Abstract
Background:
Thiopurines, azathioprine (AZA) and 6-mercaptopurine (6-MP) are common maintenance medications for inflammatory bowel disease (IBD). Excessive methylation via thiopurine methyltransferase (TPMT) frequently causes therapeutic failure. Allopurinol reduces excessive 6-methyl-mercaptopurine (6-MMP) while enhancing 6-thioguanine (6-TGN) levels. The aim of this study was to evaluate clinical, metabolic and endoscopic impact of allopurinol in combination with low-dose thiopurine in IBD.
Methods:
Retrospective review of consecutive cases treated with allopurinol. Metabolites and their ratios (6-MMP/6-TGN) were compared pre- and post-allopurinol. Clinical and endoscopic remission were assessed.
Results:
Allopurinol (n = 66) reduced mean dose of AZA by 70% (p < 0.01). Baseline levels (SD) 6-TGN, 6-MMP and 6-MMP/6-TGN were 165 (64), 9388 (5234) and 59.8 (30.3), respectively. These values improved on allopurinol to 297 (102), 896 (1031) and 3.4 (4.0), respectively (p < 0.0001). Therapeutic 6-TGN level (>235) was achieved in 49/58 cases on allopurinol combination therapy, versus 9/58 monotherapy (p = 0.0001). Among the thiopurine failure group (40 patients), clinical remission or response was observed in 65% and 22% of patients, respectively. In the asymptomatic group with excessive 6-MMP, 11/14 achieved sustained remission on allopurinol. Repeat colonoscopy (n = 28) showed mostly endoscopic remission (67.9%) or improvement (17.8%). Few had unimproved lesions (14.3%). Importantly, 46% of cases had complete mucosal healing. Two patients had cancer on combination therapy (de novo pancreatic cancer and fatal recurrence of metastatic testicular cancer). Elevated transaminases were reduced on allopurinol (48.2 versus 6.9%) (p < 0.001); no change in leukopenic or infectious events occurred.
Conclusion:
Allopurinol in combination with low-dose thiopurine corrected excessive 6-MMP levels, resulting in clinical remission and mucosal healing in the majority of cases. The potential cancer risk of allopurinol and thiopurine combination therapy needs further research.
Keywords: thiopurine, allopurinol, inflammatory bowel disease, drug metabolism
Introduction
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders. Immunomodulatory drugs are commonly used to maintain remission. Thiopurine drugs 6-mercaptopurine (6-MP) and azathioprine (AZA) remain a mainstay of therapy in IBD. A meta-analysis of adult patients showed that thiopurines help prevent relapse in quiescent UC and CD.1 A more recent Cochrane meta-analysis of six studies (489 CD participants) showed that AZA (1.0–2.5 mg/kg/day) was significantly superior to placebo for maintenance of remission over a 6- to 18-month period.2 In the AZA group, 73% of patients maintained remission compared to 62% of placebo-treated controls (RR 1.19, 95% CI 1.05–1.34). The number needed to treat for a beneficial outcome was nine.
Our group was among the first to describe how differences in metabolism of these drugs lead to individual variation in thiopurine metabolite levels that can determine their therapeutic efficacy in IBD and risk of development of adverse reactions.3,4 The parent drug needs to be metabolized to 6-thioguanine (6-TGN) to be therapeutically active. However, the metabolism of thiopurines can be diverted toward inactive metabolites via different enzymatic pathways. Xanthine oxidase shunts 6-MP toward 6-thiouracil. Also, excessive methylation via the thiopurine methyltransferase (TPMT) enzymatic route has been reported to result in the overproduction of 6-methyl-mercaptopurine (6-MMP) and sub-therapeutic 6-TGN levels, potentially causing therapeutic failure.5 Up to one-third of 285 IBD patients were reported as having excessive metabolism toward 6-MMP.6 The proportion of preferential metabolism was equal in adult and pediatric patients. However, as reviewed by Duley and Florin,7 these conclusions were based, for practical reasons, on using erythrocyte metabolite concentrations as a surrogate for leukocyte levels from IBD tissue. Moreover, the concept of excessive 6-MMP production is based on retrospective rather than prospectively collected data.
