fulminant Wilsonian crises), and in hyperoestrogenaemic states (such as pregnancy).53 Conversely, low serum caeruloplasmin is observed physiologically in infancy, 20% of heterozygotes, hypoproteinaemic states (including cirrhosis of any aetiology), Menke’s disease and acaeruloplasminaemia.8
Serum copper
In Wilson’s disease, total serum copper (copper bound and unbound to caeruloplasmin) is usually decreased in proportion to the decreased caeruloplasmin in the circulation, despite it being a disease of copper overload. Serum copper may be within normal limits in those with severe liver injury (despite decreased serum caeruloplasmin levels) or markedly elevated in the setting of Wilson’s induced acute liver failure. Serum free copper (noncaeruloplasmin bound) levels above 3.14 μmol/l (200 μg/l), has been proposed as a diagnostic test for Wilson’s disease but is limited by its dependency on the adequacy of the methods for measuring both serum copper and caeruloplasmin.54 Hence, it is of more importance in monitoring therapy rather than diagnosing the disease.31
Urinary copper excretion
24-h urinary copper excretion is increased in Wilson’s disease, reflecting the amount of serum free copper in the circulation. Consequently, it may be helpful for diagnosing Wilson’s disease and for monitoring treatment. In those symptomatic of Wilson’s disease, a urinary copper excretion in a 24-h period of >1.6 μmol (>100 μg/24 h) is considered diagnostic of the disease,55 with levels >0.6 μmol (>40 μg/24 h) as the better threshold for diagnosis because it improved sensitivity in testing for the disease.50 A penicillamine challenge study may still be a useful diagnostic test for Wilson’s disease in the paediatric population but the predictive value of this test in adults is very uncertain and thus not widely implemented.
Hepatic copper concentration
Hepatic copper content ⩾250 μg/g dry weight is considered the hallmark of Wilson’s disease and is the method of choice for the diagnosis of Wilson’s disease.31 Although, a threshold value of 70 μg/g dry weight has been suggested to improve sensitivity (from 83.3% to 96.5%) albeit at the expense of specificity (from 98.6% to 95.4%).56 The main issue with hepatic copper concentration is the heterogeneous distribution of copper within the liver in advanced stages of Wilson’s disease, with the amount of copper varying from nodule to nodule in the presence of cirrhosis. The accuracy of the measurement is improved with adequate specimen size of at least 1 cm of biopsy core length.57 Hepatic copper concentration should be obtained in cases where the diagnosis is not straightforward and in younger patients. A normal hepatic copper concentration (<50 μg/g) almost always excludes a diagnosis of Wilson’s disease.8 Increased hepatic copper concentrations can be seen in those with long-term cholestasis and therefore this result should be taken in the context of the patient’s clinical presentation.50 Critically, tissue copper content is distinct and not equivalent to copper deposition identified by microscopy.
Liver biopsy
Liver biopsy is not always needed and may have greatest utility when diagnosing indeterminate cases or if there is suspicion of other hepatic disease.57 Thought needs to be given to when to perform a liver biopsy, how to ensure that tissue is prepared appropriately and whether electron microscopy is needed.
Detection of copper in hepatocytes by routine histochemical evaluation is highly variable. In the early stages, abnormalities include mild steatosis, glycogenated nuclei in hepatocytes, and focal hepatocellular necrosis, often misdiagnosed as nonalcoholic fatty liver disease or nonalcoholic steatohepatitis.58 Liver histology may also show features consistent with autoimmune hepatitis. All children with an apparent diagnosis of autoimmune hepatitis should also be investigated for Wilson’s disease, and adults with a presumptive diagnosis of autoimmune hepatitis failing to respond rapidly and appropriately to corticosteroid therapy must be carefully evaluated for Wilson’s disease.8
Eventually, progressive parenchymal damage leads to fibrosis and subsequent cirrhosis. In the presence of acute liver failure due to Wilson’s disease, there is marked hepatocellular degeneration via apoptosis and parenchyma collapse on a background of cirrhosis.59 Ultrastructural analysis of liver parenchyma (e.g. by electron microscopy) reveals specific mitochondrial abnormalities which are pathognomonic of Wilson’s disease in the absence of cholestasis.31,60
Neuroimaging studies
All patients diagnosed with Wilson’s disease should undergo neurological evaluation. Those with neuropsychiatric manifestations should receive consultation with a neurologist or a movement disorder specialist before treatment or soon after treatment is initiated.31 Currently, there is no commonly accepted rating scale which describes neurological signs in Wilson’s disease.
In Wilson’s disease, computed tomography of the brain will show increased density around the basal ganglia, whereas magnetic resonance (MR) imaging may be more sensitive and will usually reveal hyperintensity on T2-weighted MR imaging of the basal ganglia.61 Other features include the ‘face of the giant panda’ sign and hyperintensities in the tectal-plate, central pons and the brainstem. Abnormal brain imaging may even be present in some individuals prior to the onset of symptoms.
Acute liver failure
The diagnosis of acute liver failure due to Wilson’s disease is challenging as many investigations have a turnaround time of several days, therefore one usually has to rely on eliciting clinical features. Around 3–5% of patients will present with acute liver failure.29 Timing is critical since mortality without liver transplantation is very high. Determination of which patients will not survive without a liver transplant is key to urgent listing for transplantation. A prognostic score incorporating serum bilirubin, aspartate aminotransferase, international normalized ratio, leucocyte count and serum albumin, determines survival without liver transplantation.62 A score of ⩾11, is associated with high probability of death without a liver transplant (Table 3).
Table 3.
Prognostic index in Wilson’s disease.62 A score of ⩾11, is associated with high probability of death without a liver transplant.
| Score (in points) | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Serum bilirubin (μmol/l) | 100–150 | 151–200 | 201–300 | >300 |
| AST (IU/l) | 100–50 | 151–300 | 301–400 | >400 |
| INR | 1.3–1.6 | 1.7–1.9 | 2.0–2.4 | >2.4 |
| WCC (×109/l) | 6.8–8.3 | 8.4–10.3 | 10.4–15.3 | >15.3 |
| Albumin (g/l) | 34–44 | 25–33 | 21–24 | <21 |
AST, aspartate aminotransferase; INR, international normalized ratio; WCC, white cell count.
