Abstract
Background
The present study aimed to investigate the effects of Scutellarin on proliferation, invasion and tumorigenicity in human breast carcinoma MCF-7 cells and its associated molecular mechanisms.
Material/Methods
The MCF-7 cells were cultured with varies of concentrations of Scutellarin in vitro. The proliferation, invasion, and apoptosis of MCF-7 cells were studied via CCK-8 assay, transwell assay, and flow cytometry. In vivo expression of the HIPPO pathway key proteins YAP and p-YAP of MCF-7 cells were analyzed by immunohistochemistry.
Results
The inhibition rates of Scutellarin-treated MCF-7 cells were 40.1%, 58.7%, and 70.6% for 24, 48, and 72 h, respectively. The MCF-7 cell proliferation was significantly inhibited by Scutellarin. Treating MCF-7 cells with Scutellarin led to invasion inhibition. The rates apoptotic cells were between 12.4±1.9% and 23.9±2.1% in 40–120 μM Scutellarin-administrated groups, which had a significant rise compared with the control group (7.8±1.9%, P<0.05). Scutellarin significantly inhibited MCF-7 xenograft tumor growth. Immunohistochemical analysis showed that the inhibition of tumor growth in Scutellarin-treated mice was associated with increased p-YAP and decreased YAP expression in vivo.
Conclusions
Scutellarin-treated breast carcinoma MCF-7 cells had significantly inhibited growth and induced apoptosis, which is associated with induction of autophagy through regulation of the HIPPO-YAP signaling pathway, providing support to the clinical use of Scutellarin-based medication to achieve optimized outcome in patients with breast carcinoma.
MeSH Keywords: Apoptosis, Breast Neoplasms, Cell Proliferation, Neoplasm Invasiveness
Background
Breast cancer is one the most common female cancers worldwide [1]. Multi-drug resistance has been shown in clinical trials, and acquired multi-drug resistance severely limits effective therapies for breast cancer [2]. Recently, numerous phytochemicals compounds and active alkaloids have been reported to have anticancer activity [3–5].
The HIPPO/YAP pathway is important in regulating overall tissue proliferation and growth with loss of proper control associated with oncogenesis [6]. The HIPPO signal pathway is a highly-conserved growth control signal pathway, which has a critical regulatory effect on cell proliferation and apoptosis; the regulatory effect of HIPPO signal is down-regulated in numerous human tumors, suggesting its close relationship with tumor genesis [7,8].
Scutellarin, an effective component found in the traditional medicinal herb Scutellaria barbata, has many pharmacological activities and is confirmed to have anti-inflammatory, anti-virus, anti-fibrotic, and immune regulation effects [9,10]. Recently, Scutellarin has been shown to have antitumor effects in cancer cells, such as hepatocellular carcinoma, tongue squamous cell carcinoma, and colorectal cancer [11–14]. Many studies have indicated that Scutellarin inhibits the proliferation and transfer of tumor cells, inducing apoptosis and differentiation but there are few reports on the anti-breast cancer mechanisms of Scutellarin. To explore the basis of the development of Scutellarin, we investigated effects of Scutellarin on MCF-7 human breast cancer cells in culture for proliferation, invasion, and tumorigenesis effects of Scutellarin on protein expression of the HIPPO-YAP signaling pathway.
Material and Methods
Cell culture and reagents
MCF-7 human breast cancer cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MCF-7 cells were cultured in RPMI-1640 medium (HyClone, Logan, USA). The media were supplemented with 10% fetal bovine serum, 100 μg/ml ampicillin, and 100 μg/ml streptomycin.
Cell viability assay
For the viability assays, cells were seeded in 96-well plates at 1×104 cells per well. For short-term treatments, selected drugs, compounds, and extracts were added to the wells immediately afterwards and cultured with the cells for 24 h. For long-term treatments, fresh medium with reagents were added every 24 h. The cell viability was measured by fluorescence chemistry with a spectrophotometer (Thermo, Multiscan MK3, USA) using the CCK8 kit (Lianke Bio, China) at OD 405 nm. Cell viability was calculated from the OD values in comparison with the blank. The inhibition rate was calculated by the following formula: inhibition rate (%)=100*(ODctrl–ODtreated)/ODctrl. Each treatment condition was repeated 4 times with at least 3 repeated wells each time.