Allopurinol, a xanthine oxidase inhibitor, was first shown by Chocair and colleagues in 1993 to dramatically reduce rejection in renal transplant patients treated with AZA, corticosteroids and cyclosporine.8 Sparrow and colleagues subsequently showed that allopurinol was also useful in IBD, beneficially modifying 6-MP metabolism with reduced 6-MMP production while favoring 6-TGN production.9 Retrospective data suggest that the addition of allopurinol to thiopurine therapy can improve 6-TGN levels in 6-MP hypermethylators, promoting clinical remission and correcting hepatotoxicity due to excessive 6-MMP.10–13 Allopurinol may also be used in IBD to enhance thiopurine response in the absence of hepatotoxicity. This study’s aim was to evaluate the clinical, metabolic and endoscopic healing achieved as a result of allopurinol in combination with low-dose thiopurine in IBD patients.
Patients and methods
Patient population
This study is a retrospective review of consecutive patients with IBD who received combination therapy of allopurinol and thiopurine at two tertiary hospitals and one community hospital. Patients recruited were followed at the IBD Clinics at the McGill University Health Center (MUHC). We searched a prospectively kept IBD patient database for MUHC patients (Montreal General and Montreal Children’s Hospitals) treated with allopurinol and a thiopurine between 2008 and 2015. We also recruited patients treated at a community hospital (Gatineau Hospital, Quebec). All patients or legal guardians gave their written informed consent for participation in the study, which was approved by the MUHC Research Ethics Board.
The diagnosis of IBD was based on standard clinical, biological, endoscopic and histological criteria. Indications for allopurinol were non-response to a thiopurine, hepatotoxicity due to thiopurine or asymptomatic patients with overproduction of 6-MMP and sub-therapeutic 6-TGN on maintenance therapy. Thiopurine failure was defined as steroid dependence (two or more courses of systemic steroids over 12 months despite thiopurine use), persistent endoscopic lesions or clinical symptoms or disease flare despite thiopurine for at least 4 months. Subjects’ medical records were reviewed to obtain demographic information including age, gender and age at the onset of treatment. Disease-related baseline variables were collected, including disease site and extent using the Montreal classification14 (phenotype, localization and extent of the disease), disease duration before starting thiopurine and before the adjunct of allopurinol, concomitant medications at the onset of combination treatment, in particular corticosteroids and past medical history of surgery related to IBD. Study treatment variables included dose of thiopurine before and after the addition of allopurinol and dose of allopurinol. Prior to initiating thiopurine treatment, TMPT was measured (Pharmacokinetics Laboratory, Ste Justine Hospital) by established techniques.15 The standard dose of AZA was 2–2.5 mg/kg (or 1–1.25 mg/kg of 6-MP) for pediatric or adult patients. The dose of thiopurine used was modified for patients with heterozygous TPMT activity. The addition of allopurinol led to a concomitant reduction of thiopurine dose by a factor of approximately two-thirds.
Outcome parameters
After the introduction of combination treatment, patients were followed and adverse events recorded. White blood cell (WBC) count, hemoglobin, alanine aminotransferase (ALT) and C-reactive protein (CRP) were recorded before and after the use of allopurinol. Myelosuppression was defined as a WBC count less than 4 × 109/L. Hepatotoxicity was classified according to the Common Terminology Criteria for Adverse Events v3.0, which is divided into three grades. Grade 1 is defined as ALT between the upper limit of normal (ULN) and 2.5 × ULN; grade 2 is 2.5–5 × ULN; and grade 3 is 5–20 × ULN. All metabolic outcomes, as well as levels of thiopurine metabolites, were determined at an average of 4 weeks after initiating combination therapy and subsequently every 3 months. A prospective trial of allopurinol addition to a thiopurine in pediatric IBD found that patients reached steady state for 6-MP metabolites after 3 weeks.6 The 6-TGN and 6-MMP levels were measured in erythrocytes (red blood cells, RBCs) at Sainte Justine Hospital using a validated assay as we previously reported.3,4 Levels of TGN between 235 and 450 pmol/8 × 108 RBC were first reported by our group to correlate with therapeutic response in IBD; 6-MMP levels greater than 5700 pmol/8 × 108 RBC were found to correlate with hepatotoxicity risk.3,4
IBD clinical activity was compared using the physician’s global assessment (PGA) as well as the Harvey–Bradshaw index (HBI) for CD and the Lichtiger index for UC patients. Clinical remission was defined by the PGA, an HBI or Lichtiger Index of three points or less.16,17 When patients consented to repeat colonoscopy on combination therapy, we compared the endoscopic results on thiopurine treatment alone versus after 4–9 months on combination therapy. Endoscopic remission was defined as a Mayo score of 0 in UC patients, a SES-CD ⩽6 in CD patients or a Rutgeerts score ⩽1 in postoperative patients.