Characteristic features suggesting a diagnosis of acute liver failure due to Wilson’s disease include Coombs-negative haemolytic anaemia, coagulopathy unresponsive to parenteral vitamin K, renal failure, relatively modest rises in serum aminotransferase (AST/ALT > 2.2), normal or markedly subnormal serum alkaline phosphatase (ALP), elevated serum copper and 24-h urinary copper excretion, and a ratio of alkaline phosphatase to bilirubin of <2.8 A combination of an ALP to bilirubin ratio of <4 and an AST:ALT ratio >2.2 provided a sensitivity and specificity of 100% in identifying acute liver failure due to Wilson’s disease,63 although this was not replicated by other authors.64 Furthermore, this ratio cannot be applied to the paediatric population given the elevated alkaline phosphatase contributed by growing bone. Kayser–Fleischer rings can support the diagnosis of Wilson’s disease but may be absent in 50% of patients with acute liver failure. Readily available biochemistry tests such as bilirubin, alkaline phosphatase and serum aminotransferase should therefore be used in combination with other clinical manifestations of Wilson’s disease.31
Family screening
All first-degree relatives of a patient with newly diagnosed Wilson’s disease must be screened for Wilson’s disease because the probability of finding a homozygote in siblings is 25%.8 The chance amongst the offspring is 0.5%. In such cases, liver function tests, serum copper and caeruloplasmin concentration, and 24-h urinary copper excretion analysis are performed.
Molecular genetic analysis can be useful for families where both mutations have been identified in the index patient, enabling molecular analysis for the same mutation in siblings.31 Where both mutations have not been identified, haplotype analysis may be helpful to determine whether siblings of the index patient have inherited the same pair of chromosomes.6 Genetic testing is the only reliable method to separate heterozygote from homozygote siblings.
Treatment
Drug therapy for Wilson’s disease focuses on decoppering from the use of chelators (to promote copper excretion) or zinc which reduces intestinal copper absorption, or both. These treatments were first reserved for symptomatic patients because diagnostic tests were not available to identify presymptomatic disease. With advances in diagnostics, it is recognized that significant morbidity and mortality can be prevented by treating asymptomatic patients.65 Wilson’s disease remained progressively fatal until 1951 when the first copper chelator dimercaprol was used intramuscularly;66,67 it was associated with high incidence of adverse effects limiting its therapeutic use. In 1956, Walshe demonstrated the benefits of the orally active copper chelator, penicillamine.68 In 1969, trientine was made available as an alternative chelator, especially in those who did not tolerate penicillamine.69 Ammonium tetrathiomolybdate (TM) is another chelator, used by veterinarians to treat copper poisoning in animals, shown to be beneficial in Wilson’s disease.70 Currently, the mainstay of therapy for Wilson’s disease remains lifelong drug therapy usually with an orally active copper chelator. Liver transplantation is considered in those with acute liver failure, unresponsive to medical therapy and end-stage liver disease.
There is currently a lack of high quality data on the various available therapies for Wilson’s disease.71 The recommended therapy for symptomatic patients or those with active disease is initially with chelating agents8,21,31 (Table 4). Penicillamine has been the mainstay of treatment given its large worldwide treatment experience. However, adverse effects have led to increased trientine use as primary therapy given favourable efficacy data and improved supply of this chelating agent.6
Table 4.
| Medication | Mechanism of action | Side effects | Monitoring | Dose | Other notes |
|---|---|---|---|---|---|
| Penicillamine | Copper chelator from hepatic and other stores, induces urinary excretion of copper. |
Early: fever, cutaneous eruptions, myelosuppression Late: nephrotoxicity including nephrotic syndrome, pemphigus or pemphigoid lesions, systemic lupus erythematosus, Goodpasture’s syndrome, deleterious effects on vascular collagen. |
Adequacy of treatment: 24-h urinary copper excretion, 12–32 μmol/day (750–2000 μg/day) after an initial peak. Values below 3.2 μmol/day (200 μg/day), together with serum free copper of >2.36 μmol/l (>150 μg/l) may suggest noncompliance. Serum free copper of <0.79 μmol/l (<50 μg/l) may suggest overtreatment. For toxicity: full blood count with liver and renal biochemistry, and urinalysis before initiation and then every 1–2 weeks for the first 2 months, then every 4 weeks. |
Initial: 125–250 mg/day, increased by 250 mg increments every 4–7 days, to a maximum of 1000–1500 mg/day in 2–4 divided doses Maintenance: 750–1000 mg/day, in 2–3 divided doses Children: 20 mg/kg/day, in 2–3 divided doses. |
Risk of neurological worsening in 10–20% of patients with a neurological presentation when used as initial therapy. Supplemental pyridoxine recommended (25–50 mg/day). |
| Trientine | Copper chelator, induces urinary excretion of copper. | Similar to penicillamine but at a much lower frequency. Most common is proteinuria. |
Adequacy of treatment: similar to penicillamine. For toxicity: No laboratory studies necessary but good practice to monitor counts and urinalysis as for penicillamine. |
Initial: 750–1500 mg/day, in 2–3 divided doses. Maintenance: 750–1000 mg/day, in 2–3 divided doses. Children: 20 mg/kg/day, in 2–3 divided doses. |
Risk of neurological worsening after initiating therapy is <20% and lower than the risk from penicillamine. |
| Zinc | Induces metallothionein and inhibits absorption of copper with faecal excretion. | Gastric irritation. Has an otherwise excellent safety profile. |
Adequacy of treatment: 24-h urinary copper excretion (usually <1.2 μmol/day while on maintenance therapy). Urinary zinc levels and serum free copper may also be measured. For toxicity: No laboratory studies are necessary. |
Initial: controversial and not currently recommended for initial monotherapy. Maintenance: 150 mg/day of elemental zinc for larger adults and children (75 mg/day if less than 50 kg), in three divided doses. |
Asymptomatic patients may be treated with maintenance dosages of zinc monotherapy. |
| Tetrathiomolybdate | Forms a tripartite complex with protein and copper. Acts by inhibiting copper uptake when taken with meals. When taken in between meals, it is absorbed and binds copper from plasma before being metabolized by the liver and excreted in bile. | Bone marrow suppression, anaemia and increased serum aminotransferase levels, responsive to dose reduction. | Suggested monitoring includes full blood counts and liver function tests every 2 weeks. | Has only been demonstrated in initial therapy and its utility in maintenance therapy remains uncertain. Dose: 20 mg thrice daily. | Experimental therapy that is not commercially available. Experience with the drug is limited. |
