Abstract
Preeclampsia remains a leading cause of maternal and fetal mortality, due to ineffective treatment and diagnostic strategies, compounded by the lack of clarity on the etiology of the disorder. Although several clinical and biological markers of preeclampsia have been evaluated, they have proven to be ineffective in providing a definitive diagnosis during the various stages of the disorder. Exosomes have emerged as ideal biomarkers of pathological states, such as cancer, and have more recently gained interest in pregnancy-related complications, due to their role in cellular communication in normal and complicated pregnancies. This occurs as a result of the specific placenta-derived exosomal molecular cargo, which may be involved in normal pregnancy-associated immunological events, such as the maintenance of maternal–fetal tolerance. This review provides perspectives on placenta-derived exosomes as possible biomarkers for the diagnosis/prognosis of preeclampsia. Using keywords, online databases were searched to identify relevant publications to review the potential use of placenta-derived exosomes as biomarkers of preeclampsia.
Keywords: placenta-derived exosomes, preeclampsia, biomarkers
Introduction
Preeclampsia (PE) is a multiorgan systemic hypertensive disorder of pregnancy (HDP) that is a leading cause of maternal and fetal mortality.1–3 The exact etiology of PE is difficult to establish, due to the multifactorial nature of the disorder; however, the general theoretical framework underlying the etiology of PE centers on abnormal placentation due to inadequate spiral-artery remodeling caused by poor trophoblast invasion.4,5 Moreover, it is now understood that increased placental oxidative stress is triggered by a predisposing condition, which stimulates the release of microvesicles from the syncytial layer of the placenta.6,7 These extracellular vesicles (EVs), termed syncytiotrophoblast microvesicles (STBMs), may bind to monocytes and stimulate the production of proinflammatory cytokines, which results in altered maternal systemic inflammatory response (MSIR) in normal and preeclamptic pregnancies.8–11 In addition, ex vivo studies have shown that STBMs have a key role in maternal immunomodulation that results in regulation of T-helper 1 (Th1)/Th2 immunoresponses in normal pregnancy,8 and may thus be responsible for the Th1 skewness in PE.12
It is now recognized that the exact nature and composition of STBMs has yet to be defined with regard to proportion of exosomes, apoptotic bodies, and other vesicles. In light of this, recent literature has indicated that the constituents of STBMs should be classified as EVs and thus be termed STBEVs.13 Though prior knowledge of STBEVs indicates that these vesicles in their entirety can be used as circulating STB biopsies,14 they cannot be clearly defined as biomarkers of PE, as studies have not demonstrated the objective isolation and detection of PE-specific biomarkers. In addition, studies conducted by the Obstetrics and Gynaecology Unit at the University of Oxford have shown that exosomes may be a key constituent of STBMs involved in the pathogenesis of the disorder.7,8,10,12–19 These fundamental observations support the potential of placenta-derived exosomes as a biomarker of PE. Therefore, this review aims to evaluate the potential application of exosomes as a biomarker of PE.
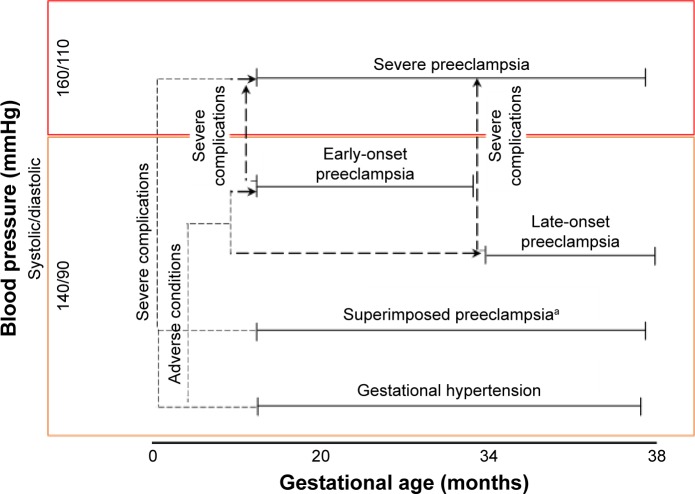
Understanding the spectrum of pregnancy-related hypertensive disorders
HDPs are classified into four groups: 1) PE and eclampsia, 2) chronic hypertension (of any cause), 3) chronic hypertension with superimposed PE, and 4) gestational hypertension. Clinical classification of the types of HDP is based primarily on gestational age, blood pressure, and compounding complications (Figure 1). PE is defined as an HDP that has been characterized extensively in clinical practice, as it is associated with an increased risk of fetal and maternal morbidity and mortality.20 Without early-detection strategies, PE is clinically diagnosed as new-onset hypertension with proteinuria,20,21 and can be further subcategorized into early-and late-onset PE.21–24 Based on gestational age, early-onset PE is defined by new-onset hypertension at <33 weeks plus 6 days of gestation and late-onset PE defined by new-onset hypertension >34 weeks of gestation.22–24 However, the severity of PE pregnancies can be differentiated by their clinical manifestations, as outlined by clinical practice guidelines that present the adverse conditions and severe complications of PE according to the end organs affected.25
Figure 1.
Classification of hypertensive disorders in pregnancy.