Statistical analysis
A one-sample Kolmogorov–Smirnov test was used to evaluate the normal distribution of 6-TGN/6-MMP metabolite concentrations. Quantitative variables are presented as percentages or means, followed by range and standard deviation. Non-parametric comparisons included Wilcoxon test for paired data or Mann–Whitney U test for unpaired data. The t test was used to compare continuous variables and a contingency table (χ2) to compare categorical variables.
Results
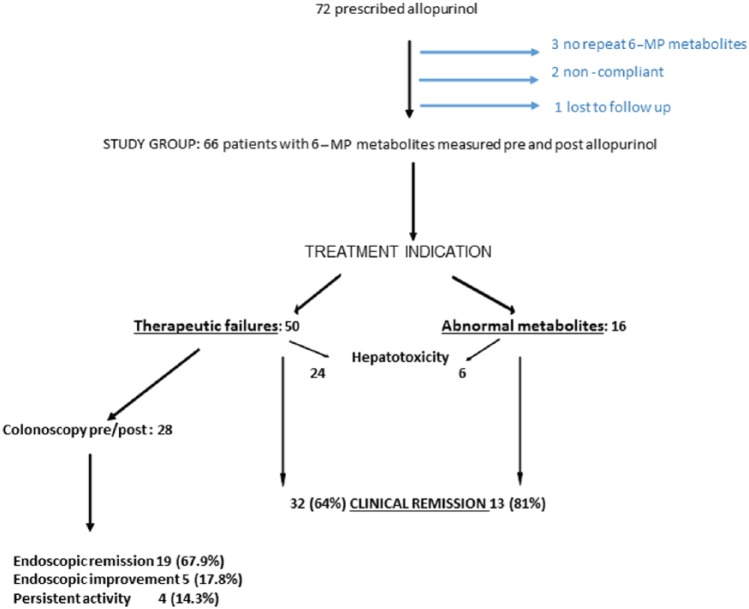
A total of 72 IBD patients (18 pediatric) received allopurinol in combination with a thiopurine at the MUHC (56) and a community hospital in Gatineau (26) between 2008 and 2015 (Figure 1). A total of six patients were excluded: three due to lack of 6-MP metabolites post-allopurinol; two for non-compliance; and one lost to follow up. Of the remaining 66 patients, 50 (75.8%) were prescribed allopurinol for thiopurine failure. Another 16 patients were asymptomatic on thiopurine alone and were prescribed allopurinol for excessive 6-MMP and sub-therapeutic 6-TGN levels. Hepatotoxicity was observed in 30 of 66 cases (45.5%): 24 in the thiopurine failure group and in 6 asymptomatic patients with an abnormal metabolic pathway (Figure 1). A colonoscopy was performed before and after treatment with allopurinol in 28 patients.
Figure 1.
Flow diagram of the allopurinol-treated IBD study patients.
The baseline demographics and disease-related characteristics of study patients are shown in Table 1. Compared to the thiopurine failure group, patients in the asymptomatic group with aberrant metabolism on maintenance thiopurine were mostly pediatric luminal CD patients with a median age at the start of thiopurine and of allopurinol of 15 and 16.5 years, respectively. The thiopurine failure group includes an older population, with two-thirds of them having CD and one-third UC. This group was at a higher risk of treatment failure due to 52% having a stricturing phenotype, including 11 that had undergone a previous bowel resection. Among the thiopurine failure group, 26 (52%) were moderately to severely ill, requiring corticosteroids at the time of allopurinol introduction (Table 1).
Table 1.
