Abstract
Disability is a common worldwide health challenge and it has been increasing over the past 3 decades. The treatment paradigm has changed dramatically in inflammatory bowel diseases (IBDs) from control of symptoms towards full control of disease (clinical and endoscopic remission) with the goal of preventing organ damage and disability. These aims are broadly similar to rheumatoid arthritis and multiple sclerosis.
Since the 1990s, our attention has focused on quality of life in IBD, which is a subjective measure. However, as an objective end-point in clinical trials and population studies, measures of disability in IBD have been proposed. Disability is defined as ‘…any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.’ Recently, after 10 years of an international collaborative effort with the World Health Organization (WHO), a disability index was developed and validated. This index ideally would assist with the assessment of disease progression in IBD.
In this review, we will provide the evidence to support the use of disability in IBD patients, including experience from rheumatoid arthritis and multiple sclerosis. New treatment strategies, and validation studies that have underpinned the interest and quantification of disability in IBD, will be discussed.
Keywords: Crohn’s disease, disability, functioning, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, ulcerative colitis
Introduction
Worldwide evidence regarding levels and trends in disease and injury incidence, prevalence and years lived with disability (YLD) is an essential component to predict and develop global, regional, and national health policies. The Global Disease Study 2013 estimates were made to quantify disability across acute and chronic diseases, and injuries for 188 countries between 1990–2013.1 In this landmark study, disease and injury are highly prevalent across these time periods. In the years studied, comorbidity rose substantially with age and in absolute terms from 1990–2013. In addition, significant subsequent chronic sequelae were largely attributable to noncommunicable diseases. Overall, the proportion of disability-adjusted life years due to YLDs increased globally from 21% in 1990 to 31% in 2013.1
The treatment paradigm has changed dramatically in inflammatory bowel disease (IBD) from control of symptoms towards full control of disease (clinical and endoscopic remission) with the ultimate target of terminating disease progression and thereby preventing bowel damage and subsequent disability. The World Health Organization (WHO) has previously approved the International Classification of Functioning, Disability and Health (ICF) which is a common conceptual framework that describes and measures the dimensions of human functioning, disability and health. The WHO defines disability as ‘…any restriction or lack (resulting from an impairment) of ability to perform an activity in the manner or within the range considered normal for a human being.’2
IBD is known to affect physical, psychological, familial and social dimensions of life.3,4 The psychological impact of IBD may also contribute to disability.3–5 Previously, until 2010, the major focus of disease impact in IBD was on quality of life, measured by the IBDQ (IBD Questionnaire), SIBDQ (short IBD Questionnaire) and the generic SF-36.6 However, in more recent years, more objective measures of disability have been considered.
We have learned from rheumatoid arthritis (RA) and multiple sclerosis (MS) that disability can be prevented by disease-modifying agents, whereas such evidence is lacking in IBD. Now that a validated disability index is available, ongoing and upcoming clinical studies will explore whether disability can also be prevented in IBD patients. In this review, we will discuss disability in other inflammatory conditions and IBD, and what treatments may potentially impact favorably on this outcome.
Review criteria
An electronic search of publications in English on PubMed up to June 2017 was performed using the following keywords: ‘Crohn’s disease’, ‘ulcerative colitis’; ‘inflammatory bowel disease’, ‘treatment’, ‘functioning’; ‘disability’; ‘treat to target’; ‘early disease’; ‘rheumatoid arthritis’; ‘multiple sclerosis’. Also, a hand search of abstracts from the annual meetings of Digestive Disease Week and United European Gastroenterology Week, the European League Against Rheumatism Conference and the European Committee for Treatment and Research in Multiple Sclerosis Congresses between 2011 and 2016/7 was performed. Clinical trials status was checked on: http://www.clinicatrials.gov and http://www.clinicaltrialsregister.eu.
Why is inflammatory bowel disease a disabling condition?
IBD is known to significantly reduce the health-related quality of life (HRQOL) of patients. HRQOL is a quantitative measurement of subjective perception of health state.7 Many previous studies and disease-specific questionnaires primarily focused on the Inflammatory Bowel Disease Questionnaire (IBDQ).6 However, this is subjective and as it was not developed and validated according to the US Food and Drug Administration (FDA) guidance for the development of patient-reported outcomes,8 it has consequences for future new drug approval in IBD.
Fatigue is a commonly reported symptom in IBD and has been shown to negatively impact the HRQOL in these patients.9–13
Pain has also been shown as prevalent in IBD patients during disease flares and also with extraintestinal manifestations of the disease.14–17 Many chronic conditions are known to lead to chronic pain which thereby impacts on HRQOL,18–21 which is also observed in IBD.3,22 A large proportion of IBD patients require opioid analgesia.23 Patients with IBD also experience frequent concomitant anxiety and depression,5 and some studies have reported that patients with Crohn’s disease (CD) have higher rates of anxiety and depression, than ulcerative colitis (UC) patients.10,24 There also appears a need to compare HRQOL indices in order to compare disease burden and impact across various disease states.3,8,25–27 These data have a high impact with regards future governmental health resource allocation.
