Abstract
Background
Numerous studies have discussed cases of concomitant temporomandibular disorders (TMD) and otologic symptoms (OS). However, attempts to determine the true origin of these symptoms combined with assessments of the condition of the organ of hearing are relatively rare.
This study aimed to evaluate the frequency and type of OS in patients with TMD, and attempted to determine the origin of the OS in the studied group of patients.
Material/Methods
246 patients, aged 40.08±11.12 years (F=147, M=99) with TMD, from the Department of Oral Rehabilitation of Poznan University of Medical Sciences. Methods: dental history interviews and clinical examinations. There were 2 groups–G1 and G2–selected on the basis of the presence or absence of OS in the medical history stage. After audiological evaluation, 2 subgroups were identified: G1.1 and G1.2.
Results
OS were observed in 36.18% (G1). In 48 patients (53.93%), the audiological evaluation found there was no impairment of the organ of hearing (G1.2). Audiological abnormalities were found in 46.07% (n=41) of the patients (G1.1). The OS which differentiated the 2 groups were a plugging sensation as well as otalgia (more frequent in group G1.2) and hearing impairment (more frequent in group G1.1).
Conclusions
1. The OS which most frequently accompany with TMD were fullness and otalgia. 2. It is recommended that a subjective assessment of hearing loss in patients with TMD and concomitant OS should be included in the medical history stage. 3. Clicking and popping are significantly more frequent in patients without concomitant hearing impairment.
MeSH Keywords: Hearing Loss, Stomatognathic System Abnormalities, Temporomandibular Joint Disorders, Tinnitus
Background
Numerous studies have discussed cases of concomitant temporomandibular disorders (TMD) and otologic symptoms (OS). The otologic symptoms of TMD within the stomatognathic system vary, but often involve otalgia (referred ear pain), tinnitus lasting for over 5 minutes, hypersensitivity to sounds, a fullness/plugging sensation, vertigo, and hearing loss [1–3]. Although the occurrence of the various OS together with functional disturbances of the stomatognathic system (SS) is unquestionable, an unambiguous explanation of this phenomenon has not been provided so far. Most probably, it is triggered by connections at the embryological, anatomical, and physiological levels between the structures of the middle ear and the SS. At present, there are several theories that attempt to explain the emergence of OS in the course of functional disorders of the SS [4–7]. These include, for instance, theories concerning the direct mechanical stimulation of the malleus through its connections to the TMJ by means of the discomalleolar ligament (Pinto’s ligament) [8]. The shared innervation of the tensor veli palatini, tympanic membrane, and masseter via the trigeminal nerve and its irritation [7], or the contraction of the blood vessels supplying the hearing receptor, or an oral parafunctional activity (bruxism) leading to microtraumas, may also account for the appearance of OS in the course of SS disorders. The aforementioned theories may partially explain the occurrence of pain, not only in the neuromuscular system and the masticatory system, but also in the region of the ear (otalgia) [6]. Otologic complaints, similarly to tension headaches, and sensations within the oral cavity and throat, as well as ocular symptoms, are non-characteristic clinical symptoms in the clinical picture of SS functional disorders since all of them are located outside the masticatory system [6,9]. Additionally, patients with concomitant OS of TMD complain of problems with falling asleep, difficulty in interpersonal communication, unclear speech, and problems with concentration, as well as anxiety, irritation, and fear connected with chronic symptoms and prolonged diagnostic procedures [2,3,10]. This leads to a deterioration in their quality of life and a decrease in their professional capacity [11].
Although many studies have explored the issue of OS coexisting with SS dysfunctions, attempts to verify the real origin of these ailments, together with an appropriate assessment of the organ of hearing, have been rare [7]. The concomitance of hearing loss or middle ear disorders may also be a source of OS localized in the periauricular region, irrespective of the disturbances and symptoms which appear in the course of SS disorders. However, the treatment of these symptoms differs depending on their primary location.