Demographic and disease-related characteristics of IBD patients treated with thiopurine and allopurinol.
| Treatment indication | ||
|---|---|---|
| Abnormal metabolism group |
Thiopurine failure group |
|
| Gender M:F | 6:10 | 17:33 |
| CD (n) | 14 | 34 |
| L1 ± L4, n (%) | 2 (12) | 25 (50) |
| L2 ± L4, n (%) | 7 (44) | 5 (10) |
| L3 ± L4, n (%) | 7 (44) | 20 (40) |
| B1, n (%) | 11 (69) | 9 (39) |
| B2, n (%) | 4 (25) | 26 (52) |
| B3, n (%) | 1 (6) | 2 (9) |
| perianal, n (%) | 3 (19) | 7 (14) |
| UC (n) | 2 | 16 |
| E2, n (%) | 0 | 7 (44) |
| E3, n (%) | 2 (100) | 9 (56) |
| Previous bowel surgery, n (%) | 1 (6) | 11 (22) |
| Age at diagnosis (median in year) | 13 | 27 |
| Age when thiopurine started (median in year) | 15 | 34 |
| Age when allopurinol added (median in year) | 16.5 | 41 |
| Allopurinol dose, mg (mean) | 72.5 | 98,4 |
| Concomitant medications | ||
| Corticosteroid n = (%) | 0 (0) | 26 (52) |
| 5-ASA n = (%) | 5 (31) | 5 (10) |
| Anti-TNF n = (%) | 2 (12) | 7 (14) |
5-ASA, 5-aminosalicylic acid; anti-TNF, anti-tumor necrosis factor; CD, Crohn’s disease; UC, ulcerative colitis.
Among the 66 patients with 6-MP metabolites measured before and after the addition of allopurinol (50–100 mg/day concomitantly with AZA), the mean dose (SD) of AZA was reduced by 70%, from 165 (57) to 49.5 (15) mg/d (p < 0.01). The 6-TGN, 6-MMP and 6-MMP/6-TGN ratios all significantly improved (p < 0.001) after allopurinol treatment (Table 2). A therapeutic 6-TGN level, defined as >235 and <450 pmol/8 × 108 RBC, was achieved initially in 51 of the 66 patients on allopurinol combination therapy (77.3%) compared to only 10 patients (15.2%) on thiopurine alone (p < 0.001).
Table 2.
Values of erythrocyte 6-TGN and 6-MMP, and their ratio while on a thiopurine alone or in combination with allopurinol.
| 6-TGN (pmol/8 × 108 RBC) | 6-MMP (pmol/8 × 108 RBC) | 6-MMP/6-TGN | |
|---|---|---|---|
| Thiopurine alone, mean (SD) | 161 (60) | 9388 (5106) | 58.7 (30.7) |
| With allopurinol, mean (SD) | 298 (104) | 893 (1033) | 3.2 (3.9) |
|
p-value 95% confidence interval |
p < 0.0001 −166.2 to −127.8 |
p < 0.0001 7226.4 to 9763.6 |
p < 0.0001 47.9 to 63.0 |
6-MMP, 6-methyl-mercaptopurine; 6-TGN, 6-thioguanine.
Clinical outcomes were assessed in all 66 patients and divided according to the clinical indication for the use of allopurinol. For the thiopurine failure group, clinical remission was observed in 32 of the 50 patients (64%). A clinical response was observed in 11 others (22%). Among these 43 responders, 33 remained on combination therapy without requiring steroids for an average of 34 months. The 7 others patients stopped treatment secondary to side effects (5) or on a voluntary basis (2). For the asymptomatic group on maintenance treatment but with excessive 6-MMP levels, 13 of the 16 patients remained in remission on therapy without steroids for an average of 27 months. Two others had recurrence of symptoms that required escalation of treatment and one patient abandoned treatment as a personal choice. Comparison of outcomes did not show significant differences between pediatric and adult-onset IBD. However, the pediatric group size was a limiting factor to a post-hoc analysis.
A repeat colonoscopy was carried out in 28 cases in the thiopurine failure group, between 4 and 9 months after initiating combination therapy (Figure 1). Endoscopic remission was observed in 19 patients (67.9%), while improvement was seen in 5 others (17.8%). Persistently active disease was observed in only 4 patients (14.3%). Complete mucosal healing was observed in 13 (46.4%) cases, as illustrated in Figure 2.
Figure 2.