Invasion assay
Cell invasion were determined using Transwell cell Matrigel invasion chambers (Matrigel-coated membrane, BD Biosciences, San Jose, CA, USA) as previously described [20]. Briefly, cells (2×103) were resuspended in 200 μl of serum-free medium and seeded on the top chamber of the 8.0-μm pore, 6.5-mm polycarbonate transwell filters (Corning, NY, USA). The full medium (600 μl) containing 10% FBS was added to the bottom chamber. The cells were allowed to migrate for 24 h at 37°C in a humidified incubator with 5% CO2. After 24 h, the cells that had invaded through the membrane and adhered to the underside of the membrane were counted in 4 randomly selected microscopic fields.
Colony formation assay
For colony formation, human breast cancer MCF-7 cells were seeded (500 cells/well) in 6-well plates overnight. After 10~14 days, the culture medium was removed, and cells were briefly rinsed with PBS. The cells were then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and colonies were counted by visual inspection.
Flow cytometry analysis
Freshly isolated MCF-7 cells from the tumor mass were collected following tissue sectioning and digestion by trypsin, and were subjected to flow cytometry by use of an apoptosis analysis kit (Beckman Coulter, USA) based on the manufacturer’s instructions. Flow cytometry analysis was performed immediately afterwards (FACSAriaTM, BD Biosciences, USA).
Western blot analysis
Total protein was extracted from the cultured cells, and quantitated using the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA) as previously described [21]. Equal amounts of protein from different samples were separated through 10% SDS-polyacrylate gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Blocking buffer, containing 5% non-fat milk, was used to block and incubate the membranes with primary antibodies overnight at 4°C. The expression of the target proteins was examined using an enhance chemiluminescence (ECL) kit (Amersham Pharmacia Biotech, Uppsala, Sweden).
Xenograft experiment
The BALB/c nude mice were purchased from the Shanghai Laboratory Animal Center (Chinese Academy of Sciences, Shanghai, People’s Republic of China). The animal experimental protocols were approved by the Animal Care Committee of the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences, as well as the Tongji University Shanghai Tenth People’s Hospital, Medical School, Tongji University. Under constant temperature and humidity, female athymic nude mice (5 weeks old) were maintained in laminar flow rooms. In our experiments, MCF-7 cells were subcutaneously injected into one flank of 5 mice. The MCF-7 cells were collected by centrifugation and resuspended in PBS with the concentration of 1×107/ml. We seeded 0.2 ml of suspension containing 2×106 cells to the auxiliary region of each mouse.
When tumors showed, mice were randomly assigned into 4 groups, with 3 mice in each group: (1) the control group was injected with saline at the same volume as that used for the combined group; (2) the 5-FU group was injected intraperitoneally (IP) once weekly with 25 mg/kg 5-fluoro-2,4 (1 h and 3 h) pyrimidinedione; (3) the Scutellarin group was injected IP once every other day with 5 mg/kg Scutellarin; and (4) the Scutellarin and 5-FU combination group was injected IP once every other day with 200 mg/kg Scutellarin and injected IP with 25 mg/kg 5-FU hydrochloride once a week. Weights of mice were recorded daily prior to drug administration. After 3 weeks of treatment, the mice were killed on day 22 by cervical luxation. The inhibition rates by different treatments were calculated based on the weight of the tumor masses based on following formula: inhibition (%)=100*(Weight ctrl–Weight treated)/Weight ctrl.
Immunohistochemistry
Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 4 mm sections, then subjected to hematoxylin-eosin (HE) staining or immunohistochemistry (IHC). For IHC, tissue sections were incubated with anti-YAP or anti-p-YAP antibodies (both from Cell Signaling Technology, USA). The positive signals were amplified by a biotinylated peroxidase-conjugated streptavidin system (Bio-Genex Laboratories, USA). All specimens were observed and photographed (BX51T-32H01, Olympus, Japan).
Statistical analysis
Each in vitro experiment was performed at least 3 times with multiple repeats, and the data were analyzed by SPSS (v13.0). The statistical significance was calculated by one-way ANOVA to compare multiple groups, or by Student’s t-test when only 2 groups were compared. P<0.05 was considered significant and P<0.01 was considered highly significant.