This study aimed to assess the prevalence of otologic signs and symptoms and the type of reported otologic complaints in patients with temporomandibular disorders, and to determine the origin of otologic symptoms in a group of patients with functional disturbances of the stomatognathic system [12].
Material and Methods
The study group comprised patients with functional disorders of the SS who presented at the Clinic of Oral Rehabilitation, Department of Prosthodontics at Poznan University of Medical Sciences (PUMS) for the diagnosis and treatment of dysfunctions of the masticatory system.
Initially, each patient underwent dental and audiological evaluation in accordance with the following protocol:
-
The dental part:
-
– History taking (the research diagnostic criteria for temporomandibular disorders – part 1) (RDC-TMD) [12]
General and dental interview – this enabled the influence of general conditions on SS dysfunctions to be eliminated.
-
– Specialist dental evaluation (RDC-TMD – part 2) [12].
The assessed factors included the symptoms of muscular hyperfunction in the region of the masticatory system and bruxism, i.e., tooth wear (according to Martin’s scale), vertical cracks in the enamel, and impressions on the lateral surfaces of the tongue.
-
-
The audiological part:
-
– Audiological survey.
The survey concerned the occurrence of tinnitus, ear fullness, otalgia, hypersensitivity to sounds, and impaired hearing. The presence or absence of otologic symptoms in the survey enabled the division of all the subjects into 2 groups: G1 – patients who reported the presence of OS in the medical history stage; and G2 (control group) – patients in the survey without OS (Figure 1).
-
– Specialist audiological evaluation.
The evaluation was performed to objectively verify the reported otologic complaints with respect to their actual origin, as well as evaluating the impairments to the organ of hearing. The performed OS diagnostics made it possible to subdivide group G1 into 2 subgroups: G1.1 (patients with abnormalities in the audiological evaluation) and G1.2 (patients without abnormalities in the audiological evaluation). The division into groups and subgroups is illustrated in Figure 1.
-
Figure 1.
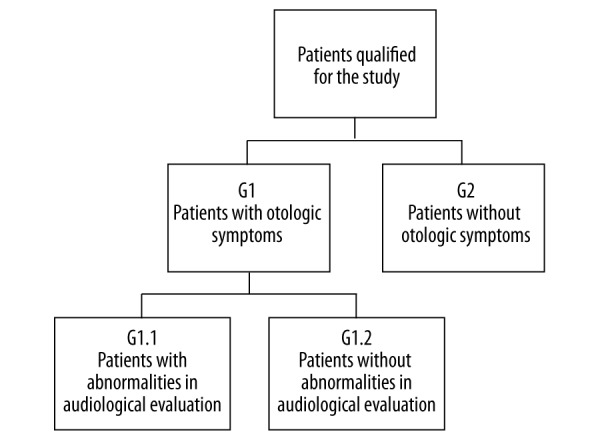
A graphical representation of patients with functional disturbances of the SS (TMD), including subjective and objective otologic symptoms.
To assess the organ of hearing, objective hearing tests and psychophysical methods were employed, all of which were performed at the Department and Clinic of Phoniatrics and Audiology. The following audiological tests were performed each time [13–15]:
Otoscopy: a visual assessment of the external auditory meatus and the tympanic membrane – an analysis of color, mobility, post-inflammatory scarring, and the presence of tympanic perforation (Heine mini 3000 otoscope with built-in light source and optical magnifying system).
Acoumetric tests: (the whisper test) the most physiological approximate assessment of hearing via air conduction and the ability to discriminate speech.
Tuning fork tests: an evaluation of the bone and air conduction of sound – Weber’s test assesses the lateralization of the vibrating tuning fork placed on the apex of the skull; Rinne’s test compares the duration of bone and air conduction in a given ear; Schwabach’s test compares the time of bone conduction in the patient and the investigator (265 Hz and 512 Hz tuning forks).