After initial chelation therapy of typically 3–6 months, usually until symptoms or biochemical abnormalities have stabilized, maintenance dosages of chelators or zinc monotherapy can be used for treatment.8,31 Asymptomatic patients may be treated with either maintenance dosages of oral chelating agents or with zinc monotherapy. Discontinuation of lifelong maintenance treatment has led to recurrent symptoms and liver failure ultimately requiring liver transplantation. Monitoring of therapy is important to ensure compliance, especially when they feel well, and for potential treatment-related side effects. Generally, serum copper and caeruloplasmin, liver biochemistry, international normalized ratio, full blood count and urinalysis (more often if on chelation therapy), and physical examination should be performed regularly, at least biannually.8,31
Penicillamine
Penicillamine has the largest experience of all the available Wilson’s disease treatments. Although widely available and relatively cheap until recently,72 penicillamine is associated with serious adverse effects.68 Penicillamine was identified as the breakdown product of penicillin with a free sulphydryl group which acts as the copper chelator from hepatic and other stores, causing urinary excretion of copper.6,21 It is well documented in showing efficacy in Wilson’s disease, usually after 2–6 months of treatment.26,73
The initial dosing of penicillamine is 125–250 mg/day to facilitate tolerability, increased by 250 mg increments every 4–7 days, to a maximum of 1000–1500 mg/day in two to four divided doses. Usually it is administered half an hour before meals or at least 2 h after meals because food inhibits its absorption.8,31 It has an oral bioavailability of 40–70%. The maintenance dose is usually 750–1000 mg/day in two or three divided doses. In the paediatric population, dosing is 20 mg/kg/day in two or three divided doses. Supplemental pyridoxine is recommended (25–50 mg/day) as penicillamine may interfere with pyridoxine metabolism. Adequacy of treatment is monitored by measuring 24-h urinary copper excretion, which settles to 12–32 μmol/day (750–2000 μg/day) after an initial peak. Values of urine copper excretion below 3.2 μmol/day (200 μg/day), together with a serum free copper of >2.36 μmol/l (>150 μg/l) may suggest noncompliance, whereas a serum free copper of <0.79 μmol/l (<50 μg/l) may suggest overtreatment.
There are numerous side effects to penicillamine and often severe requiring discontinuation in approximately 30% of patients.74,75 In the early stages, sensitivity reactions marked by fever and cutaneous eruptions may occur. If these occur, then the medication should be stopped and an alternative should be used. Late reactions include nephrotoxicity including nephrotic syndrome, pemphigus or pemphigoid lesions, systemic lupus erythematosus, Goodpasture’s syndrome, and bone marrow suppression. Monitoring is necessary for penicillamine toxicity including full blood count with liver and renal biochemistry, and urinalysis.21 Initial neurological worsening has been reported in 10–20% of patients with a neurological presentation and which in some cases cannot be reversed,76 consequently other agents are used as first-line therapy.
Trientine
Trientine was introduced in 1969, as an alternative therapy for Wilson’s disease in patients intolerant to penicillamine.69 It has a polyamine-like structure, and it chelates copper by forming a stable complex with the four constituent nitrogen atoms in a planar ring.6 Like penicillamine, trientine promotes copper excretion via the kidneys after chelation. Although there are few available pharmacokinetics data on trientine, it is poorly absorbed from the gastrointestinal tract with most of the absorbed drug metabolized and only a small fraction excreted in urine.
Trientine is an effective treatment in Wilson’s disease, especially in those intolerant to penicillamine, with decompensated liver disease, and in those with neurological presentation.77,78 The risk of neurological worsening after initiating therapy is <20% with trientine.79 Trientine has few side effects and although they are similar to penicillamine, the frequency is much lower.54,80 In general, adverse effects due to penicillamine resolve when it is substituted for trientine. Copper deficiency induced by trientine has been known to lead to reversible sideroblastic anaemia and iron overload in the liver.
Typical initial dose of trientine are 750–1500 mg/day in two or three divided doses, with 750–1000 mg/day used for maintenance.8,31 In the paediatric population, dosing is 20 mg/kg/day in two or three divided doses. Similar to penicillamine, trientine should be administered half an hour before meals or at least 2 h after meals. Monitoring is also necessary including full blood count with liver and renal biochemistry, and urinalysis.21 Adequacy of treatment is monitored, identically to penicillamine, by measuring 24-h urinary copper excretion and serum free copper levels.
Ammonium tetrathiomolybdate
TM is not commercially available and experience with the drug is limited. It is a potent decoppering agent forming a tripartite complex with protein and copper.21 When taken with meals, TM acts by inhibiting copper uptake and when administered in between meals, it is absorbed and acts by binding copper from the plasma before being metabolized by the liver and excreted in bile. Although it has a good side effect profile, adverse effects include bone marrow suppression, anaemia and increased serum aminotransferase levels, responsive to dose reduction.
The use of TM has only been demonstrated in initial therapy and its utility in maintenance therapy remains uncertain. In the initial therapy setting, TM has demonstrated no worsening of neurological symptoms and a rapid reduction in circulating free copper during the first 8 weeks of therapy (in combination with oral zinc therapy).70,81
Zinc
Zinc was first used in the 1960s to treat Wilson’s disease.82,83 Its mode of action is through inhibition of copper uptake by intestinal mucosa. Zinc induces enterocyte metallothionein which has a greater affinity for copper (than for zinc) inhibiting its portal absorption.84 Reabsorption of copper from salivary and gastric secretions is also impaired resulting in an overall mildly negative copper balance. This negative balance is not sufficient for zinc to be an effective monotherapy agent in those with symptomatic Wilson’s disease. Zinc is currently approved for maintenance therapy in those who have already been initially treated with penicillamine or trientine, although it has been used as first-line therapy in presymptomatic patients with equal efficacy as penicillamine.71 Zinc monotherapy appears to be effective and safe in neurologic Wilson’s disease and consequently may have a role as first-line therapy in this setting.85
The recommended dose is 150 mg/day of elemental zinc for larger adults and children (75 mg/day if <50 kg), in three divided doses, 30 minutes before meals.8,31 Zinc is usually well tolerated with gastric irritation a common problem, usually with the early morning dose. Elevation in serum lipase and amylase may occur without any clinical or radiological evidence of pancreatitis. No formal laboratory testing is required for monitoring of adverse effects.21
Monitoring response to therapy, including compliance, is performed by measuring 24-h urinary excretion of copper, which is <1.2 μmol (75 μg) per day while on maintenance therapy.8 In addition, urinary zinc levels may be measured on the same sample which should be >30.6 μmol/day (2.0 mg/day). Furthermore, serum free copper may also be measured.