With regards work disability in IBD a Swiss cohort study identified certain factors which contributed to this temporarily and permanently. In patients with CD, temporary work disability was associated with gender, disease duration, disease activity, C-reactive protein level, smoking, depressive symptoms, fistulas, extraintestinal manifestations and the use of steroids/immunosuppressants. In patients with UC, temporary work disability was associated with age, disease duration, disease activity, and the use of steroids/antibiotics. Overall, in this cohort study in all patients with IBD, permanent work disability was only associated with disease activity.28
In another cohort study from the IBSEN group, patients with IBD had an more of a relative risk (RR) for requiring a disability pension than the ‘normal’ population in the 10 years after disease onset (RR: 1.8 and 2.0, for UC and CD, respectively).29 Accordingly, the impact of IBD on social and professional life was included in the recently developed IBD disease severity index.27
Overall, disability is high in both CD and UC and contributes to disease burden in IBD, but a validated tool to objectively measure this was lacking.
Development and validation of the inflammatory bowel disease disability index: a World Health Organization initiative
The International Classification of Functioning, Disability and Health (ICF) is a common conceptual framework which describes and measures the dimensions of human functioning, disability and health that was approved by the WHO in 2001.30,31 The ICF framework of classifying function and disability is multidimensional and includes factors that affect functioning, such as personal and environmental factors. IBD is known to affect physical, psychological, familial and social dimensions of life.3,4
In an attempt to quantify disability, the ‘IBD Disability Index’ (IBD-DI) (Table 1) was developed in collaboration with the WHO.32,33 This international collaboration was commenced in 2007 and the IBD-DI has recently been validated in a French population-based study.33 The IBD-DI demonstrated high internal consistency, interobserver reliability and construct validity, and moderate intraobserver reliability. It comprises 14 questions, with scores ranging from 0–100. In this study, 150 patients with CD and 50 patients with UC completed the IBD-DI validation phase. IBD-DI scores were highly correlated with the IBDQ (–0.82; p < 0.001) and SF-36 (–0.61; p < 0.05) scores. Female gender (p < 0.001), clinical disease activity (p < 0.0001) and disease duration (p = 0.02) were associated with higher IBD-DI scores. The responsiveness of the IBD-DI is being evaluated in ongoing prospective studies such as CURE [EudraCT Number: 2013-003199-11] and the ICARE [ClinicalTrials.gov identifier: NCT02377258] studies. It is expected that the IBD-DI should be a major secondary endpoint in clinical practice and future disease-modification trials, but this requires further longitudinal evaluation.34
Table 1.
Disability index in inflammatory bowel disease (IBD), the IBD disability index (IBD-DI).33
| IBD-DI | |
|---|---|
| Categories | Five categories: (1) General health (2) Body functions (3) Body structures (4) Activities and participation (5) Environmental factors |
| Number of items | 27 items 17 items assessing different aspects of disability: 12 were scored on a 5-point Likert score, 2 required a dichotomous answer, 2 required continuous answers, and 1 was divided into three categories Eight items assessing environment interaction with disease Two items assessing access to social security and healthcare system |
| Scoring | (1) Sum the item values from all selected items to calculate the overall score of the IBD-DI (a higher score indicates a greater level of disability) (2) Rescale all items to range from 0 to 4. Binary items are coded as ‘0’ for ‘no’ and ‘4’ for ‘yes’ (weight loss, arthritis, blood in stools). BMI is scored as ‘0’ for a BMI > 18.5 and ‘4’ for a BMI < 18.5. Number of liquid stools are categorized into quartiles. (3) In case of <20% of missing items for an individual, the total score is then rescaled from 0 to 100 using the following formula: score × 100/(p × 4), where p represents the number of answered items (score is calculated only when at least 80% of items are answered) |
| Interpretation | Total score ranging from 0 to 100 0–20 (no disability), 20–35 (mild disability), 35–50 (moderate disability) and 50–100 (severe disability) |
| Recall period | 1 week |
| Application | Designed to be applied by an interviewer A self-reported version may also be developed |
| Languages | Validated in its French version It needs to be translated for use in other languages and assessing cross-cultural validity |
IBD-DI, inflammatory bowel disease disability index; BMI, body-mass index.