Pure tone audiometry: measuring the hearing thresholds for air and bone conduction within the frequencies 125–8000 Hz, separately for each ear; this qualitatively and quantitatively evaluates hearing loss (AD 229e manual audiometer manufactured by Interacoustic).
Impedance audiometry: evaluating the changes in acoustic impedance of the conductive system, being a function of pressure changes in the external auditory meatus (tympanometry), as well as an acoustic reflex threshold test (AT 235h impedance audiometer manufactured by Interacoustic).
Auditory brainstem responses: an evaluation of the changes in electrical activity in the individual fragments of the auditory pathway under the influence of acoustic stimuli (Vivisonic Integrity V 500).
Otoacoustic emission (DPOAE – distortion-product otoacoustic emission): an assessment of the micromechanics of the cochlea; as a result of which, the responses, otoemission signals, are recorded for individual frequencies (Vivisonic Integrity V 500).
The evaluation was approved by the PUMS Bioethical Committee, and the patients, having been informed about the objectives and the course of the test, signed an informed consent form to participate in the test.
The obtained data underwent statistical analysis with Statistica 10 PL software (StatSoft). To investigate the relevance of differences between the tested groups, non-parametric tests were used for the qualitative variables – the chi-square test and Fisher’s exact test. Tests for proportions were used as well, with the significance level being assumed at p>0.05.
Results
Having taken into consideration the proper exclusion criteria (Table 1), out of a total of 455 patients, 246 with an average age of 40.08±11.12 qualified for the research (F=147, M=99).
Table 1.
Exclusion criteria regarding participation in the clinical research.
| Exclusion criteria |
| Aged 60 or over |
| Elevated body temperature, acute infection |
| Primary, congenital defects of the stomatognathic system |
| Neoplastic processes within the oral cavity |
| Pregnancy, breastfeeding |
| Mental retardation |
| Traumas and surgeries within the facial portion of the skull |
| Epilepsy |
On the basis of the audiological survey, OS were observed in 36.18% of participants (n=89) – group G1. To verify the reported otologic symptoms and to assess their source, the group underwent specialist examinations. Objectively-found audiological abnormalities were confirmed in 46.07% (n=41) of participants (group G1). A sensorineural hearing loss was found in 25 subjects, conductive hearing loss in 11, and mixed hearing loss in 5. The causes of hearing impairment determined on the basis of audiological criteria are presented in Figure 2. The assessment of the degree of hearing loss is presented in Figure 3.
Figure 2.
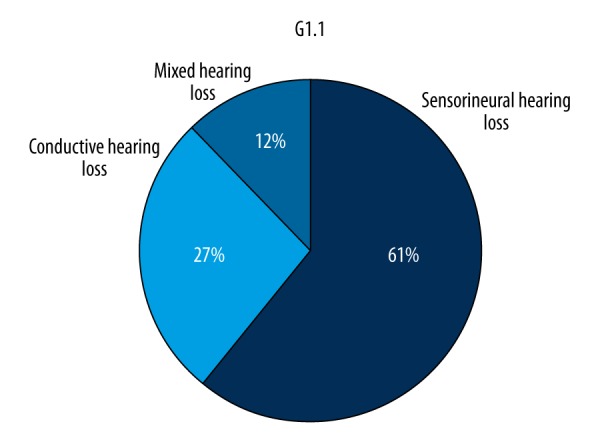
Diagnosed disorders of the organ of hearing in patients in subgroup G1.1.
Figure 3.