Diet
Dietary management is not recommended as sole therapy. High concentrations of copper are found naturally in numerous foods such as chocolate, shellfish, nuts, mushrooms, liver and soy. They are generally best avoided especially in the first year of treatment for Wilson’s disease.8 The role of diet is thought to have been overstated according to some authors who advise avoiding only shellfish and liver from a normal diet.21 The copper content of well water and water brought into the home through copper pipes may need checking if there is concern and a water purifying system is advised if the copper content is high. Most patients do not restrict their diet but consideration to avoiding copper containers can be as relevant as can running a tap to clear stagnant water in copper pipes is recommended.
Other therapies
Accumulation of copper in the liver produces oxidative stress related damage. Vitamin E levels have been found to be low in Wilson’s disease.86 Supplementation of antioxidants, such as vitamin E or N-acetylcysteine, may have a role as adjunctive therapy but no well controlled studies are available to advocate its routine use.31
Physiotherapy to maintain muscle function and management of contractures is important, especially on initiation of anticopper therapy.21 Occupational therapy has a role in identifying and eliminating environmental barriers to independence and participation in activities of daily living. Speech therapy is also helpful, initially to maintain and strengthen existing abilities, but then also to optimize any potential outcomes on recovery following medical therapy. It also has a role in assessing swallow and risk of aspiration.
Patients with hepatic disease or decompensation should be managed as with any other patient with chronic liver disease.21 Indeed, they may require optimization of diuretic therapy, treatment of encephalopathy and varices, dietary salt restriction and treatment of infection.
Recovery and prognosis
Once diagnosed, patients with Wilson’s disease have an excellent prognosis provided they remain compliant with lifelong therapy, even if cirrhosis is present at the time of diagnosis.21 Patients who discontinue treatment are at high risk of fulminant hepatic failure hence the recommendation to continue therapy for life.31 In those with neurological features, patients begin showing clinical improvement 5–6 months after initiation of anticopper therapy with most patients eventually showing substantial improvement. The greater the severity at diagnosis, the more likely that significant disability will be permanent. Residual neurological features present after 24 months of therapy is usually permanent. Dysphagia tends to improve after 1–2 years of therapy to a point where aspiration no longer occurs. The use of enteral feeding during this interim period may ensure adequate nutrition. Psychiatric and behavioural manifestations usually improve and resolve within 1–2 years of therapy, with most patients leading a normal life from a mental health point of view.21
In most patients, following normalization of liver function from anticopper therapy, the liver will remain compensated for the remainder of a normal lifespan. Further insults to the liver are best avoided, including avoiding alcohol and ensuring vaccination against hepatitis A and B.21
Specific situations
Presymptomatic patients
Usually these will be siblings of an index patient who are diagnosed as a result of family screening. Current guidelines recommend treatment with a chelating agent, such as trientine rather than penicillamine, or with zinc in preventing disease symptoms or progression.8,31 A randomized study comparing penicillamine with zinc as initial therapy found similar efficacy, although more adverse effects were reported with the penicillamine arm.87
Initial therapy
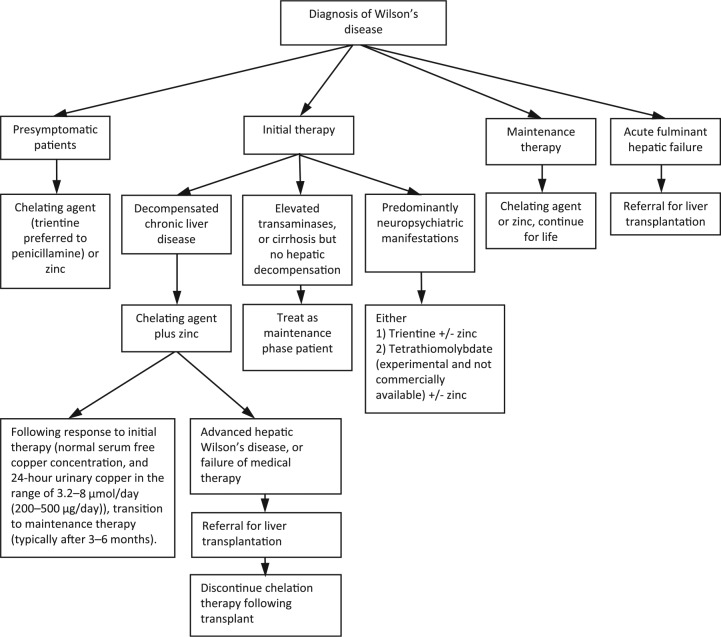
Initial therapy is recommended with a chelator, either penicillamine or trientine, and zinc in those with decompensated chronic liver disease (Figure 1 provides an overview).8,21,31 Typically this includes hypoalbuminaemia, elevated bilirubin, prolonged prothrombin time, ascites, but no encephalopathy. If a patient has elevated transaminases, or cirrhosis and transaminase elevations, but no evidence of hepatic decompensation, they may be treated as a maintenance phase patient. Trientine is often chosen over penicillamine because of improved tolerability. Chelator and zinc administration should be spaced 5–6 h apart in order to avoid chelator binding to zinc thereby negating their respective therapeutic effect.
Figure 1.
Summary of treatment recommendations and suggestions for Wilson’s disease.8,21,31
For those who respond to initial therapy, as assessed by clinical parameters or synthetic function, may be transitioned to maintenance therapy, typically after 3–6 months. Liver function begins improving 3–4 months following initiation of therapy, and usually normalize by 12 months.21 Given paucity of data, the timing of transition from initial therapy to maintenance therapy in patients with hepatic Wilson’s disease remains investigational.