Notes: Hypertensive disorders with preeclampsia subclassified into early- and late-onset preeclampsia. Adverse conditions are defined as conditions that increase the risk of severe complications. Adverse conditions and severe complications are categorized into the organ system affected (ie, central nervous system, cardiorespiratory, hematological, renal, hepatic, and fetoplacental), as described by Magee et al.25 aHypertension present either at prepregnancy or that developed at 20 weeks prior to gestation.
Adverse conditions in PE are associated with risks that increase the odds of severe maternal and fetal complications, and when exaggerated complications lead to severe (that warrant delivery).25 Diagnosis of these complications is primarily based on clinical maternal symptoms and laboratory tests. In addition, gestational hypertension, superimposed PE, and PE can progress to severe PE if one or more severe complications manifest, which thus necessitates improved clinical monitoring-management systems using defined prognostic and diagnostic methods. Thus far, the only known cure of PE is the early delivery of the fetus and placenta to prevent worsening of disease progression and associated maternal complications, which leads to prematurity and its subsequent complications.25,26 All these complications emphasize the need for a biomarker that would enable early diagnosis and development of a treatment method for PE to prevent the inherent distress induced by it.
Biomarkers of PE: the current state
Biomarkers are universally defined as biochemical alterations that can be objectively detected and quantified in human tissue, cells, or fluids and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention.27 It is thus imperative that these criteria are met when identifying potential biomarkers of PE to develop an efficient clinical test for early diagnosis and differentiation of the severity of PE. However, this is a challenge, due to the complex etiology and pathology of PE.
Clinical markers, such as maternal factors, blood pressure, proteinuria, and uterine-artery Doppler velocimetry remain the most reliable methods in PE monitoring and supportive care of the mother and fetus. However, they cannot be used in the early diagnosis of PE,21,28 but may still serve as useful markers when used in combination with other PE biomarkers. Previous studies (Table 1) have shown that combined clinical and biological markers have improved detection rates in overt PE, which could be incorporated into clinical care for early screening and assessment of patient-specific risks of PE.29–31 However, the limitation of combined clinical markers and biological markers is the inability of these markers to provide definitive differentiation of the severity of PE and assessment of the PE-disease pathway. Currently, combined biomarkers of PE include cytokines, proteins, angiogenic and antiangiogenic factors that have a fundamental role in the pathogenesis and etiology of PE, as well as other related HDPs.32–34
Table 1.
Overview of potential, combined and candidate biomarkers in preeclampsia
| Biomarker | Biomarker type | Detection stage | Role | Sensitivity | Specificity | Advantages | Disadvantages | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Potential biomarkers/combined biomarkers | ||||||||
| Cystatin C | Protein | Preclinical (first trimester: ≥11 weeks) | Protease inhibitor involved in impaired renal function and hypothesized to disrupt placental development in PE | 91.7 | 85.7 | May be a useful first-trimester biomarker due to its elevation in overt PE, with a 93% detection rate when used in combination with maternal BMI | Indirect measure of glomerular filtration impairment and may be unrelated to placental dysfunction; may have application in severe preeclampsia, though the possibility that this occurs as a result of renal impairment due to increase protein load in maternal circulation should not be excluded The study was inconclusive, as data regarding clinical markers, such as arterial pressure and Doppler PI were not been measured; inclusion of other HDPs need to be taken into consideration in future studies | 114 |
| HTRA3 | Enzyme | Clinical (second trimester: 13–14 weeks) | Serine protease found to be associated with alterations in placental oxygen transfer, and thus may be involved in blood-vessel remodeling | ND | ND | Elevation of HTRA3 in PE indicates biomarker potential; additionally, the ratio of HTRA3-L to total HTRA3 was lower in early-onset PE | Involvement of the enzyme in the pathogenesis of PE is unknown; enzyme not a marker of placental function and thus cannot be used as a specific marker of placental function in PE | 62 |
| NGAL | Glycoprotein | Clinical (late second trimester, early third trimester: 24–26 weeks) | Involved in the modulation of oxidative stress in normal cellular physiology; its dysregulation has been associated with inflammation and cancer | 75 | 94.5 | Elevated levels of NGAL obtained in PE that were positively correlated with blood pressure and proteinuria | Involvement of NGAL in pathophysiology of PE unknown; findings need to evaluated in larger population | 66 |