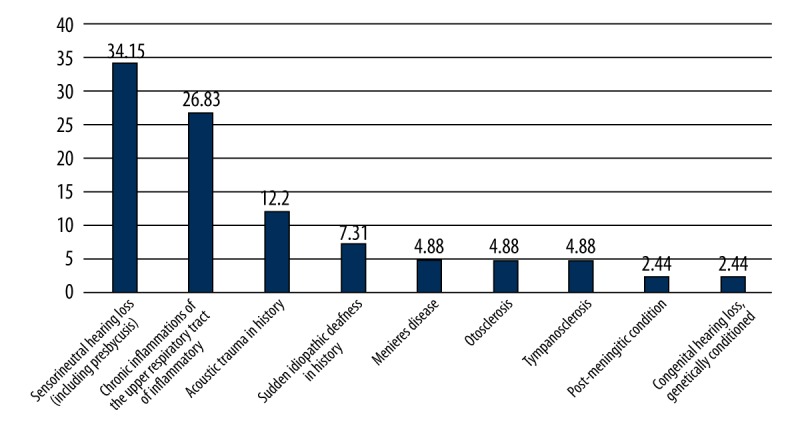
Causes of impairment of the organ of hearing in subgroup G1.1 (%).
The percentage distribution of OS in individual subgroups is presented in Table 2.
Table 2.
Percentage distribution of the frequency of otologic symptoms in subgroups and their statistical relevance, including p value (significance level p<0.05).
| Group | Otological symptom | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tinnitus | Ear fullness | Otalgia | Hypersensitivity to sounds | Hearing loss | ||||||
| G 1.1 (n=41) | n=15 | 36.59% | n=11 | 26.83% | n=18 | 43.90% | n=8 | 19.51% | n=30 | 73.17% |
| G 1.2 (n=48) | n=21 | 43.75% | n=33 | 68.75% | n=37 | 77.08% | n=18 | 37.50% | n=8 | 16.67% |
| p | p=0.478 | p=0.0001 | p=0.0012 | p=0.2206 | p=0.0000 | |||||
Statistically significant differences were found between the otologic symptoms in the 2 groups: ear fullness and otalgia were more frequent in G1.2, and progressive hearing loss was more frequent in G1.1.
The participants presented with a considerable variety and variability of symptoms regarding SS disturbances. Their type and frequency of occurrence were analyzed in the groups listed below (Table 3).
Table 3.
The most frequent symptoms of functional disturbances of SS in patients in individual groups and subgroups, including p value (significance level p<0.05).
| Symptom | G1 n=89 |
G2 n=157 |
p G1 vs. G2 |
G1.1 n=41 |
G1.2 n=48 |
p G1.1 vs. G1.2 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tenderness of SS muscles | 87 | 97.75% | 125 | 79.62% | p=0.0001 | 39 | 95.12% | 48 | 100% | p=0.1217 |
| Tooth wear | 71 | 79.78% | 129 | 82.17% | p=0.6441 | 26 | 63.41% | 45 | 93.75% | p=0.0004 |
| Impressions on tongue | 54 | 60.67% | 62 | 39.49% | p=0.0014 | 19 | 46.34% | 35 | 72.92% | p=0.0105 |
| Cracks of enamel | 50 | 56.18% | 74 | 47.13% | p=0.1727 | 17 | 41.46% | 33 | 68.75% | p=0.0097 |
| Tenderness of TMJ on palpation | 55 | 61.78% | 72 | 45.86% | p=0.0162 | 18 | 43.90% | 37 | 77.08% | p=0.0013 |
| Popping/clicking in TMJ | 47 | 52.81% | 118 | 75.16% | p=0.0003 | 21 | 51.22% | 26 | 54.17% | p=0.7813 |
| Masseter hypertrophy | 53 | 59.55% | 92 | 58.59% | p=0.8841 | 17 | 41.46% | 36 | 75% | p=0.0013 |
| S-shaped mandibular abduction path | 39 | 43.82% | 91 | 57.96% | p=0.0328 | 18 | 43.90% | 21 | 43.75% | p=0.9885 |
The normal condition of the organ of hearing was found in 48 patients (53.93% – subgroup G1.2). In these patients, the results of the audiological evaluation were within normal ranges: in pure tone audiometry, the average hearing threshold for the right ear was 12.52±5.16 decibels hearing level (dBHL), and for the left ear 10.70±6.37 (dBHL); tuning fork test results were normal, i.e., Weber’s was inside the head and Rinne’s was bilaterally positive; and in impedance audiometry there were A-type curves bilaterally as well as acoustic reflexes present for the frequencies 0.5, 1, 2, and 4 kHz.