Although a patient may have lifelong cirrhosis, this does not present any medical problems with the exception of varices which can pose a risk in the first 2–3 years of therapy. The risk of a hepatoma developing in this situation is low, at 1 in 400 lifelong, but recent studies would suggest hepatocellular carcinoma is more common in Wilson’s disease than appreciated.88–90 Some authors do not advocate routine hepatoma surveillance in cirrhosis related to Wilson’s disease.8,91 In practice however, clinicians will often opt for hepatoma surveillance, especially in the elderly.
In those with advanced hepatic Wilson’s disease, or who fail medical therapy with chelators, may require referral for liver transplantation. A prognostic index scoring system has been developed92 and later modified by Dhawan and colleagues.62 This score can be calculated using the serum bilirubin, international normalized ratio, aspartate aminotransferase, albumin and white cell count. A score of 11 or more is associated with high probability of death without liver transplantation and should be referred to a transplant centre promptly.
Initial therapy for patients presenting with predominantly neuropsychiatric manifestations of Wilson’s disease can result in paradoxical irreversible neurologic worsening of symptoms.84 Probably through mobilising hepatic copper and transiently elevating neuronal copper levels. Penicillamine has an estimated 10–20% risk of this and therefore should not be given to patients presenting with neurological Wilson’s disease.8 Neurologic worsening occurred on all three treatments used for Wilson’s disease (penicillamine, trientine and zinc), but mainly with penicillamine.31 Although not commercially available, TM does not cause neurologic worsening. Some authors have recommended TM is used in combination with zinc as initial therapy for neuropsychiatric Wilson’s disease, rather than trientine which is associated with a 20% risk of neurologic worsening.21
Maintenance therapy
Following adequate initial treatment with a chelator, therapy may be continued at a maintenance dose of the chelating agent or zinc. Trientine is often better tolerated and is often the preferred chelator over penicillamine. Surrogate measures indicating when maintenance therapy may begin include a normal serum free copper concentration, and 24-h urinary copper in the range of 3.2–8 μmol/day (200–500 μg/day). Zinc may be preferred over penicillamine or trientine because of its fewer side effects and it is more selective for removing copper than other trace metals in the body.8,31
Pregnancy
Medical therapy must be maintained throughout pregnancy with cases of acute liver failure being reported from discontinuation of therapy during pregnancy.93 Satisfactory outcomes have been observed in those pregnant with Wilson’s disease treated with penicillamine, trientine and zinc. There are some concerns over the teratogenicity associated with penicillamine, the risks of discontinuing treatment however outweigh those of continuing it. Reports of birth defects during treatment for Wilson’s disease are rare making it difficult to establish a true increased risk in this population. It is important to note that copper deficiency is teratogenic and therefore 24-h urinary copper and serum free copper should be closely monitored during pregnancy.94
The dosage of zinc is maintained throughout pregnancy without adjustment; although, chelating agent doses should be reduced by 25–50%, to promote better wound healing after pregnancy.8,31 Breast feeding under chelation therapy is not recommended, although little is known about the safety of trientine and zinc in breast milk.
Regarding contraception, spermicides, barrier contraceptives and progesterone only preparations are recommended. Many intrauterine devices containing copper are relatively contraindicated. Yet the reported absorption of copper amounts to less than that ingested daily in a normal diet. Therefore, its contribution may be considered insignificant.95 The advice to avoid oestrogen containing contraceptives is from historic articles due to the risk of provoking or enhancing cholestasis.96
Liver transplantation
Liver transplantation is indicated in those with Wilson’s disease who present with acute fulminant liver failure, and in patients with decompensated liver disease unresponsive to medical therapy (usually assessed after 3 months of initiation).8,31 Given the metabolic defects lie mainly in the liver in Wilson’s disease, liver transplantation corrects the underlying problem. Consequently, oral chelation therapy may be discontinued following transplantation. The 1-year survival following transplantation ranges from 79% to 87%, with those who survive this early period continuing to survive long term.46 Outcomes are much better for adults than children and also following transplantation for chronic liver disease than acute fulminant hepatic failure. Live donor transplantation is possible in addition to transplantation of cadaveric donors, even if the donor is a heterozygous carrier.8 Less definite indications for liver transplantation exist for patients with severe neurological disease as these are rarely eliminated. Consequently, liver transplantation as a cure for neurological disease is not advocated. Furthermore, patients with neuropsychiatric manifestations tend to have poorer compliance and adherence to medical regimens after liver transplantation.31
The future
Future curative treatments for Wilson’s disease in the form of stem cells or ex vivo modification of cells by gene therapy, may obviate the difficulty of immunosuppression associated with current liver transplantation.6 Animal model studies have shown cell therapy will correct genetic disorders characterized by organ damage, however suitable mechanisms for inducing transplanted cell proliferation will be critical for therapeutic success.97
At present, an effective early diagnostic test for Wilson’s disease is lacking. The current clinical practice guidelines for Wilson’s disease, has positive gene detection as the highest weight in the diagnosis of the disease.31 Gene detection methods for Wilson’s disease currently include direct gene sequencing and haplotyping. A novel sequencing technique has been developed recently which enables sequencing of all exons, even including the whole genome.98 In the future, the diagnosis of Wilson’s disease with genetic analysis will be more practical and efficient.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Mohmadshakil Kathawala, Centre for Liver Research, NIHR Birmingham Liver Biomedical Research Unit, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, UK.
Gideon M. Hirschfield, Centre for Liver Research, NIHR Birmingham Liver Biomedical Research Unit, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK.
References
- 1. Schilsky ML. Wilson disease: genetic basis of copper toxicity and natural history. Semin Liver Dis 1996; 16: 83–95. [DOI] [PubMed] [Google Scholar]
- 2. Wilson SAK. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain 1912; 34: 295–507. [DOI] [PubMed] [Google Scholar]
- 3. Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 1993; 5: 327–337. [DOI] [PubMed] [Google Scholar]
- 4. Tanzi RE, Petrukhin K, Chernov I, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 1993; 5: 344–350. [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun 1993; 197: 271–277. [DOI] [PubMed] [Google Scholar]
- 6. Ala A, Walker AP, Ashkan K, et al. Wilson’s disease. Lancet 2007; 369: 397–408. [DOI] [PubMed] [Google Scholar]
- 7. Rosencrantz R, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis 2011; 31: 245–259. [DOI] [PubMed] [Google Scholar]
- 8. Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology 2008; 47: 2089–2111. [DOI] [PubMed] [Google Scholar]
- 9. Lutsenko S, Barnes NL, Bartee MY, et al. Function and regulation of human copper-transportin ATPases. Physiol Rev 2007; 87: 1011–1046. [DOI] [PubMed] [Google Scholar]
- 10. Holtzman NA, Gaumnitz BM. Studies on the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J Biol Chem 1970; 245: 2354–2358. [PubMed] [Google Scholar]
- 11. Pfeiffer RF. Wilson’s disease. Handb Clin Neurol 2011; 100: 681–709. [DOI] [PubMed] [Google Scholar]
- 12. Patil M, Sheth KA, Krishnamurthy AC, et al. A review and current perspective on Wilson disease. J Clin Exp Hepatol 2013; 3: 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson Disease Mutation Database. http://www.wilsondisease.med.ualberta.ca/database.asp (2010, accessed 10 July 2016).