Interestingly, in a recent large French nationwide survey of patient-reported outcomes in IBD, 1185 patients were included in the analysis. In total, half of patients reported poor quality of life (SIBDQ < 45:53%), severe fatigue (Functional Assessment of Chronic Illness Therapy (FACIT) < 30:47% and/or depression (hospital anxiety and depression (depression component) (HAD)-D > 7:49%). One third of patients reported anxiety (Hospital Anxiety and Depression (HAD)-A > 7:30%) and /or moderate (22%) or severe (12%) disability. Around half of patients had moderate-to-severe loss of work productivity and loss of activity. This study demonstrates that disease burden is very high in IBD, and disability is prevalent in one third of patients.26
In a recent study of 84 patients with restorative proctocolectomy, the IBDQ was compared with the IBD-DI, and they found them to be highly correlated (r = 0.84, p < 0.01).33,35 Worse quality of life and disability were found in those who had their position affected at work (both p < 0.01) and those who had more than 100 days off work in the last year (p < 0.01 for quality of life and p = 0.012 for disability). Lower quality of life and disability scores were associated with higher indirect and total costs (p < 0.01).35
Patients with UC, and familial adenomatous polyposis and subsequent ileoanal pouch anastomosis had less disability than those with CD (as a final diagnosis). This led to several conclusions, including the finding of higher disability in patients with CD versus UC and lower disability scores in patients with restorative proctocolectomy with ileal pouch anal anastomosis compared with medical-treated patients.35,36 In another study, HRQOL and the IBD-DI was compared in patients with UC (86 who had surgery and 119 patients who were receiving anti-TNF therapy). The authors reported that pouch patients had better overall health compared with the anti-TNF group, and patients who had surgery had more gastrointestinal symptoms than those treated with anti-TNF therapy. However, overall median IBD-DI scores were similar between the groups.37
Despite perceived improvements in quality of life with pouch surgery,38 bowel functioning and quality of life are not normal in many patients, as patients frequently experience unwanted side effects, for example, leakage, fecal urgency, and pouchitis, indicating that surgery does not prevent future disability in many of these patients.39 More recently, a self-reported version of the IBD Disability Index (IBD-DI) has been validated in a population-based cohort of IBD patients.40 Disability, in general, is associated with lower earnings, indirectly and directly, it also has been associated with reducing access to education and can lead to social exclusion. This impact of disability on society is challenging for health promotion, as disability can be preventable or ameliorated.41 Disability is a major factor of disease burden in IBD, and the main associations with disability of disease severity are: the impact of the disease on the individual, disease burden, and the disease course. A disease severity classification including disability is being developed and validated by the The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD).42
The inflammatory bowel disease disk, a simple tool for routine practice
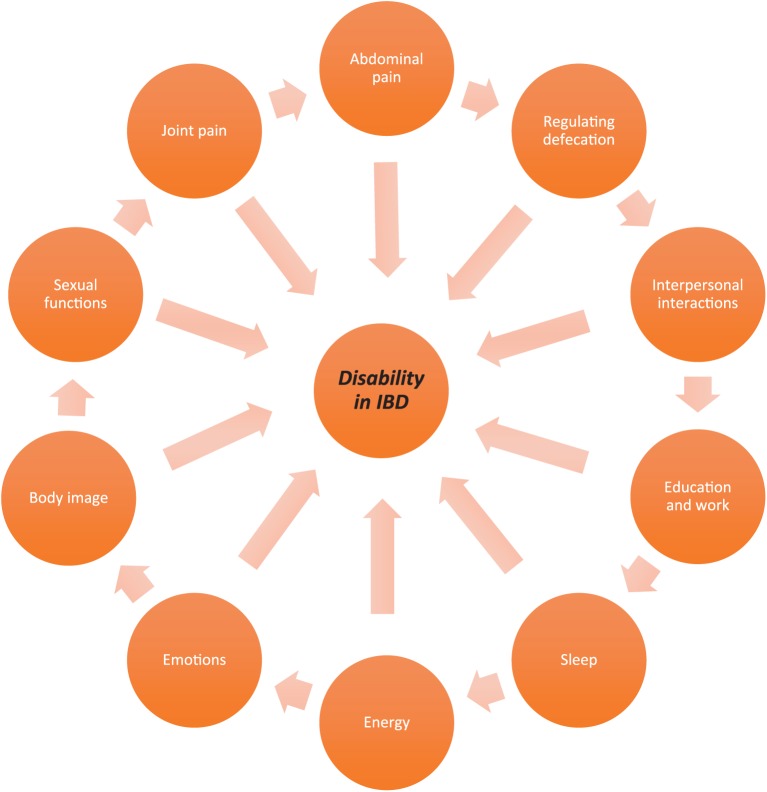
As the IBD-DI evaluates functional status it is used mainly in the clinical trial setting. A more recent IBD disk (see Figure 1 and Table 2) was developed, which is a shortened, self-administered adaptation of the validated IBD-DI. This should permit immediate visual representation of patient-reported The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD)-related disability. In the preparatory phase, the IBD CONNECT group (30 health care professionals) ranked IBD-DI items in the perceived order of importance.
Figure 1.
Disability in inflammatory bowel disease (IBD) components of disability according to the IBD disk consensus agreement.43
Table 2.