Discussion
Many authors point to the fact that OS are concomitant in patients with SS dysfunctions [1,2,4,16–18]. The concomitance of both these disorders fluctuates within a wide range (2% to 82.4%) [19,20]. The literature includes infrequent reports of simultaneous otolaryngological and audiological evaluations of patients with SS disturbances in order to verify the reported OS [7]. In the present study, we performed diagnostic procedures that made it possible to reliably connect SS dysfunction symptoms with OS through an appropriate selection and standardization of the groups of studied patients.
We found otologic symptoms in more than one-third of the participants (G1=36.18%, n=89) (Figure 1). The frequency of the occurrence of OS with hearing impairment was 46.07% in the G1.1 subgroup (16.67% of the total number of the evaluated subjects). These patients were diagnosed with certain audiological disturbances (Figures 2, 3). In 48 patients (53.93% of the subjects with OS) no objective abnormalities were recorded in the audiological tests (G1.2), although all of them had subjective complaints. This suggests that there is a possibility of SS dysfunctions coexisting with impairments to the organ of hearing, as well as the necessity to distinguish the source of the troublesome symptoms [20]. In contrast, in 48 patients (53.93% – 19.51% of the total number of subjects), no objective abnormalities were recorded in the audiological tests (G1.2).
The most frequent OS in the G1.2 group was referred ear pain, i.e., otalgia (Table 2), which is the symptom often mentioned in the literature [2,17,20–23]. Kuttiula noted the occurrence of otalgia in 40–49% of patients with TMD [21] and De Felicio found this in almost 60% [20]. In the present study, the percentage was lower and amounted to 22.37% of the group as a whole (n=246). However, in the group with S (G1), the percentage was comparable and was 61.79% (n=89). The overall figure obtained of 22.37% may be the result of a more detailed interview as well as the audiological evaluation, in which no indicators of otitis were found in any of the cases. The excessive tenderness of the stomatognathic region, as well as the temporomandibular joint (TMJ) region, but not the ear, which was found significantly more often in the control group (G2) as compared to the G1 group (Table 3), points to the fact that otalgia may be of articulatory origin.
An equally frequent OS was ear fullness/ear discomfort – 17.89% (n=246); and 49.44% in group G1 (n=89) (Table 2). Only a few authors have analyzed this OS: Kuttiula et al. reported ear fullness in 5–9% of cases [21] and Kaygusuzi et al. reported this in 13.6% of cases [23]. Nevertheless, if the scope of the survey question is extended to include non-specific symptoms that are still located within the ear (ear fullness and/or ear discomfort, but differentiating and excluding pain), the percentage of reported OS is as high as 8.4% of the 4528 patients studied in Cooper’s research [19]. In the present research, ear fullness with otalgia but without hearing impairment were the symptoms that differentiated the subgroups G1.1 and G1.2 (p=0.0001 and p=0.0012, respectively) (Table 2). These results may confirm the anatomical relationships between the organ of hearing and the SS and they may also point to the transmission of tensions or overloads due to the closeness of their developmental origin.
Tinnitus was present in 14.63% (n=246) of cases, which is 40.45% of the reported OS in group G1. According to the literature, the range in tinnitus is wide. In an article by Tuz, out of 200 patients with SS disturbances, 155 patients reported OS, 91 of whom (45.4%) reported tinnitus [2]. In an analysis by Buergers et al., tinnitus was 8 times more frequent in patients with TMD (30 out of 82; 36.6%) than in the group without such disorders (38 out of 869; 4.4%) [24]; while De Felicio’s study found it in 74.4% of cases [20].