| PP13 | Protein | Preclinical (first trimester: 6–10 weeks) | Immunoregulatory protein involved in placental development | 80 | 20 | Significantly reduced levels of PP13 found in PE in early pregnancy and thus may have application as potential early predictor of PE | Marker has poor detection sensitivities, and detection has to be based on predetermined cutoff values | 72 |
| Serum lipids | Oxidized cholesterol and glycerophosphocholine | Preclinical (first trimester: 6–10 weeks) | Lipids like oxidized cholesterol contribute to vascular atherosclerosis | 91 | 82 | Combined lipid biomarkers show good ability to detect PE in early pregnancy | Role of these lipids still unknown in pathogenesis of PE; in addition, further studies required to determine if these markers are specific for PE or associated with all types of pregnancy-related complications | 76 |
| IL10, TNFα, and IFNγ | Cytokines | Clinical (second trimester: 14–18 weeks) | IL10 is an important anti- inflammatory cytokine in pregnancy that inhibits upregulation of MMP2 and MMP9 and promotes the termination of Th1 inflammatory rejection reactions against the fetal placental unit TNFα involved in systemic endothelial cell activation IFNγ associated with idiopathic recurrent spontaneous abortion | ND | ND | Reduced levels of these cytokines may be involved in the modulation of etiopathogenesis of PE and thus show promise as early clinical stage biomarker | Larger population size required to validate biomarker potential | 55, 56 |
| RASSF1A | cfDNA/cffDNA | Clinical (second trimester: 17–30 weeks) | It is hypothesized that the reason cffDNA increases in maternal circulation before onset of the clinical symptoms is increased placental perfusion caused by inadequate spiral-artery remodeling; oxidative stress, apoptosis, and necrosis the main physiological processes that disrupt placental development, leading to increase levels of cfDNA and cffDNA in maternal circulation | 87.5/100 | 75/50 | Reliable predictor of PE with ROC (AUC) values of 0.94 and 0.83 for cfDNA and cffDNA, respectively; predictive capability of woman with risk of PE who developed PE and woman at risk who did not develop PE had an ROC (AUC) value of 0.78 and 0.81 for cfDNA and cffDNA, respectively; combined cfDNA and cffDNA quantification of RASSF1A may be used as a late preclinical stage biomarker in women with high risk of PE | Cannot be used as a very early biomarker (0–16 weeks of gestation) cffDNA has low specificity and thus needs to be used in combination with cfDNA The exact physiological role of these molecules in PE has not been elucidated | 50, 51 |
| Combined clinical markers/potential biomarkers | ||||||||
| MFs, MAP, UtA PI, PGF, PAPPA | Clinical measurements, angiogenic factor, and protein | Preclinical (first trimester: 11–13 weeks) | Clinical markers (MFs, MAP, and UtA PI) direct measures of cardiac malfunction PGF an angiogenic factor required for normal placental development PAPPA involved in cellular proliferation | ND | ND | Combined markers have potential to detect severe EOPE at detection rate of 93% with false- positive rate of 5% Algorithm for the detection of severe EOPE may serve as a useful screening tool Method has a greater detection rate of PE than previous traditional methods, which relied entirely on maternal history | Poor detection rates for LOPE (35%) and GH (18.3%) obtained; this proves that the combined marker cannot differentiate between PE subtypes | 31 |
| MFs, MAP, UtA PI, PAPPA, free βhCG | Clinical measurements, protein, and hormone | Preclinical (first trimester: 8–12 weeks) | βhCG involved in implantation and blastocyst development and replaced PGF in Poon et al31 | ND | ND | At a false-positive rate of 5%, 10% detection rates for EOPE were 69.2% and 80.8%; however, LOPE-detection rates were lower at 29.4% and 39.6% May be a useful tool in early screening of EOPE | Cannot differentiate between PE subtypes Cannot be used as screening test, as larger studies with inclusion of angiogenic factors are required to validate potential to be used in differential diagnosis of PE | 29 |
| Combined candidate biomarkers | ||||||||
| sFlt1 and PGF | Angiogenic and antiangiogenic factors | Clinical (third trimester: 20–33 weeks) | Directly involved in the pathogenesis of PE; PGF and angiogenic factor required for normal placental development and sFlt1 an antiangiogenic factor | 88–95 | 94–99.5 | Ability to detect PE with high sensitivity and specificity. In addition, differential phase cutoffs improve the diagnostic accuracy of the ratio Favorable sensitivity and specificity are attributed to their direct involvement in normal pregnancy | Larger studies required to validate differential diagnosis, due to high constructed incidence of PE in this study and multifactorial nature of the disorder Cannot be used as an early biomarker of PE | 38 |
Abbreviations: BMI, body-mass index; EOPE, early-onset PE; GH, gestational hypertension; HDPs, hypertensive disorders of pregnancy; LOPE, late-onset PE; MAP, mean arterial pressure; MFs, maternal factors; ND, not determined; PE, preeclampsia; UtA PI, uterine-artery pulsatility index; βhCG, β-human chorionic gonadotropin.