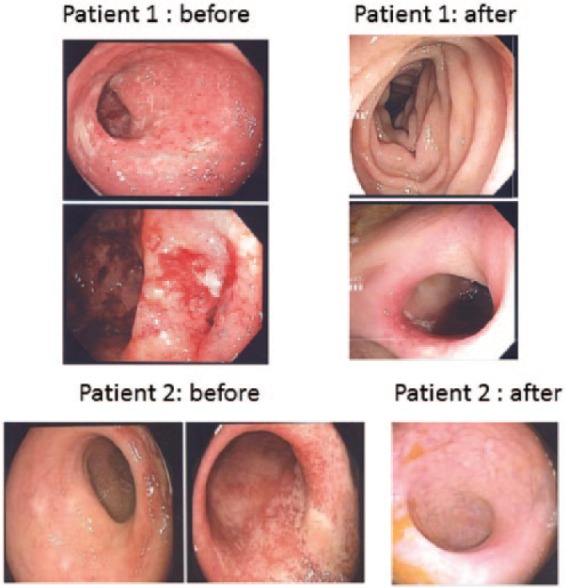
Representative endoscopic images of two Crohn’s disease patients. The initial colonoscopy was obtained while failing thiopurine therapy. Endoscopic appearance after the addition of allopurinol is shown as well.
Hepatotoxicity due to thiopurine therapy, defined by an elevated ALT on a thiopurine with high 6-MMP levels in the absence of other causes, was observed in 30 of 66 patients (44.5%) overall. The severity of hepatotoxicity was grade 1 in 22, grade 2 in 6, and grade 3 in 2. On combination therapy with allopurinol, 5 (7.6%) had hepatotoxicity (p < 0.001), all grade 1.
Mild leukopenia (2000–4000) was observed in 6 and 8 cases before and after the use of allopurinol, respectively (p = NS). No serious infections occurred. Two patients were diagnosed with cancer on combination therapy. One was a fatal case of de novo pancreatic cancer at the age of 65. The other was a fatal recurrence of metastatic testicular cancer at the age of 48, after a 14-year remission.
Discussion
Our study provides further evidence that allopurinol favorably alters the metabolism of thiopurines, with improved IBD clinical outcomes, a high rate of mucosal healing and correction of 6-MMP/6-TGN ratios (Table 2). For the thiopurine failure group, clinical remission was achieved in 64%, and a clinical response in another 22%. Among responders, three-quarters had a sustained clinical response on combination therapy, without relapse or need for steroids for an average of 34 months. We and others have reported that 6-TGN levels over the ‘threshold’ of 235 pmol/8 × 108 RBC favor clinical remission.4,18 In this study, therapeutic 6-TGN levels were achieved in 77.3% of cases on allopurinol combination therapy compared with 15.2% on a thiopurine alone (p < 0.001).
Between 14 and 31% of both pediatric and adult IBD patients preferentially metabolize 6-MP away from the active 6-TGN metabolites and toward potentially hepatotoxic 6-MMP metabolites.6,19 In our recent study in 238 pediatric CD patients,15 steroid-free remission and lower relapse rates were seen in patients whose 6-MMP/6-TGN ratios were 4 to 24 (p = 0.001). Hepatotoxicity was associated with high 6-MMP levels (>3919 pmol/8 × 108 RBC) and 6-MMP/6-TGN ratios >24 (p = 0.001).
The addition of allopurinol to thiopurine therapy has been shown to reduce 6-MMP while bolstering 6-TGN production, resolving hepatotoxicity in the majority.10–13,20,21 Our study shows similar results, with resolution of the hepatotoxicity in 83.3% of cases. In the remaining five patients with persistent hepatotoxicity, it was mild (grade 1) in all cases. Moreover, three were subsequently found to have non-alcoholic fatty liver disease with steatosis on abdominal ultrasound.
The use of allopurinol in combination with low-dose thiopurine can also be beneficial regardless of 6-MMP levels. Patients started on combination treatment as a first-line therapy responded very well.13 In a study published in abstract form, Pavlidis and colleagues recently showed that even TPMT-deficient patients who cannot produce high methylated mercaptopurine metabolite levels can develop hepatotoxicity.22 This adverse event responded well to allopurinol, suggesting an independent hepatocyte protective effect of allopurinol. Using rat hepatocyte cultures it has been shown that thiopurines can induce hepatocyte cell death via oxidative stress, mitochondrial injury and loss of ATP.23 Allopurinol was shown to reduce the hepatotoxicity in vitro.