Results
Scutellarin inhibited human breast cancer MCF-7 cells proliferation in vitro
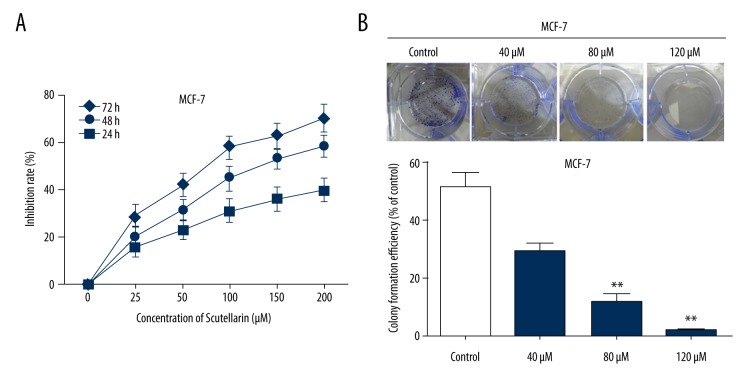
After Scutellarin treatment for 72 h, the CCK-8 assay showed that growth inhibition rates of MCF-7 were significantly elevated compared with MCF-7 treated with DMSO or the untreated group at 24 h, 48 h, and 72 h (Figure 1A). In addition, the total numbers of non-adherent sphere cells in the Scutellarin-treated group were calculated at different points in time (3, 7, and 10 days). We found that the total numbers of non-adherent sphere cell in the Scutellarin-treated group were obviously reduced compared to the control (DMSO-treated) group at each time point (Figure 1B).
Figure 1.
Scutellarin cytotoxicity in MCF-7 cells. (A) Cells were treated with various concentrations of Scutellarin and incubated for 24, 48, and 72 h. Scutellarin cytotoxicity was detected using CCK-8 assay, shown by absorbance at 450 nm. (B) The colony formation assay results showed that the colony formation rate of MCF-7 cells treated with Scutellarin was significantly lower than in the Control group. Data represent the mean ±SEM of 3 independent experiments. ** P<0.01 versus control.
Scutellarin suppressed invasion of human breast cancer MCF-7 cells in vitro
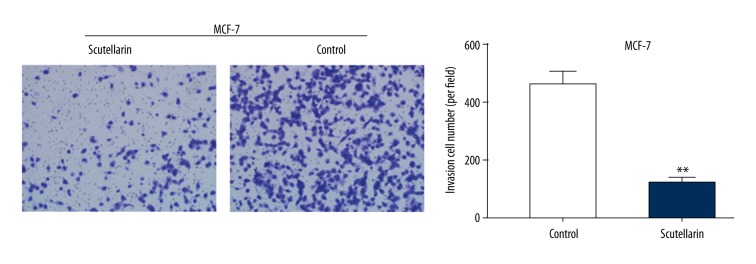
The transwell migration assay showed that there were fewer invading MCF-7 cells per well in the Scutellarin group than in the DMSO-treated group or the untreated group (Figure 2). These data indicate that Scutellarin suppressed the proliferation and invasion capabilities of human breast cancer MCF-7 cells.
Figure 2.
Scutellarin impaired the invasion ability of human breast cancer MCF-7 cells. MCF-7 cells were cultured with moderate Scutellarin concentrations (80 μM) of Scutellarin in the upper Transwell chambers. Following 16 h of incubation, Matrigel was coated on the Transwell chambers. After 24 h, the invaded cells were photographed (×200) and quantified by a microplate reader. All data are represented as mean ±SD of 3 independent experiments. ** P<0.01, Scutellarin-treated group compared with the untreated control group.
Scutellarin-induced cell apoptosis of human breast cancer MCF-7 cells in vitro
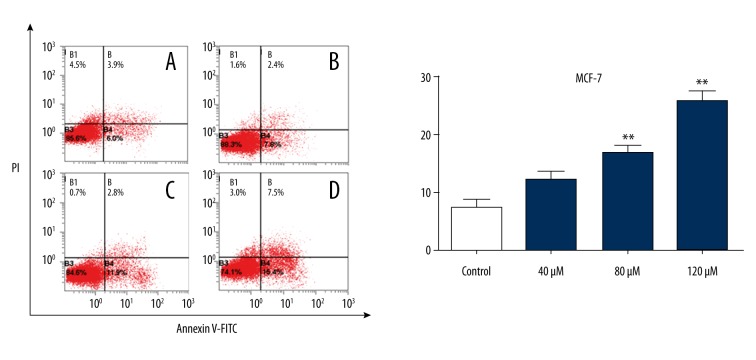
We analyzed the change in apoptotic rate in Scutellarin-treated human breast cancer MCF-7 cells. Flow cytometry was carried out to detect the apoptotic cells induced by Scutellarin after co-staining of annexin V-FITC and PI (Figure 3). After treatment for 24 h, 40–80 μM Scutellarin did not induce a significant increase of apoptotic cells. After cells were treated with 120 μM Scutellarin, cell apoptosis was significantly increased. Apoptotic rates analyzed by morphological observation and flow cytometry were comparable. Figure 4 shows that the apoptotic rate of human breast cancer MCF-7 cells from the control group was 7.8±1.9% and increased to 23.9±2.1% after being treated with Scutellarin (40, 80, and 120 μM).