Hypersensitivity to sounds was reported by 10.57% (n=246) of patients, 19.51% in group G1 (Table 2). This is not surprising, taking into consideration the characteristic, usually emotional, nature of such patients [25].
It is worth mentioning that symptoms such as tinnitus or hypersensitivity to sounds were not differentiated for the groups with and without hearing impairment (p>0.05) (Table 2). These symptoms may often be conditioned by audiological, dental, and psychogenic factors [3,6].
Hearing impairment was reported in the audiological survey by 15.45% of the 246 patients (42.70% of group G1) (Figure 3, Table 2). However, verification by means of the audiological evaluation revealed normal hearing thresholds for 48 patients in group G1.2 (Figure 1). The subjective sensation of deteriorated hearing in this group may result from the discomfort of the preauricular and articulatory region, as well as from concomitant complaints such as ear fullness/ear plugging. Similar observations were made by De Felicio [20].
It should be emphasized that in the group of patients with TMD, the differentiating factor is hearing loss. It is more often considered to be connected with certain otologic disorders than with functional disturbances of the SS (p=0.0000, Table 2). Therefore, in questionable cases, the origin of OS should be determined by means of a hearing test.
The literature points to the fact that patients with TMD and concomitant OS present with greater tenderness on palpation, as well as increased soreness, as compared with patients with SS disturbances but not suffering from OS [1,2,5,16,17]. In this research, the presence of OS in the G1.2 subgroup was related to the greater frequency of dental symptoms such as impressions on the tongue, cracks in the enamel, tenderness of the TMJ, tooth wear, and masseter hypertrophy (Table 3). Therefore, OS may suggest a greater intensity of symptoms connected with the masticatory system and more advanced changes in comparison with the control group. In contrast, clicking and a disturbed path of mandibular abduction were significantly more frequent in those cases of functional disturbances of the TMJ without OS (G2). It is possible that the presence of OS may indicate a different degree in the intensity of changes, a different mechanism leading to SS dysfunction, or an individual adaptive capacity of the SS.
The studied group presented mainly with a slight degree of hearing loss, both conductive (connected with lesions in the middle ear – post-inflammatory lesions, allergic lesions – along with disturbances in the function of the eustachian tubes) and sensorineural, resulting from damage to the inner ear (Figures 2, 4). Slight hearing loss is related to aging, the so-called presbycusis. This phenomenon manifests itself through disturbances in hearing and understanding speech as a result of involutional changes during the aging process, and is observed mostly in people over 65 years of age. However, the aging process in the organ of hearing is not always identical and is characterized by considerable individual variability [26].
Figure 4.
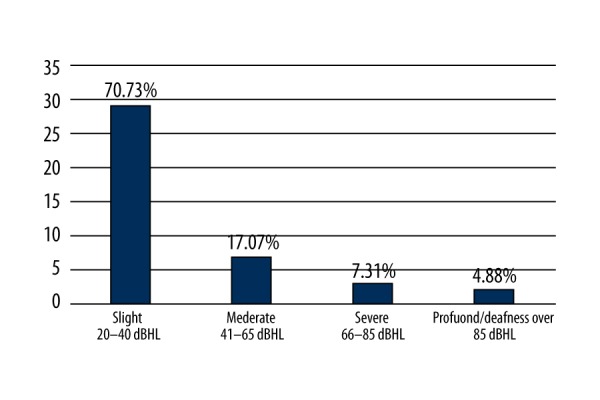
The degree of hearing loss in group G1.1 (n=41).
Slight high- or low-frequency hearing loss may remain unnoticed in its early stages in everyday life since it does not significantly influence the understanding of speech. Thus, it only tends to be revealed by means of specialist hearing tests. Nevertheless, any existing impairment to the organ of hearing may lead to tinnitus and discomfort, as well as ear fullness. Moreover, disturbances in muscular activity and pathological changes within the TMJ may at first develop imperceptibly, the only manifestations being OS. Therefore, patients are not usually aware of them or they wrongly attribute the observed symptoms to other diseases, which is reflected both in our material and in the relevant literature [6]. There were various reasons for the hearing loss (Figure 3). It is worth noting that many of them can be treated by drugs or by using a hearing aid. As a result, OS in patients with SS can be verified after audiological/laryngological consultation.