Angiogenic and antiangiogenic factors
Thus far, antiangiogenic sFlt1 and angiogenic PGF in combination serve as the most promising biomarkers for the diagnosis of PE.35–38 This is mainly attributed to the synergistic role of these molecules in the pathogenesis of PE, whereby an altered angiogenic balance induced by sFlt1, inhibits PGF signaling, resulting in vascular endothelial dysfunction, a key feature of PE.32,37–39 Although the precise physiologic role of these factors is not fully understood, the elevated circulating levels of sFlt1 and decreased PGF observed in overt PE highlight their combined biomarker potential,33,37,38,40 Furthermore, a recent study presented the differential diagnostic potential of the sFlt1:PGF ratio in PE.37,38,41,42 This ratio proved to be effective in the diagnosis of overt PE in the third trimester of pregnancy, together with differential diagnosis of severe gestational and chronic hypertension at ≥34 weeks’ gestation.35,31 Regardless of the favorable sensitivity and specificity of the sFlt1:PGF ratio, its clinical usage is restricted as a late-pregnancy diagnostic and management tool for severe or overt HDP.42 A case–control multicenter study showed that use of differential cutoffs for the various gestational phases enhanced the diagnostic potential of the sFlt1:PGF ratio.38 This study, however, did not show false-positive or false-negative values, due to the high constructed incidence of PE and variability of the disorder. Therefore, validation of the sFlt1:PGF ratio in a clinical setting with a larger, homogeneous population is required. sEng, a key antiangiogenic factor implicated in PE, has been found to be elevated in maternal circulation in the second trimester in PE-predisposed patients.43–45 Although sEng values alone or in combination with other pro- and antiangiogenic factors are significantly altered in PE serum, it has proven to be a poor biomarker of the disorder.46
Cell-free fetal DNA
cfDNA and cffDNA are also probable combined biomarkers of PE, mainly due to their sensitivity and specificity in biological fluids.47,48 The identification of the methylated RASSF1A promoter gene, which is elevated in early-PE pregnancies,49–51 offers a promising alternative to the quantification of cffDNA, using Y-chromosome-specific sequences.52 Notably, high doses of human cffDNA did not induce PE-like symptoms in a murine in vivo model,53 reinforcing the notion that the increase in RASSF1A methylation is a consequence rather than a cause of PE. Nevertheless, when used in combination with other markers, cffDNA RASSF1A methylation may have potential in monitoring PE development in the second trimester.49–51
Cytokines
Cytokines are significantly involved in the pathophysiology of PE, as they are instrumental in the maintenance of the Th1–Th2 balance in normal pregnancy.9 During normal pregnancy, there is a shift toward Th2 immunity that results in the synthesis of IL4, IL5, IL6, and IL10, whereas in PE there is a distinct shift toward Th1 immunity, resulting in the production of IL2, TNFα, and IFNγ.54 It is this shift toward Th1-associated immunity in PE that results in the exaggerated MSIR. Therefore, Th1- and Th2-associated cytokines have gained interest as potential PE biomarkers. Research conducted in patients in the early second trimester of pregnancy has indicated that IL10-, TNFα-, and IFNγ-expression levels were significantly altered in PE during weeks 14–18 of gestation.55–58 Therefore, these cytokines in combination may serve as potential early biomarkers of PE if validated in a larger sample population. In addition, GDF15, a macrophage-inhibiting cytokine possessing cardioprotective and biomarker potential in cardiovascular disease, has been found to be significantly reduced in PE women.59 However, GDF15 cannot be used as a biomarker of PE in the first trimester of pregnancy, as no marked differences between normotensive and PE pregnancies have been observed. Although cytokines may be involved in the pathophysiology of PE, their application as possible biomarkers is questionable, since key cell-mediated factors are involved in their regulation during pregnancy.
High-temperature-requirement A3 enzyme
HTRA3, a pregnancy-related serine protease expressed by placental extravillous trophoblast cells, may have biomarker potential in the early detection of PE.60 It has been found to be associated with altered placental oxygen transfer and blood-vessel remodeling in the first trimester.61 Elevated levels of HTRA3 in weeks 13–14 of gestation have been observed in PE-predisposed patients.62 Although the potential application of HTRA3 as a biomarker is promising, it has limited application, since factors responsible for altered expression of this enzyme in PE are still unclear. Additionally, this enzyme is not a specific marker of placental function, and thus cannot be used an indicator of placental maladaptation in PE.
Pregnancy-associated plasma protein A
PAPPA, a more relevant, pregnancy-related protease involved in early placental development, has been found to be dysregulated in intrauterine growth restriction, PE, placental abruption, and premature birth.63 PAPPA is responsible for the cleavage of IGFBP4, hence regulating the synthesis of IGF, a key growth factor in the regulation of placental and fetal growth.64 Dysregulation of this enzyme may be linked to a variety of pregnancy-related complications associated with generalized placental function. Due to the aspecificity of this enzyme, it cannot be used as a marker of PE, but may have diagnostic application in combination with other clinical measurements and biomarkers.65
Neutrophil gelatinase-associated lipocalin
NGAL, a lipocalin-type glycoprotein involved in iron sequestration normally associated with inflammation, neoplastic transformation, and renal damage, has been found to be linked to PE.66 Increased serum NGAL is proportional to the severity of PE in the late second trimester of pregnancy, with sensitivity and specificity of 75% and 94.5%, respectvely.67 However, NGAL is not linked exclusively to placental maladaptation, and its role in PE is unclear. Additionally, maternal serum AFP, in combination with maternal factors, the uterine-artery pulsatility index, and mean arterial pressure, has shown potential in the diagnosis of PE in weeks 19–24 of gestation in combination with other biomarkers.68
Placental protein
PP13 has been reported extensively in early placental development,69,70 and may be involved in T-cell and mac rophage apoptosis, regulation of maternal immunoresponses, and maternal–fetal immunomodulation.70,71 Due to its association with early placental development, it may be useful in detecting PE during early pregnancy.72 A significantly lower concentration of PP13 in weeks 6–10 of gestation has been found in PE-predisposed patients, which was attributed to impaired trophoblastic regulation and spiral-artery remodeling in PE.72 Studies involving PP13 as a biomarker are limited, due to the lack of sample-population homogeneity and poor detection sensitivity; in addition, sensitivity and specificity for detection has to be determined using predefined cutoff values. Interestingly, STBEVs have been shown to be immunopositive for PP13,70 suggestive of an immunomodulatory role. Therefore, future studies must evaluate PP13-biomarker potential in association with STBEVs and exosomes.