Figure 3.
Apoptosis of MCF-7 cells were analyzed using flow cytometry assay using FITC-Annexin-V/PI staining. Scutellarin induced the apoptosis of MCF-7 cells: (A) Control without Scutellarin treatment; (B) 40 μM, (C) 80 μM, (D) 120 μM Scutellarin treatment.
Figure 4.
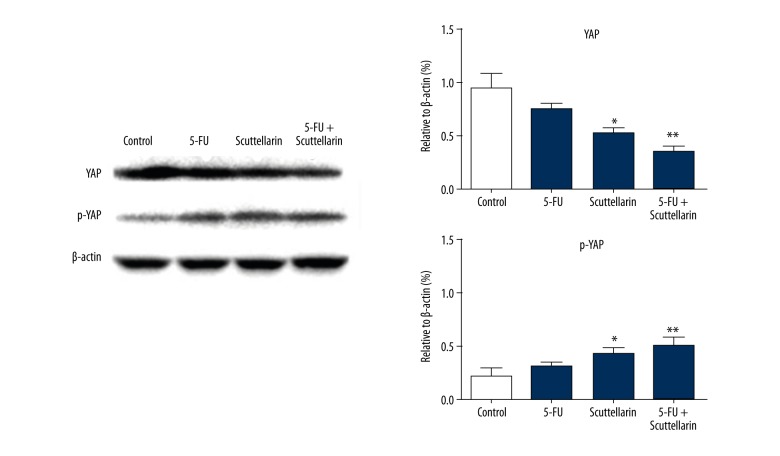
Scutellarin regulates the expression of YAP and phosphorylation of YAP in MCF-7 cells.
Scutellarin regulated the expression of YAP and p-YAP in human breast cancer MCF-7 cells
Western blotting confirmed these observations by demonstrating that p-YAP in Scutellarin-treated MCF-7 cells was up-regulated compared with the control group, while the level of YAP was down-regulated, which was consistent with experiment results of IHC.
Scutellarin inhibited human breast cancer MCF-7 cells xenograft growth
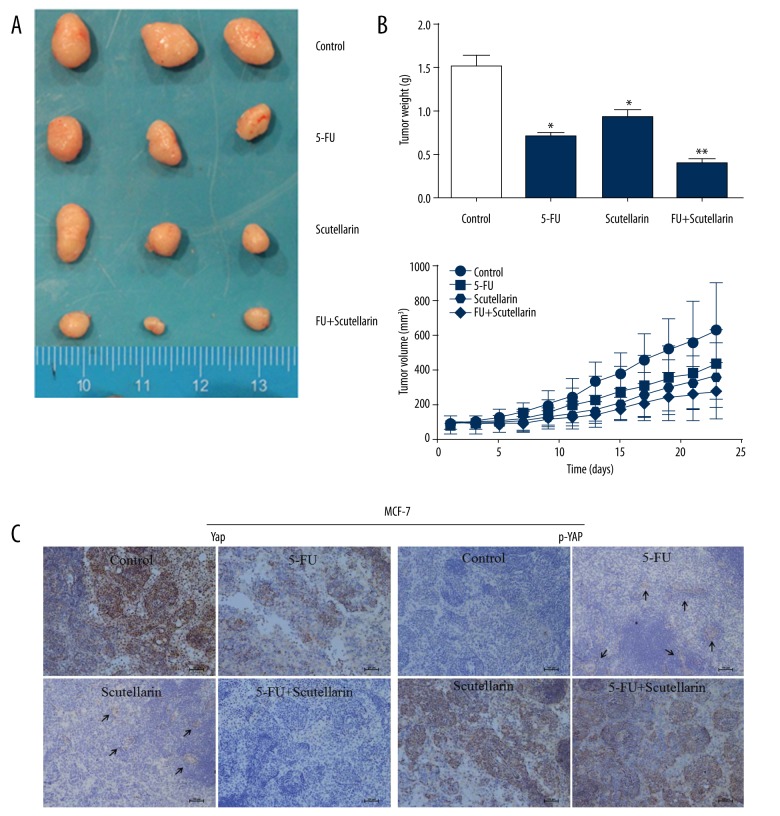
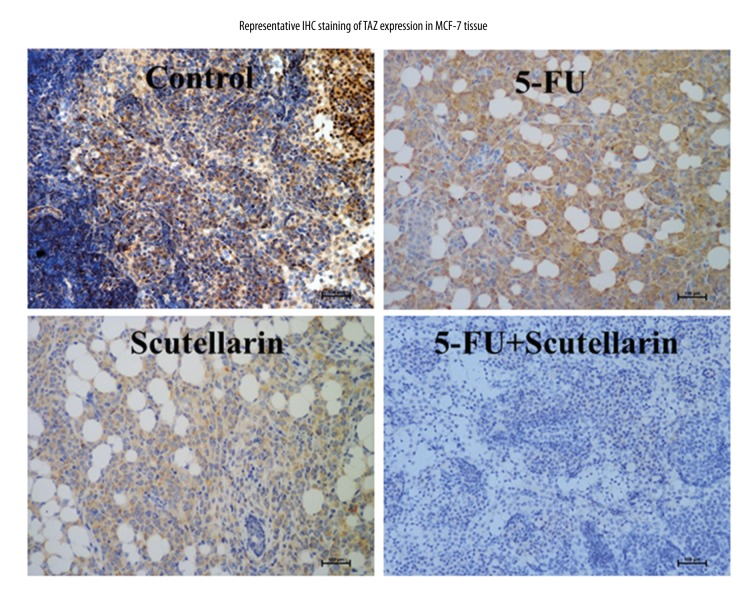
For the in vivo and mechanistic studies, the human breast cancer MCF-7 cells were inoculated subcutaneously into the BALB/c nude mice, and treated with PBS, Scutellarin, 5-FU or combination therapy, respectively. As shown in Figure 5A, in the Scutellarin-treated group, the tumor volumes were substantially reduced compared to those in saline treated group. Although mice in both groups eventually developed tumors, those formed in the Scutellarin-treated group grew more slowly than those formed in the saline -treated group (Figure 5B). In Figure 5A, after 10 days of continuous Scutellarin treatment at 200 mg/kg in mice, the human breast cancer MCF-7 tumor mass was significantly decreased. The inhibition rate was 32.4%, comparable with 25 mg/kg 5-FU treatment (35.4%). There was no significant difference between the 5-FU group and the Scutellarin groups at different doses. Tumors from to the combination group were significantly lighter than those from Scutellarin/5-FU-treated group (Figure 5B). Moreover, P-YAP and YAP were detected by IHC, shown in Figure 5C. In MCF-7 tumors with saline treatment, 81% samples were YAP positive and 7% samples were P-YAP positive. After treatment by Scutellarin or 5-FU, the YAP positive rate dropped to 33.5% and 18.5%, respectively, while P-YAP positive rate increased to 64.6%. The changes were comparable with the saline treated group. In summary, xenografts formed by MCF-7 cells treated with Scutellarin were smaller, with reduced proliferation and invasion capacities. Moreover, we also explored the effect of Scutellarin on other components of HIPPO signaling pathway, such as TAZ (Figure 6).
Figure 5.
Effect of Scutellarin on xenografted MCF-7 tumor growth in nude mice. (A) Representative tumors from mice from Control, 5-U, Scutellarin and Combination groups. (B) Growth curve of MCF-7 tumors indicating means and the standard error. * P<0.05, ** P<0.01 vs. control. (C) Immunohistochemistry staining of p-YAP and YAP in xenografts tissue. Under the magnification ×200 microscope field, Expression levels of p-YAP were up-regulated in Scutellarin-treated and Combination groups, while YAP was lower in Scutellarin-treated groups than in the control. Bar, 100 μm.
Figure 6.
Immunohistochemistry staining of TAZ in xenografts tissue. Under the magnification ×200 microscope field, Expression levels of TAZ were down-regulated in Scutellarin-treated and combination groups. Bar, 100 μm.