Due to the untypical radiating localization of pain symptoms in the course of TMD, patients do not associate them with symptoms related to the abnormal functions of the SS and tend to seek the help of other specialists rather than a dentist (e.g., otorhinolaryngologists, audiologists, neurologists, psychiatrists or ophthalmologists) [6]. It is estimated that every day, on average, 2–3 patients visiting an audiological-laryngological practice are patients with OS concomitant with functional disturbances of the SS, but without objective indicators of changes within the external ear, and without any impairment of the organ of hearing in audiological evaluations. Kent, on the basis of his longstanding experience from his own otolaryngological practice, reports that patients with OS concomitant with SS complaints spend, on average, approximately 6 years looking for effective medical (i.e., dental) assistance [16]. This suggests that SS dysfunctions are often overlooked in general diagnoses, and the frequency of their occurrence in the population is underestimated.
Conclusions
The otologic symptoms which are most frequently concomitant with TMD are plugging and fullness sensations in the ear, as well as otalgia.
It is recommended that a subjective assessment of hearing loss in patients with TMD and concomitant OS should be included in the medical history stage. Hearing loss may be related to objective hearing impairment and produce otologic symptoms unrelated to the condition of the masticatory system.
Dental examination proves that clicking and popping are significantly more frequent in patients without concomitant hearing impairment.
Footnotes
Conflicts of interest
None.
Source of support: Self-financed
References
- 1.Ferendiuk E, Zajdel K, Pihut M. Incidence of otolaryngological symptoms in patients with temporomandibular joint dysfunctions. Bimed Res Int. 2014;(2):824684. doi: 10.1155/2014/824684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuz HH, Onder EM, Kisnisci R. Prevalence of otologic complaints in patients with temporomandibular disorder. Am J Orthod Dentofacial Orthop. 2007;6:620–23. doi: 10.1016/s0889-5406(03)00153-7. [DOI] [PubMed] [Google Scholar]
- 3.Maciejewska-Szaniec Z, Maciejewska B, Mehr K, et al. The otological symptoms among patients with the temporomandibular disorders. Forum Med Rodz. 2015;2:85–87. [Google Scholar]
- 4.Camparis CM, Formigoni G, Teixeira MJ, de Siqueira JTT. Clinical evaluation of tinnitus in patients with sleep bruxism: Prevalence and characteristics. J Oral Rehabil. 2005;32:808–14. doi: 10.1111/j.1365-2842.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 5.Hilgenberg PB, Saldanha DD, Cunha CO, et al. Temporomandibular disorders, otologic symptoms and depression levels in tinnitus patients. J Oral Rehabil. 2012;39:239–44. doi: 10.1111/j.1365-2842.2011.02266.x. [DOI] [PubMed] [Google Scholar]
- 6.Okeson JP. Management of temporomandibular disorders and occlusion. 7th ed. Elsevier; 2013. [Google Scholar]
- 7.Sobhy OA, Koutb AR, Abdel-Baki FA, et al. Evaluation of aural manifestarion in temporo-mandibular joint dysfunction. Clin Otolaryngol. 2004;29:382–85. doi: 10.1111/j.1365-2273.2004.00842.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez ALM, Sandoval OGP, Ballesteros LE. Theories on otic symptoms in TMD: Past and present. Int J Morphol. 2005;23(2):141–56. [Google Scholar]
- 9.Michalak M, Wysokińska-Miszczuk J, Wilczak M, et al. Correlation between eye and ear symptoms and lack of teeth, bruxism and other parafunctions in population of 1006 patients in 2003–2008. Arch Med Sci. 2012;8(1):104–10. doi: 10.5114/aoms.2012.27289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totta T, Santiago G, Goncales ES, et al. Auditory characteristic of individuals with temporomandibular dysfunctions and dentofacial deformities. Dental Press J Orthod. 2013;18(5):70–77. doi: 10.1590/s2176-94512013000500013. [DOI] [PubMed] [Google Scholar]
- 11.Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study – the OPPERA study. J Pain. 2011;12(11):T4–T11.e2. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohrbach R. Research diagnostic criteria for temporomandibular disorder. Clinical protocol and assessment instruments. International Consortium Network [Version 3Dec2014] Available from: URL: http://www.rdc-tmdinternational.org.