Syncytiotrophoblast extracellular vesicles
STBEVs may play a role in maternal–fetal immunoadaptation.73 In addition, in vitro research has indicated that PE-derived STBEVs differentially affect immune cells, endothelial cells, and platelet activation, suggesting a key role in maintenance of the MSIR.74 STBEVs have been identified in maternal circulation in the first trimester of normal pregnancy and also found to be significantly elevated in early-onset PE.75 Moreover, it is known that STBEVs contain subpopulations of EVs.16 As such, it is difficult to utilize the entire STBEV population as a biomarker of PE solely, as there are a multitude of biological factors associated with each EV subtype.
Lipids
New developments in lipidomic research have led to the identification of multimarker lipid biomarkers of PE. Lipid biomarkers with a mass:charge ratio (m/z) of 383, 784, 796, 798, and 920 were able to identify PE with sensitivity of 91% and specificity of 82%. Lipids with an m/z of 383 have been identified as oxidized cholesterol, which has been shown to contribute to vascular atherosclerosis in PE.76 However, lipids with an m/z of 784, 796, 798, and 920 belong to the glycerophosphocholine-lipid class, which may be elevated in PE pregnancies due to enhanced cellular apoptosis.76 Although lipidomics provide a new avenue for the identification of PE biomarkers, further studies are needed to determine if these markers are specific for PE.
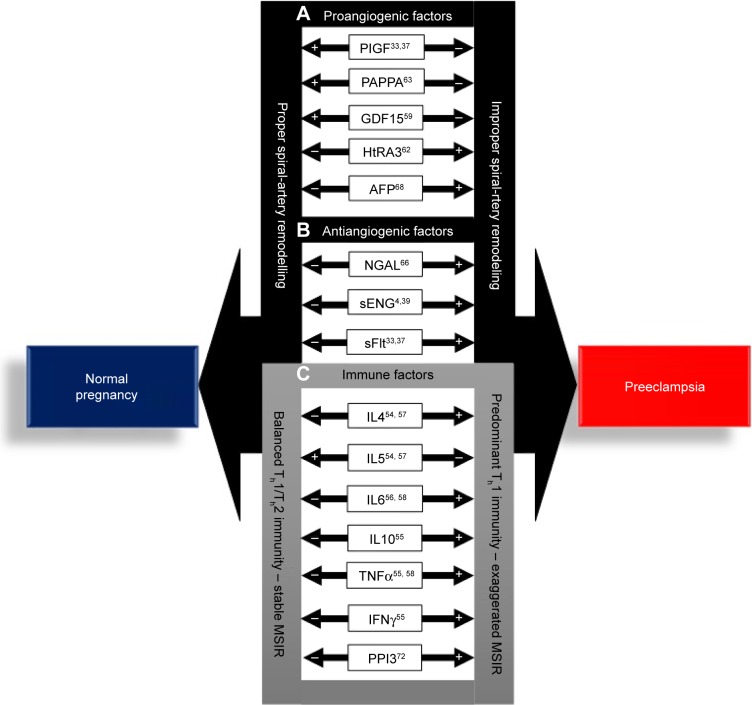
In summary, biomarkers of PE can be described as angiogenic and antiangiogenic factors involved in placental maladaptation, improper spiral-artery remodeling, and immune factors that are synthesized as a result of the predominant Th1 immunoshift in PE (Figure 2). The dysregulation of these factors in PE indicates that they may be involved in the etiopathogenesis of the disorder. However, the upregulation of proangiogenic factors, AFP, and HTRA3 indicates that these factors are consequences of the disorder, rather than the cause. Consolidation of the current state of biomarkers in PE indicates that there is no single biomarker of the disorder that can be objectively utilized as a definitive early biomarker of PE. However, placenta-derived exosomes, a key constituent of STBEVs, may best fit the required biomarker criterion of PE and provide key evidence supporting the etiology and pathogenesis of the disorder. This review thus aims to evaluate the potential of placenta-derived exosomes as biomarkers of PE within the approved US Food and Drug Administration (FDA) biomarker-criterion framework.
Figure 2.
Potential biomarkers involved in the pathogenesis of preeclampsia (PE).
Notes: (A) Proangiogenic and (B) anti-angiogenic factors associated with improper spiral-artery remodeling in PE. (C) Immune factors associated with the pathogenesis of PE as a result of the predominant Th1 immunity of the disorder. +Significantly increased expression in comparison to normal pregnancy; −significantly lowered expression in comparison to PE pregnancies.
Abbreviations: MSIR, maternal systemic inflammatory response; Th, T-helper.