- 14. Coffey AJ, Durkie M, Hague S, et al. A genetic study of Wilson’s disease in the United Kingdom. Brain 2013; 136: 1476–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferenchi P. Phenotype-genotype correlations in patients with Wilson’s disease. Ann N Y Acad Sci. 2014; 1315: 1–5. [DOI] [PubMed] [Google Scholar]
- 16. Okada T, Shiono Y, Kaneko Y, et al. High prevalence of fulminant hepatic failure among patients with mutant alleles for truncation of ATP7B in Wilson’s disease. Scand J Gastroenterol 2010; 45: 1232–1237. [DOI] [PubMed] [Google Scholar]
- 17. Houwen RH, Juyn J, Hoogenraad TU, et al. H714Q mutation in Wilson disease is associated with late, neurological presentation. J Med Genet 1995; 32: 480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stapelbroek JM, Bollen CW, Van Amstel JK, et al. The H1069Q mutation in ATP7B is associated with late and neurologic presentation in Wilson disease: results of a meta-analysis. J Hepatol 2004; 41: 758–763. [DOI] [PubMed] [Google Scholar]
- 19. Vrabelova S, Letocha O, Borsky M, et al. Mutation analysis of the ATP7B gene and genotype/phenotype correlation in 227 patients with Wilson disease. Mol Genet Metab 2005; 86: 277–285. [DOI] [PubMed] [Google Scholar]
- 20. Frydman M. Genetic aspects of Wilson’s disease. J Gastroenterol Hepatol 1990; 5: 483–490. [DOI] [PubMed] [Google Scholar]
- 21. Brewer GJ, Askari FK. Wilson’s disease: clinical management and therapy. J Hepatol 2005; 42(Suppl. 1): S13–S21. [DOI] [PubMed] [Google Scholar]
- 22. Schilsky ML. Liver transplantation for Wilson’s disease. Ann N Y Acad Sci 2014; 1315: 45–49. [DOI] [PubMed] [Google Scholar]
- 23. Kido J, Matsumoto S, Momosaki K, et al. Plasma exchange and chelator therapy rescues acute liver failure in Wilson disease without liver transplantation. Hepatol Res. Epub ahead of print 23 March 2016. DOI: 10.1111/hepr.12711. [DOI] [PubMed] [Google Scholar]
- 24. Akyildiz BN, Yildirim S, Kondolot M, et al. Is plasma exchange effective in prevention of hepatic transplantation in fulminant Wilson disease with hepatic failure? J Pediatr Gastroenterol Nutr 2011; 52: 778–780. [DOI] [PubMed] [Google Scholar]
- 25. Motobayashi M, Fukuyama T, Nakayama Y, et al. Successful treatment of fulminant Wilson’s disease without liver transplantation. Pediatr Int 2014; 56: 429–432. [DOI] [PubMed] [Google Scholar]
- 26. Scheinberg IH, Sternlieb I. Wilson’s disease. In: Smith JL. (ed.) Major problems in internal medicine. Vol. 23 Philadelphia, PA: WB Saunders, 1984, pp.25–35. [Google Scholar]
- 27. Saito T. Presenting symptoms and natural history of Wilson disease. Eur J Pediatr 1987; 146: 261–265. [DOI] [PubMed] [Google Scholar]
- 28. Walshe JM. The liver in Wilson’s disease. In: Schiff L, Schiff ER. (eds) Diseases of the liver. 6th ed. Philadelphia, PA: J B Lippincott, 1987, pp.1037–1050. [Google Scholar]
- 29. Ostapowicz G, Fontana RJ, Schiødt FV, et al. ; U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 947–954. [DOI] [PubMed] [Google Scholar]
- 30. Korman JD, Volenberg I, Balko J, et al. ; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008; 48: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Association for Study of Liver. EASL clinical practice guidelines: Wilson’s disease. J Hepatol. 2012; 56: 671–685. [DOI] [PubMed] [Google Scholar]
- 32. Walshe JM. Wilson’s disease. The presenting symptoms. Arch Dis Child 1962; 37: 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zimbrean PC, Schilsky ML. Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry 2014; 36: 53–62. [DOI] [PubMed] [Google Scholar]
- 34. Svetel M, Kozic D, Stefanova E, et al. Dystonia in Wilson’s disease. Mov Disord 2001; 16: 719–723. [DOI] [PubMed] [Google Scholar]
- 35. Taly AB, Meenakshi-Sundaram S, Sinha S, et al. Wilson disease: description of 282 patients evaluated over 3 decades. Medicine (Baltimore) 2007; 86: 112–121. [DOI] [PubMed] [Google Scholar]
- 36. Merle U, Schaefer M, Ferenci P, et al. Clinical Presentation, diagnosis and long-term outcome of Wilson disease – a cohort study. Gut 2007; 56: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seniów J, Mroziak B, Czlonkowska A, et al. Self-rated emotional functioning of patients with neurological or asymptomatic form of Wilson’s disease. Clin Neuropsychol 2004; 17: 367–373. [DOI] [PubMed] [Google Scholar]
- 38. Svetel M, Potrebic A, Pekmezovic T, et al. Neuropsychiatric aspects of treated Wilson’s disease. Parkinsonism Relat Disord 2009; 15: 772–775. [DOI] [PubMed] [Google Scholar]
- 39. Golding DN, Walshe JM. Arthropathy of Wilson’s disease. Study of clinical and radiological features in 32 patients. Ann Rheum Dis 1977; 36: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Azizi E, Eshel G, Aladjem M. Hypercalciuria and nephrolithiasis as a presenting sign in Wilson disease. Eur J Pediatr 1989; 148: 548–549. [DOI] [PubMed] [Google Scholar]
- 41. Factor SM, Cho S, Sternlieb I, et al. The cardiomyopathy of Wilson’s disease. Myocardial alterations in nine cases. Virchows Arch [Pathol Anat] 1982; 397: 301–311. [DOI] [PubMed] [Google Scholar]