Description of the inflammatory bowel disease disk: a simple way to capture the patients’ perspective?43.
| The IBD disk: what is it? |
|---|
| • Highly visual tool for assessing disease burden in patients with IBD |
| • Based on the “Psodisk” used by patients with psoriasis |
| • 10-item scale; each disease impact item rated from 1–10 |
| How to complete the IBD disk? |
| • Completed by patient before or during their consultation with the healthcare practitioner |
| Potential benefits of the IBD disk |
| • Provides an easy-to-use visual tool for IBD patients to effectively communicate disease burden to their healthcare practitioner |
| • Allows prompt discussion on specific issues in a consultation |
| • Aids in setting short- and long-term goals with patient |
| • Tracks changes in disease burden over time |
| • Helps assess and monitor treatment efficacy in terms of disease burden |
| • Monitors and encourages medication adherence |
IBD, inflammatory bowel disease.
The steering committee then selected 10 items from the IBD-DI to take forward for inclusion in the IBD disk. In the consensus phase, the items were refined and agreed by the IBD Disk Working Group (comprising 14 gastroenterologists) using an online Delphi consensus process. After consensus agreements, 10 items were agreed for inclusion in the IBD disk including: abdominal pain, body image, education and work, emotions, energy, interpersonal interactions, joint pain, regulating defecation, sexual function and sleep.
The IBD disk tool lacks validation but has the potential to be a valuable outcome measure for use during routine clinical visits and may be able to accurately capture specific disability-related issues and correlate changes in disability over time.44
Can we prevent disability? Lessons from disease-modification trials in non-inflammatory bowel disease conditions
Rheumatoid arthritis
Rheumatic diseases (including RA) are chronic and progressive and they cause damage to the locomotor system leading to disability in patients.45 In patients with RA, around half received legal disability status, with around one third considered to be severely disabled in the long term.46 The healthcare costs associated with sick leave and permanent incapacity in RA are significant.47 Over the past 20 years the management of RA has improved dramatically as a consequence of earlier initiation of disease-modifying antirheumatic drugs (DMARDs), better markers, and closer monitoring of disease activity and biologic therapies.48
Joint damage has been shown as a potential predictor of disability. A longitudinal relationship between radiographic progression and disability score (HAQ) was observed in a post hoc analysis of the TEMPO trial, evaluating etanercept in a population of patients with established RA (mean disease duration 6.4 years).49 In the CONCERTO trial, early biologic and methotrexate-naïve RA patients were randomized to adalimumab plus weekly blinded methotrexate (MTX) (at doses of 2.5, 5,10 or 20 mg). The authors reported that increasing doses of MTX in combination with adalimumab was associated with a significant trend in improved clinical outcomes. In addition, disease measures of both functional and radiographic outcomes did not demonstrate statistical difference between treatment groups suggesting that any dose of MTX in combination with adalimumab is sufficient to determine these two important aspects of RA treatment.50 In a large meta-analysis of anti-TNF trials (748 patients), joint damage (described as joint erosion and joint space narrowing) was associated with increasing disability scores (HAQ).51 In a post hoc analysis of the BeSt study, evaluating the components of joint damage (joint erosions and joint space narrowing), found that erosion of the wrists was the most important independent predictor of functional disability.52
The 2010 Rheumatoid Arthritis Classification Criteria was published by a collaborative initiative of the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR). The new classification redefined the treatment objectives of RA at high risk of chronicity and erosive damage by focusing on features at earlier stages of disease. It has been reported that patients treated early in the disease course with a DMARD and biological DMARD improve not only clinical outcomes but also have reductions in joint damage and long-term disability.50,53–56
Although structural joint damage progression is associated with the presence of clinical disease activity, several reports have underscored the presence of radiological progression in some patients with apparent clinical remission.57,58 These observations have introduced concepts and definitions such ‘complete or deep remission’.59
The concept of treating to reach an objective target improves outcomes and has been studied in several chronic conditions like hypertension and diabetes.60 The treat-to-target recommendations for RA were formulated in 2010 and updated in 2014.48,61 The agreed target for treatment of RA is clinical remission, defined as the absence of signs and symptoms of significant inflammatory disease activity.61 Interestingly, studies have reported that tight control (of disease activity) by means of close monitoring of patients with objective scoring tools, and adjusting treatments towards a target endpoint, results in greater improvement and a higher percentage of patients achieving low disease activity and better outcomes in RA patients.62–66
In summary, the objectives of rheumatology physicians are to identify and treat patients earlier in their disease course to reduce inflammation, improve joint functioning and prevent or ameliorate joint damage and thereby long-term disability.
Multiple sclerosis
Disability is also common in MS.67–69 Disability in MS can be measured by several methods including: The Kurtzke Expanded Disability Status Scale (EDSS) (based on neurological examination and assessment of a range of neurological functions); the Multiple Sclerosis Functional Composite (MSFC); The Nine Hole Peg Test; and the Sloan Low Contrast Letter Acuity.68
Several important phase III trials have confirmed the benefit of certain drugs in reducing MRI activity in MS patients with Fingolimod (sphingosine-1-phosphate receptor modulator),70 natalizumab (anti-beta integrin monoclonal antibody)71 and rituximab (anti CD-20 monoclonal antibody),72 however none of these agents reduced progression of disability in these patients.