Studies have identified mucosal healing on endoscopy as a key prognostic parameter in the management of IBD, highlighting the important role of endoscopy for monitoring disease activity and response to therapy.24 Mucosal healing has emerged as a key treatment goal in IBD, predicting sustained clinical remission and preventing resections. It is well established that clinical scores are often not in accordance with endoscopic findings or non-invasive markers of mucosal healing such as fecal calprotectin. A recent study showed that the Harvey–Bradshaw Index was not able to distinguish active from inactive CD.25 Despite the established utility of endoscopic reassessment, previous studies on the usefulness of allopurinol combination with a thiopurine in IBD have rarely assessed patients for mucosal healing. One study reported endoscopic remission on combination therapy in 9 of 11 IBD patients with hepatotoxicity due to thiopurine treatment.26 However, no endoscopic assessment was performed prior to the use of allopurinol. In a more recent similar study, at the end of follow up no inflammation was identified macroscopically in 98/180 (54%) cases.27 Improvement was seen when compared with the previous endoscopy in the other 82 cases (46%).
In our study, 28 of the 48 adult patients consented to undergo endoscopic reassessment after a minimum of 4 months on combination therapy. Endoscopic remission was achieved in 67.9% and improvement in 17.8%. Only 14.3% of cases were found to have persistently active endoscopic disease. Complete mucosal healing was observed in 46.4%. These endoscopic healing results are impressive when compared to the SONIC trial in which the AZA monotherapy group achieved mucosal healing in only 16.5% of cases.28 However, in the latter randomized controlled study, 6-MP metabolite testing was not done to optimize response to thiopurine therapy, as is now more often recommended.29,30 A recent study in IBD patients followed at tertiary care centers showed that 52% did not achieve therapeutic 6-TGN levels.31 Our higher endoscopic remission and healing rates are in accordance with other prospective studies using AZA. In UC, 60% of patients have complete mucosal healing (Mayo score 0).32 In CD 83% of AZA-treated patients achieved complete or near complete healing.33
Regarding the safety profile of allopurinol in combination with a thiopurine, the most frequent major concern is the risk of leukopenia. This risk is usually related to a high 6-TGN level generated by the addition of allopurinol. However, we routinely decreased the dose of the thiopurine to 25% of the previous dose. Consequently, we did not observe an increase in the number of leukopenic events after using combination therapy, and no serious infections were observed. In the largest reported study to date, only 1% of 300 patients on combination therapy developed myelotoxicity.28 Unfortunately, two patients in our study were diagnosed with cancer on combination therapy. One was a fatal case of de novo pancreatic cancer at the age of 65. This patient was on AZA alone for 5 years before starting allopurinol and was on combination therapy for another year before the diagnosis of cancer. The other case was a fatal recurrence of metastatic testicular cancer after a 14-year remission. This patient was 48 years old and was on AZA alone for 3 years and only 2 months on combination treatment. An increased incidence of cancer has not been previously reported in patients receiving both allopurinol and AZA. Cancers have not been associated with the use of allopurinol per se. There is an increased risk of lymphoproliferative disorder,34 and, although rare, cases of hepatosplenic T-cell lymphoma with thiopurine treatment for 2 or more years.35 Using a database of over 600 IBD patients treated with 6-MP, Disanti and colleagues reported that a statistically significant increase in the number of hematologic malignancies occurred in the group with sustained leukopenia (p = 0.014).36 However, 6-TGN metabolites were not measured. Moreover, none were treated with allopurinol. Due to the limited cohort in this study, conclusions should not be drawn about the risk of cancer subsequent to the addition of allopurinol. Excluding non-melanoma skin cancers, no significant increase in risk of malignancy has been attributed to thiopurine therapy.37–39
A recent retrospective study in patients with IBD and a history of cancer, exposure to an anti-TNF agent or an antimetabolite after cancer was not associated with an increased risk of incident cancer, compared with patients who did not receive immunosuppression.40 However, the study cohort (n = 300) was not considered large enough to exclude this possibility. Large prospective studies are needed to evaluate whether the addition of allopurinol increases the risk of cancer in thiopurine-treated IBD patients.