Placenta-derived exosomes as potential biomarkers of PE
Placenta-derived exosomes are synthesized by STB cells via the lysosomal pathway, whereby multivesicular bodies fuse with the cell membrane via exocytosis, resulting in the release of placental exosomes into maternal circulation. During the process of exosome biogenesis, selective packaging of molecules into exosomes occurs within the originating cell. These native molecules contained within the exosome thus consist of genetic and proteomic information that could serve as direct markers of pregnancy-related complications/placental function. However, the rationale for presenting exosomes as a candidate biomarker of PE is yet to be evaluated in terms of the FDA-approved biomarker criterion.27 In this review, we thus incorporate the FDA-biomarker criterion in evaluating placenta-derived exosomes as biomarkers of PE, whereby placenta-derived exosomes were evaluated in view of the following criteria. Can they be objectively isolated, detected, and quantified from biological fluids? Have they been shown to increase with an increase in gestational age in normal pregnancy? Have they been shown to be significantly altered in PE? Do they contain key molecular markers that can improve biomarker sensitivity and specificity? Can they be used as a biotherapeutic agent to reprogram the dysfunctional placenta?
Isolation, detection, and quantification of placenta-derived exosomes in maternal circulation
Blood is the biofluid of choice when isolating exosomes for research and diagnostic purposes; however, various factors, such as the choice of anticoagulant, venipuncture, blood handling, circadian systems, time of draw, fasting status, platelet activation, and hemolysis, affect the composition and characteristics of exosomes in downstream sample processing and may result in inaccurate results.77 It is thus a requirement that when investigating the use of placenta-derived exosomes as biomarkers of PE that standardized blood-collection protocols as recommended by the International Society for Extracellular Vesicles be considered to prevent the artificial release of exosomes, which would result in false positives and negatives.77 Exosomes from maternal circulation are generally isolated based on their buoyant density of 1.13–1.19 g/mL using density-gradient or static-gradient ultracentrifugation and characterized by microscopic morphology and proteomic identification of exosome-associated tetraspanins, such as CD63, CD9, CD81, and HSP70.78 Furthermore, PLAP is used as a placenta-specific marker for the isolation and quantification of placenta-derived exosomes.79–81 Alternative technologies, such as analytical size-exclusion chromatography and immunoaffinity capture,82,83 for the isolation of PLAP+ exosomes from maternal circulation have been shown elsewhere; however, these methods, especially the latter, result in analytical challenges with regard to the reproducibility and precision of the quantification of placenta-derived exosomes in maternal circulation. Alternatively, the direct quantification of placenta-derived exosomes from total purified exosomes using PLAP as a placenta-specific marker has been achieved.79–85 In contrast to immunocapture methods, this method eliminates inaccuracies attributed to the lower exosomal yield obtained using immunocapture techniques. However, the direct quantification of placenta-derived exosomes in maternal circulation is largely dependent on the purity of the exosomal fraction. It is thus advisable that density-gradient ultracentrifugation and analytical size-exclusion exosome-isolation methods be utilized in combination to improve the purity and yield of maternal exosomes, thus enhancing the objective identification of placenta-derived exosomes.
The objective isolation of exosomes in maternal circulation coupled with recent advancements in nanotechnology, such as nanoparticle-tracking analysis, enables the accurate characterization and quantification of exosomal populations based on particle-size distribution using dynamic light scattering and Brownian motion.80,85,86 Additionally, this technology enables the use of placenta-specific fluorescent probes, such as PLAP and other exosome-specific protein markers, for the quantification of placenta-derived vesicles.86,87 With advances in exosome-isolation and -detection technologies, the expected challenges would not only include the discovery of specific PE exosomal markers but also how to incorporate these markers into regulatory decision-making and clinical practice.
Placenta-derived exosomes in normal and complicated pregnancies
Altered levels of circulating placenta-derived exosomes in PE
Previously, studies have focused on supraphysiological levels of enriched STBEVs in ex vivo systems.12,19,88 These studies have demonstrated an increase in STBEVs in ex vivo placental perfusates in PE in comparison to normal pregnancies. Furthermore, it has been established that STBEVs have a key role in immunomodulation during normal and complicated pregnancies,12,83,89 and not as purified populations of constituent vesicles, such as exosomes. Exosomes are regarded as a key constituent of STBEVs, due to their ability to reprogram cells and consequently alter normal cellular physiology, which thereby contributes to various pathological states. Although STBEVs in normal pregnancies have been shown to play a role in immunoregulation12 and placenta-derived exosomes have been shown to increase with gestational age and alter in vitro endothelial cell migration,80,90 there is a lack of knowledge in understanding the role of placenta-derived exosomes in complicated pregnancies, such as those with PE, as well as the relative proportion of these vesicles in relation to other STBEVs.
Role of placenta-derived exosomes in maternal immunomodulation
Placenta-derived exosomes were initially identified from placental tissue by Frängsmyr et al, and have been shown to contain the transmembrane protein FasL, which suggests that placenta-derived exosomes are involved in immunoregulation during pregnancy by initiating the local deletion of activated maternal lymphocytes that identify placental paternal antigens.91 In addition, a similar study by Abrahams et al indicated that exosome-mediated secretion of FasL promotes immunoprivilege of semi-allograph invading trophoblastic cells by inducing apoptosis in Fas-bearing immune cells during implantation and pregnancy.92 However, a more recent study showed that B7 immunomodulatory molecules and HLAG5 are secreted via exosomes from early and term placentas.93 This study offered a novel perspective in which human placenta-derived exosomes may have served directly as key mediators of maternal immunotolerance to the semiallogeneic fetus. The identification of these key immunoregulatory proteins bound to placenta-derived exosomes supports the fact that exosomes have an immunomodulatory role in preventing the degradation of invading trophoblastic cells by inducing maternal T-cell apoptosis, which could play a key role in maternal complications, such as PE, fetal rejection, and intrauterine growth restriction.