Discussion
Recently, many anti-cancer drugs are derived from natural resources [15]. Many traditional plant medicines have shown potential antitumor effects. Scutellarin is kinds of glycoside components found in the Scutellaria barbata, which is considered have anti-inflammatory and anti-virus effects in Chinese traditional medicine.
Scutellaria barbata is a plant that belongs to the Labiatae (mint) family and has anti-inflammatory, anti-virus, anti-fibrotic and immune regulation properties, relieving swelling and pain, and inducing diuresis and hemostasis. It is commonly used in treating sore throat, ulcerative carbuncle, snake bite, and edema. The formulation of Scutellaria barbata with other herbal medicine can better relieve all kinds of cancers, such as pancreatic cancer, gastric cancer, rectal cancer, lung cancer and gynecological tumors [6,7]
In this study, we demonstrated that Scutellarin inhibits proliferation, invasion, and induce apoptosis in human breast cancer MCF-7 cells in vitro. Induction of apoptosis is accompanied by increased P-YAP level and decreased YAP level. Other studies have been shown that Scutellarin reduces the expression of MMP-2 and -9, and integrin αvβ6 in human tongue squamous carcinoma (SAS) cells, possibly through the regulation of expression of transcription factor AP-1 [10]. Numerous studies have shown that Scutellarin can suppress the growth of human colorectal cancer cells and tumor xenografts in vivo through cell cycle arrest and induction of apoptosis [11]. We also observed Scutellarin-induced DNA damage and STAT3 expression and this is in agreement with other reports demonstrating that the STAT3 signal pathway is required for Scutellarin-mediated apoptosis [8].
In this study, cell viability was measured using the CCK-8 assay kit. The growth of MCF-7 cells was significantly inhibited in a dose- and time-dependent manner when treated with Scutellarin. The result is consistent with a previous study [16]. Thus, we speculate that Scutellarin inhibits the proliferation of MCF-7 cells via cytoplasm and nucleic acid metabolism of breast cancer cells in vitro.
It has also been verified in previous research that Scutellarin can effectively inhibit the growth and survival of multiple cancer cells and induces cell apoptosis, but effects of Scutellarin on the tumor-associated signal pathway have not been reported yet. The HIPPO-YAP is a signal pathway that was extensively investigated in recent years; it plays a key role in regulating cell proliferation and apoptosis, as well as controlling organ size. YAP is a transcriptional co-activator discovered in fruit flies by Sudol et al. in 1994 [17]. The HIPPO/YAP pathway is a signal transduction pathway controlling organ size and development, as well as tumor formation. The key link of the pathway in mammals is a kind of transcription activator YAP yes-associated protein located downstream of HIPPO [7]. The large tumor suppressor 1 (LATS1) in the HIPPO pathway restricts it within the cytoplasm through the phosphorylation of YAP, while the dephosphorylated YAP is transferred to the cell nucleus. YAP binds with a series of transcription factors (including apoptosis negative regulatory factors and mitosis-promoting factors) after it enters into the cell nucleus, and eventually inhibits cell apoptosis, promotes cell proliferation, and enlarges organs. Therefore, the HIPPO-YAP pathway controls YAP through phosphorylation, which gives rise to balanced cell proliferation and apoptosis, and thus regulates development of organ size [8].
Because the HIPPO pathway, especially the downstream effector protein YAP/TAZ, plays an extremely important role in carcinogenesis, drugs targeting the HIPPO pathway are of important clinical significance. The HIPPO signal pathway was discovered in research on fruit flies in recent years, which is constituted by the core components, as well as upstream and downstream components, and it is a highly-conserved pathway that is involved in regulating organ size, cell cycle, and apoptosis. As a downstream transcriptional activator, YAP plays a crucial role in tumor derivation. YAP is highly expressed in human liver cancer cells, is closely related to hepatocarcinogenesis, and has oncogene characteristics [18]. The HIPPO-YAP pathway controls YAP through phosphorylation, and more YAP proteins can be retained in cytoplasm only through enhancing YAP phosphorylation level, which gives rise to reduced YAP entering into the cell nucleus, thus achieving the objectives of promoting cell apoptosis and inhibiting cell proliferation. YAP expression is up-regulated in multiple tumors, such as gastric cancer, esophageal cancer, lung cancer, and liver cancer, and excessively-expressed YAP promotes liver tissue hyperplasia and eventually develops into hepatocellular carcinoma. Survival analysis of YAP expression level and patient survival indicates that YAP is an independent prognostic factor for patients with hepatocellular carcinoma, and that YAP expression level is related to the differentiation of hepatocellular carcinoma [19].