In summary, the combination of allopurinol and low-dose thiopurine is a clinically effective immunomodulation therapy for patients with IBD. This combination offers a solution for the well-recognized limitations of thiopurine monotherapy, including modest response rates and side effects such as hepatotoxicity. The addition of allopurinol was associated with reduced 6-MMP levels. Moreover, clinical as well as endoscopic improvements ensued in the majority of cases. Complete mucosal healing was observed in almost half of the patients after the addition of allopurinol. Long-term follow-up shows that the combination therapy is safe and permits steroid cessation for the majority of cases. Based on the current literature, changes in the risk of cancer subsequent to the addition of allopurinol appears to be negligible. Ideally, this needs to be assessed by a large prospective study.
Footnotes
Funding: Dr Ernest Seidman is supported by a Canada Research Chair in Immune Mediated Gastrointestinal Disorders and the B Kaufman Endowed Chair of IBD at McGill.
Author contributions: B Moreau: study concept and design, acquisition of data, analysis and interpretation of data, drafting manuscript.
Pierre Clement: acquisition of data, analysis and interpretation of data, manuscript review.
Yves Theoret: acquisition of data, analysis and interpretation of data, manuscript review.
Ernest G. Seidman: study supervision, study concept and design, analysis and interpretation of data, drafting and revising manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Brigitte Moreau, Advanced IBD Fellow, Division of Gastroenterology, McGill University Health Center, Departments of Medicine and Pediatrics, Montreal, QC, Canada.
Pierre Clement, Division of Gastroenterology, Gatineau Hospital, Gatineau, QC, Canada.
Yves Theoret, Pharmacology Laboratory, Sainte Justine Hospital, Montreal, QC, Canada.
Ernest G. Seidman, McGill Center for IBD, Research Institute of McGill University Health Center, MGH Campus, C10.145, 1650 Cedar Avenue, Montreal, Quebec, H3G 1A4, Canada.
References
- 1. Khan KJ, Dubinsky MC, Ford AC, et al. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 630–642. [DOI] [PubMed] [Google Scholar]
- 2. Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2015; CD000067. DOI: 10.1002/14651858.CD000067.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuffari C, Theoret Y, Latour S, et al. 6-mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 1996; 9: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000; 118: 705–713. [DOI] [PubMed] [Google Scholar]
- 5. Dubinsky MC, Yang H, Hassard PV, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 2002; 122: 904–915. [DOI] [PubMed] [Google Scholar]
- 6. Gerich ME, Quiros JA, Marcin JP, et al. A prospective evaluation of the impact of allopurinol in pediatric and adult IBD patients with preferential metabolism of 6-mercaptopurine to 6-methylmercaptopurine. J Crohns Colitis 2010; 4: 546–552. [DOI] [PubMed] [Google Scholar]
- 7. Duley JA, Florin TH. Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit 2005; 27: 647–654. [DOI] [PubMed] [Google Scholar]
- 8. Chocair P, Duley J, Simmonds HA, et al. Low-dose allopurinol plus azathioprine/cyclosporin/prednisolone, a novel immunosuppressive regimen. Lancet 1993; 342: 83–84. [DOI] [PubMed] [Google Scholar]
- 9. Sparrow MP, Hande SA, Friedman S, et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther 2005; 22: 441–446. [DOI] [PubMed] [Google Scholar]
- 10. Sparrow MP, Hande SA, Friedman S, et al. Effect of allopurinol on clinical outcomes in inflammatory bowel disease non-responders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol 2007; 5: 209–214. [DOI] [PubMed] [Google Scholar]
- 11. Leung Y, Sparrow MP, Schwartz M, et al. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis 2009; 1: 162–167. [DOI] [PubMed] [Google Scholar]
- 12. Ansari A, Patel N, Sanderson J, et al. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2010; 31: 640–647. [DOI] [PubMed] [Google Scholar]
- 13. Smith MA, Blaker P, Marinaki AM, et al. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohn Colitis 2012; 6: 905–912. [DOI] [PubMed] [Google Scholar]
- 14. Silverberg M, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular, and serological classification of inflammatory bowel disease: report of a working party of the Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 15. Kopylov U, Amre D, Theoret Y, et al. Thiopurine metabolite ratios for monitoring therapy in pediatric Crohn disease. J Pediatr Gastroenterol Nutri 2014; 59: 511–515. [DOI] [PubMed] [Google Scholar]