In planned subgroup analyses with rituximab, participants younger than 50 years and those with gadolinium-enhanced lesions at baseline showed benefit on disability progression.72 Based on these results, a humanised anti-B lymphocyte monoclonal antibody, ocrelizumab, was evaluated in a phase III trial. In a recently reported trial, ocrelizumab appeared to reduce the risk of disability progression in MS by 24%.73 This trial enrolled relatively young patients (mean age of 45 years) with short disease duration (mean disease duration of 6.4 years) and a relatively high proportion of participants had gadolinium-enhanced lesions at baseline (26%). The subgroup with gadolinium-enhanced lesions at baseline seemed to have a greater reduction in risk of disability progression, although the difference was not significant [hazard ratio (HR) 0.65; for those with enhanced lesions versus 0.84 those without]. The different results that have been reported in trials of progressive MS seem not to relate to anti-inflammatory drug effects. The result suggests that trials enrolling a study population with younger age, shorter disease duration, and greater inflammatory lesion activity tend to exhibit greater benefit.
Interestingly, in a recent study of MS patients with no disease activity at 2 years, this was associated with a positive predictive value of 78% for absence of disability at 7 years.74 In addition, other studies have reported that clinical and radiological (magnetic resonance imaging, MRI) parameters had a better predictive value for disability progression than individual measures in MS75–77 and an inclusion of patient-reported outcome measures in these trials may further improve the accuracy of this tool in MS patients.67
The findings abovementioned have led to greater interest in the concept of deep remission, tight control and potentially disease modification, with reduction or prevention of long-term disability in IBD as in other Immune mediated inflammatory disease (IMIDs).78–80
Can we identify early disease and modify the disease course in inflammatory bowel disease to prevent disability?
The main focus on identifying early IBD to aggressively arrest inflammation, thereby preventing bowel damage and future disability, has been based on the identification of early CD. Previous studies have demonstrated that in patients with earlier CD, there appeared to be greater efficacy with regards anti-TNF therapy than in those with later or more established disease.78,81 The reasons for this disparity are unclear but should suggest processes are a greater inflammatory burden in earlier disease and differing cytokine profiles in the two stages of disease.82–85 An international consensus has proposed the ‘Paris Definition for Early Crohn’s Disease’ that defines it as disease duration ⩽ 18 months after diagnosis and without previous exposure to immunosuppressants or biological therapies.86
The concept of identifying early disease and then modifying the natural history with drugs to avoid later disease complications and prevent disability is gaining traction in current opinion. A term that was previously introduced into the literature in 2013 was DMAIDs, that is, disease-modifying anti-inflammatory bowel disease drugs and its premise is based on DMARDs in RA.87,88 Despite their widespread use in IBD management, thiopurines have proved ineffective for disease modification in CD.89,90 The previous treatment goal for CD was to induce and then ensure steroid-free remission; this lends itself to the concept of deep remission, whereby reducing inflammation may lead to reduced surgeries, hospitalizations and ultimately, disability. Whether current drugs are able to achieve these goals will require further investigation.
In the recently reported Randomised Evaluation of an Algorithm for Crohn’s Treatment (REACT) study, community gastroenterology practices from Belgium and Canada were randomly assigned to early combined immunosuppression (Early combined immunosuppression (ECI), an anti-TNF and antimetabolite drug) or conventional management.91 It was the first disease-modification trial in CD.91 The primary outcome of remission rate at month 12 was similar for both groups (66% versus 62%, p = 0.52).91 However, the 24-month composite rate of major adverse outcomes (defined as surgical intervention, hospital admission, or serious disease-related complications) was lower at ECI practices than at conventional management practices (27.7% versus 35.1%, respectively; HR: 0.73; 95% confidence interval 0.62–0.86, p = 0.0003].91 Unfortunately the REACT study did not assess disability as an endpoint and this may have led to more informative outcomes of different treatment strategies on long-term disability.
Perspectives
Disability is prevalent in IBD and is seen in patients with UC (before and after surgery) and in CD. The IBD-DI should become a major secondary endpoint in clinical trials.34 This index needs to be expanded to include worldwide comparisons of disability among IBD patients in a longitudinal fashion. Future disease-modification trials in IBD should consider the inclusion of patients with early IBD, the use of the Lémann index (in CD) and the IBD-DI as defined endpoints,92 and stratifying patients with disease severity.27,93
The recent development of the IBD disk has the potential to be a valuable tool for use during routine clinical visits and may be able to accurately capture specific disability-related issues and correlate changes in disability over time (Figure 1 and Table 2).44
Future clinical trials will move away from simply targeting response and remission to ensure deep remission, improvement in defined patient-reported outcomes and ultimately preventing and improving disability in IBD.
The costs and safety of these new therapeutic strategies in IBD that are being developed to change the natural history of disease with earlier and effective treatments in order to reduce long-term disability in IBD patients, remain to be established; however, the potential patient and public health benefits achieved warrant consideration in their own right.88,94
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: PBA: consulting fees from Merck, Abbvie, Janssen, Ferring, Tillots, Celgene, Takeda, Norgine, GSK, Allergan. Lecture fees from Merck, Abbvie, Janssen, Ferring, Tillots, Celgene, Takeda, Norgine, GSK, Allergan.