- 13.Katz J, Burkard RF, Medwetsky L. Handbook of clinical audiology. 7th ed. Baltimore: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 14.Hall JW. ABR Parametres, protocols and procedures. In: Hall JW, editor. New Handbook for Auditory Evoked Responses. 1st ed. Pearson; 2007. pp. 171–211. [Google Scholar]
- 15.Robinette MS, Glattke TJ. Otoacoustic emissions: Clinical applications. 3rd ed. Stuttgart (NY): Thieme; 2011. [Google Scholar]
- 16.Kent W, Cox MD. Temporomandibular disorder and new aural symptoms. Arch Otolaryngol Head Neck Surg. 2008;134(4):389–93. doi: 10.1001/archotol.134.4.389. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez ALM, Ballesteros LE, Sandoval GP. Topical review: Temporomandibular disorders in an integral otic symptom model. Int J Audiol. 2008;47(4):215–27. doi: 10.1080/14992020701843137. [DOI] [PubMed] [Google Scholar]
- 18.Riga M, Xenellis, Peraki E, et al. Aural symptoms in patients with temporomandibular joint disorders: multiple frequency tympanometry provides objective evidence of changes in middle ear impedance. Otol Neurotol. 2010;31(9):1359–64. doi: 10.1097/MAO.0b013e3181edb703. [DOI] [PubMed] [Google Scholar]
- 19.Cooper BC, Kleinberg I. Examination of large patient population for the presence of symptoms and signs of temporomandibular disorders. Cranio. 2007;25(2):114–26. doi: 10.1179/crn.2007.018. [DOI] [PubMed] [Google Scholar]
- 20.De Felicio CM, PimentaF CL, De Oliveira M. Otologic symptoms of temporomandibular disorder and effect of orofacial myofunctional therapy. J Craniomandibular Pract. 2008;26(2):118–25. doi: 10.1179/crn.2008.016. [DOI] [PubMed] [Google Scholar]
- 21.Kuttila S, Kuttila M, Le Bell Y, et al. Aural symptoms and signs of temporomandibular disorder in association with treatment need and visits to a physician. Laryngoscope. 1999;109(10):1669–73. doi: 10.1097/00005537-199910000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Wright EF. Otologic symptom improvement through TMD therapy. Quintessence Int. 2007;38(9):564–71. [PubMed] [Google Scholar]
- 23.Kaygusuz I, Karlidag T, Keles E, et al. Ear symptoms accompanying temporomandibular joint diseases. Kulak Bueun Bogaz Ihtis Derg. 2006;16(5):205–8. [PubMed] [Google Scholar]
- 24.Buergers R, Kleinjung T, Behr M, Vielsmeier V. Is there a link between tinnitus and temporomandibular disorders? J Prosthet Dent. 2014;111(3):222–27. doi: 10.1016/j.prosdent.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Fillingim RB, Ohrbach R, Greenspan JD, et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amstrong D, Stoney P, Hawke H, Farkashidy J. Presybacusis: Correlations of clinical audiology with morphological changes in the cochlea and ventral cochlear nocleus. J Otolaryngol. 1992;21(5):343–48. [PubMed] [Google Scholar]