MSIR and placenta-derived exosomes in maternal circulation
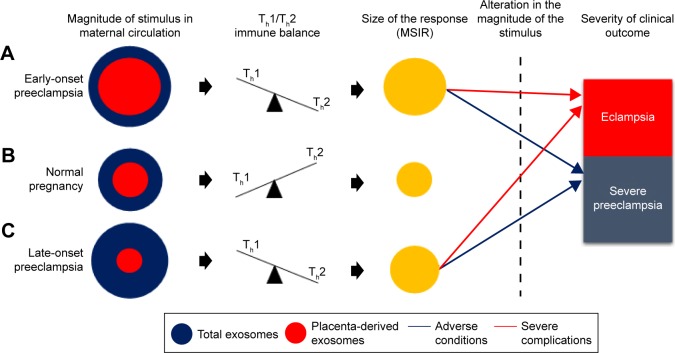
According to Redman and Sargent, in PE, placental oxidative stress as a result of abnormal placentation induces the release of STBEVs that may control cellular apoptotic and necrotic events in circulation, thus resulting in an exaggerated MSIR.7,15 In addition, these authors postulated that in normal pregnancy, the process of regeneration of placental STB cells is dependent on cellular apoptosis, which is modulated by STBEVs.7,15 In PE, the increase in STBEVs results in an exaggerated MSIR,7,10,86 which is induced when the maternal immune system reacts adversely in an exaggerated manner upon exposure to STBEVs.17 Since placenta-derived exosomes are a key constituent of STBEVs, we and others16,94 thus hypothesize that the marked differences in magnitude of placenta-derived and total exosomes in PE in comparison to normal pregnancies may control Th1/Th2 immunity during pregnancy, which in turn has a direct relationship with the size of the MSIR (Figure 3). In particular, this model proposes that a possible alteration in magnitude of placenta-derived and total exosomes during the pathological progression of early- or late-onset PE may cause adverse conditions or severe complications, which manifest in either severe PE or eclampsia, respectively. Additionally, this hypothesis can be authenticated by the incorporation of a mathematical algorithm whereby the rate of change of placenta-derived exosomes, in relation to total exosomes, within a specified gestational age would enable accurate and reliable diagnosis of PE throughout gestation. This is based on the theory that placenta-derived and total exosomes increase throughout gestation in normal pregnancy,80,81 which have been found to be altered in early-and late-onset PE.85 This model represents the classification of early- and late-onset PE based on the ratio of the rate of change of total exosomes to placenta-derived exosomes within a specified gestational age range represented by the following elementary formulae:
where PLAP+ exosomes are placenta-derived exosomes, CD63+ total exosomes, and G gestational age.
Figure 3.
Hypothetical model of exosomes in relation to immunological and clinical outcomes in EOPE, LOPE, and normal pregnancy.
Notes: (A) In EOPE, an increase in placenta-derived and total exosomes in comparison to normal pregnancy mediates a shift toward Th1 immunity and thus results in an exaggerated MSIR. (B) In normal pregnancy, there is a balance between placenta-derived and total exosomes that mediates a shift toward Th2 immunity, thereby maintaining a balanced MSIR, a requirement for successful pregnancy. (C) In LOPE, the increase in total and decrease in placenta-derived exosomes in comparison to normal pregnancy mediates a shift toward Th1 immunity, which exaggerates the MSIR to a degree that does not exceed the MSIR in EOPE. (A, C) Alteration in the magnitude of placenta-derived and total-exosomes during the manifestation of LOPE or EOPE results in adverse conditions or severe complications that lead to the clinical manifestation of severe PE or eclampsia.
Abbreviations: EOPE, early-onset preeclampsia; LOPE, late-onset PE; MSIR, maternal systemic inflammatory response; Th, T-helper.
With this hypothetical model, at any specified time period during pregnancy, the calculation of the rate of change in total exosomes to placenta-derived exosomes can be used to assess placental function, impending PE, and the severity of the complication. The model presents the rate of change of ΔPLAP+ exosomes/ΔCD63+ exosomes as a relative measure of impending PE during the preclinical and clinical phases of PE. However, this model is still to be evaluated in a clinical setting, in order to determine the appropriate rate of change for diagnosis/prognosis.