CGR: speaker fees for Takeda France, MSD France and Ferring France.
LPB: Consulting fees from Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Therakos, Pharmacosmos, Pilège, BMS, UCB-pharma, Hospira, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, Pfizer, HAC-Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis. Lecture fees from Merck, Abbvie, Takeda, Janssen, Takeda, Ferring, Norgine, Tillots, Vifor, Therakos, Mitsubishi, HAC-pharma.
Contributor Information
Patrick B. Allen, Department of Medicine and Gastroenterology, SE Trust, Belfast N. Ireland, UK
Corinne Gower-Rousseau, Public Health Unit, Epimad Registry, Maison Régionale de la Recherche Clinique, Lille University Hospital, France INSERM UMR 995, LIRIC, Team 5: From epidemiology to functional analysis, Lille University, France.
Silvio Danese, Department of Gastroenterology, IBD Center, Istituto Clinico Humanitas, Humanitas University, Milan, Italy.
Laurent Peyrin-Biroulet, INSERM U954 and Department of Hepatogastroenterology, Nancy University Hospital, Lorraine University, Allée du Morvan, F-54511 Vandoeuvre-lès-Nancy, France.
References
- 1. Global Burden of Disease Study 2013 Collaborators; Barber RM, Bell B, Bertozzi-Villa A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. International classification of impairments, disabilities and handicaps of the World Health Organisation. 1976. [Google Scholar]
- 3. Casellas F, Lopez-Vivancos J, Vergara M, et al. Impact of inflammatory bowel disease on health-related quality of life. Dig Dis 1999; 17: 208–218. [DOI] [PubMed] [Google Scholar]
- 4. Allen PB, Kamm MA, Peyrin-Biroulet L, et al. Development and validation of a patient-reported disability measurement tool for patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013; 37: 438–444. [DOI] [PubMed] [Google Scholar]
- 5. Moser G. Depression and anxiety in inflammatory bowel disease. Gastroenterol Hepatol 2009; 32(Suppl. 2): 9–12. [DOI] [PubMed] [Google Scholar]
- 6. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804–810. [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). Health Related Quality of Life, https://www.cdc.gov/hrqol/index.htm (accessed 1 June 2017).
- 8. Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol 2014; 12: 1246–1256.e6. [DOI] [PubMed] [Google Scholar]
- 9. Jelsness-Jørgensen L-P, Bernklev T, Henriksen M, et al. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 106–114. [DOI] [PubMed] [Google Scholar]
- 10. Romberg-Camps MJL, Bol Y, Dagnelie PC, et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis 2010; 16: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 11. Minderhoud IM, Samsom M, Oldenburg B. Crohn’s disease, fatigue, and infliximab: is there a role for cytokines in the pathogenesis of fatigue? World J Gastroenterol 2007; 13: 2089–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 1882–1889. [DOI] [PubMed] [Google Scholar]
- 13. Vogelaar L, van’t Spijker A, van Tilburg AJP, et al. Determinants of fatigue in Crohn’s disease patients. Eur J Gastroenterol Hepatol 2013; 25: 246–251. [DOI] [PubMed] [Google Scholar]
- 14. Wagtmans MJ, Verspaget HW, Lamers CB, et al. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol 1998; 27: 129–133. [DOI] [PubMed] [Google Scholar]
- 15. Aghazadeh R, Zali MR, Bahari A, et al. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol 2005; 20: 1691–1695. [DOI] [PubMed] [Google Scholar]
- 16. Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol 1996; 23: 29–34. [DOI] [PubMed] [Google Scholar]
- 17. Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kukreja M, Bryant AS, Cleveland DC, et al. Health-related quality of life in adult survivors after the fontan operation. Semin Thorac Cardiovasc Surg 2015; 27: 299–306. [DOI] [PubMed] [Google Scholar]
- 19. Luo J, Hendryx M, Safford MM, et al. Newly developed chronic conditions and changes in health-related quality of life in postmenopausal women. J Am Geriatr Soc 2015; 63: 2349–2357. [DOI] [PubMed] [Google Scholar]
- 20. Hussain KB, Fontana RJ, Moyer CA, et al. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol 2001; 96: 2737–2744. [DOI] [PubMed] [Google Scholar]
- 21. Naliboff BD, Kim SE, Bolus R, et al. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: a structural equation modeling analysis. Am J Gastroenterol 2012; 107: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irvine EJ. Quality of life in inflammatory bowel disease: biases and other factors affecting scores. Scand J Gastroenterol Suppl 1995; 208: 136–140. [DOI] [PubMed] [Google Scholar]
- 23. Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis 2009; 15: 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simrén M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol 2002; 97: 389–396. [DOI] [PubMed] [Google Scholar]
- 25. Maska L, Anderson J, Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment. Arthritis Care Res 2011; 63(Suppl. 11): S4–S13. [DOI] [PubMed] [Google Scholar]
- 26. Williet N, Sarter H, Gower-Rousseau C, et al. Patient-reported outcomes in a French nationwide survey of inflammatory bowel disease patients. J Crohn’s Colitis 2016; 11: 165–174. [DOI] [PubMed] [Google Scholar]