- 42. Carpenter TO, Carnes DL, Jr, Anast CS. Hypoparathyroidism in Wilson’s disease. N Engl J Med 1983; 309: 873–877. [DOI] [PubMed] [Google Scholar]
- 43. Klee JG. Undiagnosed Wilson’s disease as cause of unexplained miscarriage. Lancet 1979; 2: 423. [DOI] [PubMed] [Google Scholar]
- 44. Tarnacka B, Rodo M, Cichy S, et al. Procreation ability in Wilson’s disease. Acta Neurol Scand 2000; 101: 395–398. [DOI] [PubMed] [Google Scholar]
- 45. Cairns JE, Williams HP, Walshe JM. “Sunflower cataract” in Wilson’s disease. Br Med J 1969; 3: 95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schilsky ML, Scheinberg IH, Sternlieb I. Liver transplantation for Wilson’s disease: indications and outcome. Hepatology 1994; 19: 583–587. [DOI] [PubMed] [Google Scholar]
- 47. Gollan JL, Gollan TJ. Wilson disease in 1998: genetic, diagnostic and therapeutic aspects. J Hepatol 1998; 28(Suppl. 1): 28–36. [DOI] [PubMed] [Google Scholar]
- 48. Deguti MM, Tietge UJ, Barbosa ER, et al. The eye in Wilson’s disease: sunflower cataract associated with Kayser-Fleischer ring. J Hepatol 2002; 37: 700. [DOI] [PubMed] [Google Scholar]
- 49. Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003; 23: 139–142. [DOI] [PubMed] [Google Scholar]
- 50. Nicastro E, Ranucci G, Vajro P, et al. Re-evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology 2010; 52: 1948–1956. [DOI] [PubMed] [Google Scholar]
- 51. Perman JA, Werlin SL, Grand RJ, et al. Laboratory measures of copper metabolism in the differentiation of chronic active hepatitis and Wilson disease in children. J Pediatr 1979; 94: 564–568. [DOI] [PubMed] [Google Scholar]
- 52. Hedera P. Update on the clinical management of Wilson’s disease. Appl Clin Genet 2017; 10: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Merle U, Eisenbach C, Weiss KH, et al. Serum ceruloplasmin oxidase activity is a sensitive and highly specific diagnostic marker for Wilson’s disease. J Hepatol 2009; 51: 925–930. [DOI] [PubMed] [Google Scholar]
- 54. Brewer GJ. Wilson’s disease: a clinician’s guide to recognition, diagnosis, and management. Boston, MA: Kluwer Academic Publishers, 2001. [Google Scholar]
- 55. Ferenci P, Czlonkowska A, Merle U, et al. Late onset Wilson disease. Gastroenterology 2007; 132: 1294–1298. [DOI] [PubMed] [Google Scholar]
- 56. Ferenci P, Steindl-Munda P, Vogel W, et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s Disease. Clin Gastroenterol Hepatol 2005; 3: 811–818. [DOI] [PubMed] [Google Scholar]
- 57. Ludwig J, Moyer TP, Rakela J. The liver biopsy diagnosis of Wilson’s disease. Methods in pathology. Am J Clin Pathol 1994; 102: 443–446. [DOI] [PubMed] [Google Scholar]
- 58. Strohmeyer FW, Ishak KG. Histology of the liver in Wilson’s disease: a study of 34 cases. Am J Clin Pathol 1980; 73: 12–24. [DOI] [PubMed] [Google Scholar]
- 59. Strand S, Hofmann WJ, Grambihler A, et al. Hepatic failure and liver cell damage in acute Wilson’s disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med 1998; 4: 588–593. [DOI] [PubMed] [Google Scholar]
- 60. Sternlieb I. Mitochondrial and fatty changes in hepatocytes of patients with Wilson’s disease. Gastroenterology 1968; 55: 354–367. [PubMed] [Google Scholar]
- 61. Van Wassenaer-Van Hall HN, Van Den Heuvel AG, Algra A, et al. Wilson disease: findings at MR imaging and CT of the brain with clinical correlation. Radiology 1996; 198: 531–536. [DOI] [PubMed] [Google Scholar]
- 62. Dhawan A, Taylor RM, Cheeseman P, et al. Wilson’s disease in children: 37-year experience and revised King’s score for liver transplantation. Liver Transplant 2005; 11: 441–448. [DOI] [PubMed] [Google Scholar]
- 63. Berman DH, Leventhal RI, Gavaler JS, et al. Clinical differentiation of fulminant Wilsonian hepatitis from other causes of hepatic failure. Gastroenterology 1991; 100: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 64. Sallie R, Katsiyiannakis L, Baldwin D, et al. Failure of simple biochemical indexes to reliably differentiate fulminant Wilson’s disease from other causes of fulminant liver failure. Hepatology 1992; 16: 1206–1211. [PubMed] [Google Scholar]
- 65. Sternlieb I, Scheinberg IH. Prevention of Wilson’s disease in asymptomatic patients. N Engl J Med 1968; 278: 352–359. [DOI] [PubMed] [Google Scholar]
- 66. Cumings JN. The effects of BAL in hepatolenticular degeneration. Brain 1951; 74: 10–22. [DOI] [PubMed] [Google Scholar]
- 67. Denny-Brown D, Porter H. The effects of BAL (2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson’s disease). N Engl J Med 1951; 245: 917–925. [DOI] [PubMed] [Google Scholar]
- 68. Walshe JM. Wilson’s disease; new oral therapy. Lancet 1956; 267: 25–26. [DOI] [PubMed] [Google Scholar]
- 69. Walshe JM. The management of penicillamine nephropathy in Wilson’s disease: a new chelating agent. Lancet 1969; 294: 1401–1402. [DOI] [PubMed] [Google Scholar]
- 70. Brewer GJ, Hedera P, Kluin KJ, et al. Treatment of Wilson’s disease with ammonium tetrathiomolybdate III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch Neurol 2003; 60: 379–385. [DOI] [PubMed] [Google Scholar]