- 16. Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010; 8: 357–363. [DOI] [PubMed] [Google Scholar]
- 17. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841–1845. [DOI] [PubMed] [Google Scholar]
- 18. Moreau AC, Paul S, Del Tedesco E, et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis 2014; 20: 464–471. [DOI] [PubMed] [Google Scholar]
- 19. Appell ML, Wagner A, Hindorf U. A skewed thiopurine metabolism is a common clinical phenomenon that can be successfully managed with a combination of low-dose azathioprine and allopurinol. J Crohns Colitis 2013; 7: 510–513. [DOI] [PubMed] [Google Scholar]
- 20. Rahhal RM, Bishop WP. Initial clinical experience with allopurinol–thiopurine combination therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1678–1682. [DOI] [PubMed] [Google Scholar]
- 21. Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long-term allopurinol–thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 363–369. [DOI] [PubMed] [Google Scholar]
- 22. Pavlidis P, Reynolds R, Ansari A. Allopurinol abrogates thiopurine induced liver injury without the need for metabolite measurements. Gut 2016; 65(Suppl. 1): a259. [Google Scholar]
- 23. Tapner MJ, Jones BE, Wu WM, et al. Toxicity of low dose azathioprine and 6-mercaptopurine in rat hepatocytes: roles of xanthine oxidase and mitochondrial injury. J Hepatol 2004; 40: 454–463. [DOI] [PubMed] [Google Scholar]
- 24. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61: 1619–1635. [DOI] [PubMed] [Google Scholar]
- 25. Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis 2015; 21: 824–831. [DOI] [PubMed] [Google Scholar]
- 26. Ansari A, Elliott T, Baburajan B, et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther 2008; 28: 734–741. [DOI] [PubMed] [Google Scholar]
- 27. Pavlidis P, Stamoulos P, Abdulrehman A, et al. Long-term safety and efficacy of low-dose azathioprine and allopurinol cotherapy in inflammatory bowel disease: a large observational study. Inflamm Bowel Dis 2016; 22: 1639–1646. [DOI] [PubMed] [Google Scholar]
- 28. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 29. Goel RM, Blaker P, Mentzer A, et al. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis 2015; 6: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amin J, Huang B, Yoon J, et al. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis 2015; 21: 445–452. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg R, Moore G, Cunningham G, et al. Thiopurine metabolite testing in inflammatory bowel disease. J Gastroenterol Hepatol 2016; 31: 553–560. [DOI] [PubMed] [Google Scholar]
- 32. López-Palacios N, Mendoza JL, Taxonera C. Mucosal healing for predicting clinical outcome in patients with ulcerative colitis using thiopurines in monotherapy. Eur J Intern Med 2011; 22: 621–625. [DOI] [PubMed] [Google Scholar]
- 33. Mantzaris GJ, Christidou A, Sfakianakis M. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis 2009; 15: 375–382. [DOI] [PubMed] [Google Scholar]
- 34. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 347: 1617–1625. [DOI] [PubMed] [Google Scholar]
- 35. Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2011; 9: 36–41. [DOI] [PubMed] [Google Scholar]
- 36. Disanti W, Rajapakse RO, Korelitz BI, et al. Incidence of neoplasms in patients who develop sustained leukopenia during or after treatment with 6-mercaptopurine for inflammatory bowel disease. Clin Gastroenterol Hepatol 2006; 4: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 37. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for non-melanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011; 141: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 38. Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol 2013; 11: 389–394. [DOI] [PubMed] [Google Scholar]
- 39. Masunaga Y, Ohno K, Ogawa R, et al. Meta-analysis of risk of malignancy with immunosuppressive drugs in inflammatory bowel disease. Ann Pharmacother 2007; 41: 21–28. [DOI] [PubMed] [Google Scholar]
- 40. Axelrad J, Bernheim O, Colombel JF, et al. Risk of new or recurrent cancer in patients with inflammatory bowel disease and previous cancer exposed to immunosuppressive and anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016; 14: 58–64. [DOI] [PubMed] [Google Scholar]