- 27. Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut. Epub ahead of print 25 October 2016. DOI: 10.1136/gutjnl-2016-312648. [DOI] [PubMed] [Google Scholar]
- 28. Siebert U, Wurm J, Gothe RM, et al. Predictors of temporary and permanent work disability in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 847–855. [DOI] [PubMed] [Google Scholar]
- 29. Høivik ML, Moum B, Solberg IC, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut 2013; 62: 368–375. [DOI] [PubMed] [Google Scholar]
- 30. Coenen M, Cieza A, Stamm TA, et al. Validation of the international classification of functioning, disability and health (ICF) core set for rheumatoid arthritis from the patient perspective using focus groups. Arthritis Res Ther 2006; 8: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Disability in inflammatory bowel diseases: developing ICF Core Sets for patients with inflammatory bowel diseases based on the International Classification of Functioning, Disability, and Health. Inflamm Bowel Dis 2010; 16: 15–22. [DOI] [PubMed] [Google Scholar]
- 32. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012; 61: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gower-Rousseau C, Sarter H, Savoye G, et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut 2017; 66: 588–596. [DOI] [PubMed] [Google Scholar]
- 34. Peyrin-Biroulet L, Gower-Rousseau C. The IBD disability index should become a major secondary endpoint in clinical practice and in clinical trials. J Crohns Colitis 2016; 10: 1375–1377. [DOI] [PubMed] [Google Scholar]
- 35. Lee Y, McCombie A, Gearry R, et al. Disability in restorative proctocolectomy recipients measured using the inflammatory bowel disease disability index. J Crohn’s Colitis 2016; 10: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 36. Peyrin-Biroulet L, Gower-Rousseau C. The IBD disability index should become a major secondary endpoint in clinical practice and in clinical trials. J Crohns Colitis 2016; 10: 1375–1377. [DOI] [PubMed] [Google Scholar]
- 37. Van Gennep S, Sahami S, Buskens CJ, et al. Comparison of health-related quality of life and disability in ulcerative colitis patients following restorative proctocolectomy with ileal pouch-anal anastomosis versus anti-tumor necrosis factor therapy. Eur J Gastroenterol Hepatol 2017; 29: 338–344. [DOI] [PubMed] [Google Scholar]
- 38. Lichtenstein GR, Cohen R, Yamashita B, et al. Quality of life after proctocolectomy with ileoanal anastomosis for patients with ulcerative colitis. J Clin Gastroenterol 2006; 40: 669–677. [DOI] [PubMed] [Google Scholar]
- 39. Peyrin-Biroulet L, Germain A, Patel AS, et al. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther 2016; 44: 807–816. [DOI] [PubMed] [Google Scholar]
- 40. Peyrin-Biroulet L, LeBlanc C, Sarter H. Independent validation of a self-report version of the IBD Disability Index (IBDDI) in a population-based cohort of IBD patients. IBD Press. 2017. [DOI] [PubMed] [Google Scholar]
- 41. Disability, poverty and development. World Hosp Health Serv 2002; 38: 21–33. [PubMed] [Google Scholar]
- 42. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016; 14: 348–354.e17. [DOI] [PubMed] [Google Scholar]
- 43. Ghosh S, Louis E, Beaugerie L, et al. Development of the IBD disk. Inflamm Bowel Dis 2017; 23: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghosh S, Louis E, Beaugerie L, et al. Development of the IBD disk: a visual self-administered tool for assessing disability in inflammatory bowel diseases. Inflamm Bowel Dis 2017; 23: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anyfanti P, Triantafyllou A, Panagopoulos P, et al. Predictors of impaired quality of life in patients with rheumatic diseases. Clin Rheumatol 2016; 35: 1705–1711. [DOI] [PubMed] [Google Scholar]
- 46. Raciborski F, Kłak A, Kwiatkowska B. Indirect costs of rheumatoid arthritis. Reumatologia 2015; 53: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huscher D, Mittendorf T, von Hinüber U, et al. Evolution of cost structures in rheumatoid arthritis over the past decade. Ann Rheum Dis 2015; 74: 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010; 69: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van der Heijde D, Landewé R, van Vollenhoven R, et al. Level of radiographic damage and radiographic progression are determinants of physical function: a longitudinal analysis of the TEMPO trial. Ann Rheum Dis 2008; 67: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burmester G-R, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015; 74: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aletaha D, Funovits J, Smolen JS. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis 2011; 70: 733–739. [DOI] [PubMed] [Google Scholar]
- 52. Koevoets R, Dirven L, Klarenbeek NB, et al. Insights in the relationship of joint space narrowing versus erosive joint damage and physical functioning of patients with RA. Ann Rheum Dis 2013; 72: 870–874. [DOI] [PubMed] [Google Scholar]
- 53. Furst DE, Emery P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology 2014; 53: 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365: 2205–2219. [DOI] [PubMed] [Google Scholar]