- 71. Wiggelinkhuizen M, Tilanus ME, Bollen CW, et al. Systematic review: clinical efficacy of chelator agents and zinc in the initial treatment of Wilson disease. Aliment Pharmacol Ther 2009; 29: 947–958. [DOI] [PubMed] [Google Scholar]
- 72. Schilsky ML, Roberts EA, Hahn S, et al. Costly choices for treating Wilson’s disease. Hepatology 2015; 61: 1106–1108. [DOI] [PubMed] [Google Scholar]
- 73. Walshe JM, Yealland M. Chelation treatment of neurological Wilson’s disease. Q J Med 1993; 86: 197–204. [PubMed] [Google Scholar]
- 74. Walshe JM. Copper chelation in patients with Wilson’s disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med 1973; 42: 441–452. [PubMed] [Google Scholar]
- 75. Medici V, Trevisan CP, D’Inca R, et al. Diagnosis and management of Wilson’s disease: results of a single center experience. J Clin Gastroenterol 2006; 40: 936–941. [DOI] [PubMed] [Google Scholar]
- 76. Brewer GJ, Terry CA, Aisen AM, et al. Worsening of neurologic syndrome in patients with Wilson’s disease with initial penicillamine therapy. Arch Neurol 1987; 44: 490–493. [DOI] [PubMed] [Google Scholar]
- 77. Walshe JM. The management of Wilson’s disease with trienthylene tetramine 2HC1 (Trien 2HC1). Prog Clin Biol Res 1979; 34: 271–280. [PubMed] [Google Scholar]
- 78. Scheinberg IH, Jaffe ME, Sternlieb I. The use of trientine in preventing the effects of interrupting penicillamine therapy in Wilson’s disease. N Engl J Med 1987; 317: 209–213. [DOI] [PubMed] [Google Scholar]
- 79. Brewer GJ, Schilsky M, Hedera P, et al. Double blind study of initial therapy of neurological Wilson’s disease (abstract). J Invest Med 2003; 51(Suppl. 2): S369. [Google Scholar]
- 80. Brewer GJ, Fink JK, Hedera P. Diagnosis and treatment of Wilson’s disease. Semin Neurol 1999; 19: 261–270. [DOI] [PubMed] [Google Scholar]
- 81. Brewer GJ, Askari F, Lorincz MT, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch Neurol 2006; 63: 521–527. [DOI] [PubMed] [Google Scholar]
- 82. Hoogenraad TU, Koevoet R, De Ruyter Korver EG. Oral zinc sulphate as long-term treatment in Wilson’s disease (hepatolenticular degeneration). Eur Neurol 1979; 18: 205–211. [DOI] [PubMed] [Google Scholar]
- 83. Hoogenraad TU, Van Hattum J, Van Den Hamer CJ. Management of Wilson’s disease with zinc sulphate. Experience in a series of 27 patients. J Neurol Sci 1987; 77: 137–146. [DOI] [PubMed] [Google Scholar]
- 84. Brewer GJ, Yuzbasiyan-Gurkan V, Young AB. Treatment of Wilson’s disease. Semin Neurol 1987; 7: 209–220. [DOI] [PubMed] [Google Scholar]
- 85. Walshe JM, Munro NA. Zinc-induced deterioration in Wilson’s disease aborted by treatment with penicillamine, dimercaprol, and a novel zero copper diet. Arch Neurol 1995; 52: 10–11. [DOI] [PubMed] [Google Scholar]
- 86. Sinha S, Christopher R, Arunodaya GR, et al. Is low serum tocopherol in Wilson’s disease a significant symptom? J Neurol Sci 2005; 228: 121–123. [DOI] [PubMed] [Google Scholar]
- 87. Czlonkowska A, Gajda J, Rodo M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J Neurol 1996; 243: 269–273. [DOI] [PubMed] [Google Scholar]
- 88. Iwadate H, Ohira H, Suzuki T, et al. Hepatocellular carcinoma associated with Wilson’s disease. Intern Med 2004; 43: 1042–1045. [DOI] [PubMed] [Google Scholar]
- 89. Kumagi T, Horiike N, Abe M, et al. Small hepatocellular carcinoma associated with Wilson’s disease. Intern Med 2005; 44: 439–443. [DOI] [PubMed] [Google Scholar]
- 90. Pfeiffenberger J, Mogler C, Gotthardt DN, et al. Hepatobiliary malignancies in Wilson disease. Liver Int 2015; 35: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 91. van Meer S, de Man RA, van den Berg AP, et al. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long-term follow-up. J Gastroenterol Hepatol 2015; 30: 535–539. [DOI] [PubMed] [Google Scholar]
- 92. Nazer H, Ede R, Mowat ARW. Wilson’s disease: clinical presentation and use of prognostic index. Gut 1986; 27: 1377–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shimono N, Ishibashi H, Ikematsu H, et al. Fulminant hepatic failure during perinatal period in a pregnant woman with Wilson’s disease. Gastroenterol Jpn 1991; 26: 69–73. [DOI] [PubMed] [Google Scholar]
- 94. Keen CL, Lonnerdal B, Hurley LS. Teratogenic effect of copper deficiency and excess. Inflammatory disease and copper. Clifton: Humana Press, 1982, pp.109–121. [Google Scholar]
- 95. Mishell DR., Jr. Contraceptive use and effectiveness, oral steroid contraceptives and intrauterine devices. In: Mishell DR Jr, Davajan V, Lobo RA. (eds) Infertility, contraception and reproductive endocrinology. Boston: Blackwell Scientific Publications, 1991, pp.832–909. [Google Scholar]
- 96. Haimov-Kochman R, Ackerman Z, Anteby EY. The contraceptive choice for a Wilson’s disease patient with chronic liver disease. Contraception 1997; 56: 241–244. [DOI] [PubMed] [Google Scholar]
- 97. Dong Q-Y, Wu Z-Y. Advance in the pathogenesis and treatment of Wilson disease. Transl Neurodegener 2012; 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen C, Shen B, Xiao J-J, et al. Currently clinical views on genetics of Wilson’s disease. Chin Med J (Engl) 2015; 128: 1826–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]