Conclusions
In conclusion, our results indicate that Scutellarin inhibits viability and promotes apoptosis of breast cancer MCF-7 cells via regulating the expression of the HIPPO-YAP signaling pathway. Scutellarin also inhibits the proliferation of MCF-7 cells in vitro, although only 1 breast cancer MCF-7 cell line was investigated in the present study. These findings suggest that Scutellarin has potential to be developed as a new natural anti-breast carcinoma agent, which could be used as an alternative to other treatments or in a combined regimen against breast carcinoma.
Footnotes
Conflict of interests
None.
Source of support: This study was supported by a grant from the National Natural Science Foundation of China (No. 81272240)
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–19. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vtorushin SV, Khristenko KY, Zavyalova MV, et al. The phenomenon of multi-drug resistance in the treatment of malignant tumors. Exp Oncol. 2014;36(3):144–56. [PubMed] [Google Scholar]
- 3.Cao DH, Jiang J, Tsukamoto T, et al. Canolol inhibits gastric tumors initiation and progression through COX-2/PGE2 pathway in K19-C2mE transgenic mice. PLoS One. 2015;10(3):e0120938. doi: 10.1371/journal.pone.0120938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Li PP, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2017;8(8):13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y, Ma DC, Wang HL, et al. Matrine suppresses the ER-positive MCF cells by regulating energy metabolism and endoplasmic reticulum stress signaling pathway. Phytother Res. 2017;31(4):671–79. doi: 10.1002/ptr.5785. [DOI] [PubMed] [Google Scholar]
- 6.Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63(6):1491–501. doi: 10.1016/j.jhep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20(6):638–46. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2727–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thao do T, Phuong do T, Hanh TT, et al. Two new neoclerodane diterpenoids from Scutellaria barbata D.Don growing in Vietnam. J Asian Nat Prod Res. 2014;16(4):364–69. doi: 10.1080/10286020.2014.882912. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Cai Q, Lin J, et al. Chloroform fraction of Scutellaria barbata D. Don promotes apoptosis and suppresses proliferation in human colon cancer cells. Mol Med Rep. 2014;9(2):701–6. doi: 10.3892/mmr.2013.1864. [DOI] [PubMed] [Google Scholar]
- 11.Xu HT, Zhang SY. Scutellarin-induced apoptosis in HepG2 hepatocellular carcinoma cells via a STAT3 Pathway. Phytother Res. 2013;27(10):1524–28. doi: 10.1002/ptr.4892. [DOI] [PubMed] [Google Scholar]
- 12.Chan JY, Tan BK, Lee SC. Scutellarin sensitizes drug-evoked colon cancer cell apoptosis through enhanced caspase-6 activation. Anticancer Res. 2009;29(8):3043–47. [PubMed] [Google Scholar]
- 13.Li HX, Huang DY, Gao ZXZ, et al. Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int J Oncol. 2013;42(5):1674–81. doi: 10.3892/ijo.2013.1873. [DOI] [PubMed] [Google Scholar]
- 14.Yang N, Zhao YY, Wang ZP, et al. Scutellarin suppresses growth and causes apoptosis of human colorectal cancer cells by regulating the p53 pathway. Mol Med Rep. 2017;15(2):929–35. doi: 10.3892/mmr.2016.6081. [DOI] [PubMed] [Google Scholar]
- 15.Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CZ, Li XL, Wang QF, et al. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17(1):63–68. doi: 10.1016/j.phymed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn BH, Shim JJ, Kim SB. Inactivation of hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22(5):1256–64. doi: 10.1158/1078-0432.CCR-15-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115(19):4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu F, Lv ML, Li D, et al. MiR-506 over-expression inhibits proliferation and metastasis of breast cancer cells. Med Sci Monit. 2015;21:1687–92. doi: 10.12659/MSM.893522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo T, Yue SB, Tian H, et al. Effect of Fu-Zheng-Xiao-Liu granules on expression of human epidermal growth factor receptor 2 (HER-2) and proliferation and apoptosis of breast cancer cell line SKBR-3. Med Sci Monit. 2016;22:5068–73. doi: 10.12659/MSM.898685. [DOI] [PMC free article] [PubMed] [Google Scholar]