- 55. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 56. Schoels M, Aletaha D, Smolen JS, et al. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor alpha inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis 2012; 71: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 57. Molenaar ETH, Voskuyl AE, Dinant HJ, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004; 50: 36–42. [DOI] [PubMed] [Google Scholar]
- 58. Mulherin D, Fitzgerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol 1996; 35: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 59. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011; 70: 404–413. [DOI] [PubMed] [Google Scholar]
- 60. Atar D, Birkeland KI, Uhlig T. “Treat to target”: moving targets from hypertension, hyperlipidaemia and diabetes to rheumatoid arthritis. Ann Rheum Dis 2010; 69: 629–630. [DOI] [PubMed] [Google Scholar]
- 61. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010; 69: 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364: 263–269. [DOI] [PubMed] [Google Scholar]
- 64. Mäkinen H, Kautiainen H, Hannonen P, et al. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol 2007; 34: 316–321. [PubMed] [Google Scholar]
- 65. Verstappen SMM, Jacobs JWG, van der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007; 66: 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Allaart CF, Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, et al. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol 2006; 24(6 Suppl. 43): S77–82. [PubMed] [Google Scholar]
- 67. Van Munster CEP, Uitdehaag BMJ. Outcome measures in clinical trials for multiple sclerosis. CNS Drugs 2017; 31: 217–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ontaneda D, Thompson AJ, Fox RJ, et al. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet 2017; 389: 1357–1366. [DOI] [PubMed] [Google Scholar]
- 69. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 70. Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016; 387: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 71. Steiner D, Arnold D, Freedman M, et al. Natalizumab versus placebo in patients with secondary progressive multiple sclerosis (SPMS): results from ASCEND, a multicenter, double-blind, placebo-controlled, randomized phase 3 clinical trial. In: 68th annual meeting of the American Academy of Neurology, Vancouver, Canada, 15–21 April 2016 Cambridge, MA: Biogen Idec Inc. [Google Scholar]
- 72. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66: 460–471. [DOI] [PubMed] [Google Scholar]
- 73. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 74. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 75. Sormani MP, De Stefano N. Defining and scoring response to IFN-β in multiple sclerosis. Nat Rev Neurol 2013; 9: 504–512. [DOI] [PubMed] [Google Scholar]
- 76. Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84:1082–1091. [DOI] [PubMed] [Google Scholar]
- 77. Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology 2013; 81: 1759–1767. [DOI] [PubMed] [Google Scholar]
- 78. Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease–a SONIC post hoc analysis. Aliment Pharmacol Ther 2015; 41: 734–746. [DOI] [PubMed] [Google Scholar]
- 79. Colombel J-F, Louis E, Peyrin-Biroulet L, et al. Deep remission: a new concept? Dig Dis 2012; 30(Suppl. 3): 107–111. [DOI] [PubMed] [Google Scholar]
- 80. Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep 2013; 15: 315. [DOI] [PubMed] [Google Scholar]
- 81. Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010; 105: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 82. Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010; 139: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002; 20: 495–549. [DOI] [PubMed] [Google Scholar]
- 84. Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn’s disease. Gastroenterology 1997; 113: 118–126. [DOI] [PubMed] [Google Scholar]
- 85. Kugathasan S, Saubermann LJ, Smith L, et al. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut 2007; 56: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol 2012; 107: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 87. Peyrin-Biroulet L. Disease-modifying anti-inflammatory bowel disease drugs (DMAIDs): the missing term in the literature. Am J Gastroenterol 2013; 108: 859–860. [DOI] [PubMed] [Google Scholar]
- 88. Allen PB, Peyrin-Biroulet L. Moving towards disease modification in inflammatory bowel disease therapy. Curr Opin Gastroenterol 2013; 29: 397–404. [DOI] [PubMed] [Google Scholar]
- 89. Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 2013; 145: 758–765.e2. [DOI] [PubMed] [Google Scholar]
- 90. Panés J, López-Sanromán A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 2013; 145: 766–774.e1. [DOI] [PubMed] [Google Scholar]
- 91. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386: 1825–1834. [DOI] [PubMed] [Google Scholar]
- 92. Peyrin-Biroulet L, Danese S. Early combined immunosuppression in Crohn’s disease: acting rather than REACTing. Gastroenterology 2016; 150: 1040–1041. [DOI] [PubMed] [Google Scholar]
- 93. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016; 14: 348–354.e17. [DOI] [PubMed] [Google Scholar]
- 94. Allen PB, Olivera P, Emery P, et al. Review article: moving towards common therapeutic goals in Crohn’s disease and rheumatoid arthritis. Aliment Pharmacol Ther 2017; 45: 1058–1072. [DOI] [PubMed] [Google Scholar]