Molecular markers of placenta-derived exosomes in normal and preeclamptic pregnancies
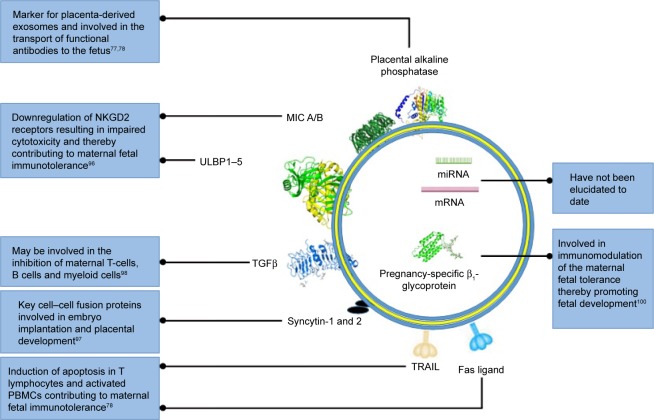
Exosomes contain cargo, such as mRNA, miRNA, cytosolic proteins, membrane-bound proteins, and lipids, which are selectively packaged during exosomal biogenesis. Exosomes usually contain a variety of molecules that are native to the originating cell and enriched with endosome-associated protein markers, such as tetraspanins (CD63, CD81, CD82, CD9, CD37), HSP70, RAB proteins, ALIX, TSG101, endocytic proteins, and cell-specific proteins, which provide information regarding their cellular origin.95 Pregnancy-associated exosomes have been found to contain a number of membrane-bound protein markers, such as NKGD2 ligands,96 FasL,82,83 TRAIL,82 and syncytin 1,97 which suggests that placenta-derived exosomes play a key role in maintaining maternal–fetal tolerance (Figure 4). Other specific pregnancy-associated exosomal proteins, such as TGFβ, indicate that placenta-derived exosomes are involved in the control of STB growth, proliferation and differentiation.98 Although these exosomal membrane-bound proteins are associated with pregnancy, they cannot be used as definitive markers of placenta-derived exosomes, due to their lack of placental specificity; therefore, placental ALP remains the marker of choice when isolating and identifying placenta-derived exosomes.79–81,85,90,99,100 Even though it is known that exosomes contain mRNA and miRNA, which are key molecular mediators in translational and posttranscriptional modification, the exact composition of these exosomal constituents has not been elucidated in placenta-derived exosomes. Therefore, future studies should incorporate identification of novel nucleic acid sequences in placenta-derived exosomes in normal and PE pregnancies to enhance the specificity and sensitivity of these vesicles as biomarkers of PE.
Figure 4.
Schematic representation of key pregnancy-associated exosomal molecular cargo and their function.
Notes: This figure highlights key transmembrane and cytosolic proteins involved in immunomodulation during normal pregnancy. Key pregnancy-associated exosomal nucleic acids have not been identified to date. Common exosomal markers are not shown.
Abbreviation: PBMCs, peripheral blood mononuclear cells.
Perspectives on engineered exosomes in PE therapeutics
Stem cell (SC)-derived exosome therapy is of emerging clinical importance as an alternative to other gene therapies, since exosomes have no risk of aneuploidy and a lower rate of immunorejection following allogeneic administration.101 In addition, they serve as ideal vectors for the reprogramming of diseased cells in various pathological states, such as PE, since they have the ability to change the phenotype of the target cell though various signaling cascades and transfer of RNA.102,103 Mesenchymal SCs (MSCs) are rich in their application as regenerative agents of damaged or diseased cells, due to their unique complement of proteins, which promote biological processes and pre-miRNA that facilitate intercellular communication.104–106 MSC-derived exosomes have been shown to act as promising therapeutic agents in cardiovascular disease,107,108 kidney injury,109 immune disease,110 tumor growth,111 and neurological diseases.112,113 However the use of MSC-derived exosomes to treat PE has its challenges, due to the complexity of the pathogenesis of the disease. It is thus a prerequisite to determine the role of placenta-derived exosomes in the pathogenesis of PE through isolation and characterization of exosomal cargo and functional activity, and to understand the mechanism of biogenesis of placenta-derived exosomes in normal and PE pregnancies.
Engineering MSC-derived exosomes for the specific treatment of PE has potential, based on the understanding that exosomes may be primary-role players in the etiopathogenesis of PE. This process would involve the in vitro genetic manipulation of isolated MSCs using plasmid-based vectors to package dsRNA selectively during in vitro MSC-exosome biogenesis to enable the silencing of specific genes involved in the pathogenesis of PE and to engineer the surface proteins of MSC exosomes to result in their specific delivery to STB cells, allowing for more targeted exosome-cargo delivery. This would enable the effective targeted delivery of RNAi for reprogramming of the diseased placenta.
Conclusion
The future of the diagnosis of PE is dependent on the identification of novel biomarkers, which in turn may lead to personalized medicine during pregnancy in the form of liquid biopsies. Exosomes are the future of liquid biopsies, as they contain molecular cargo that can be used to diagnose such diseases as cancer without the need for a conventional biopsy. Although there is much controversy regarding the collection, isolation, and characterization of exosomes, the concept of these vesicles as novel biomarkers of the disease remains promising. We project that these shortcomings will be resolved with the implementation of carefully regulated exosomal standardization and validation methods. Additionally, rapid advances in such technologies as nanoparticle-tracking analysis, immunocapture, and next-generation sequencing would improve the efficiency of exosomal isolation and characterization and lead to the identification of novel placenta-derived exosomal protein and nucleic acid markers. The development of algorithms based on the model presented would add value in creating a personalized approach in managing PE and other pregnancy-related complications. These perspectives form the fundamental basis for the use of placenta-derived exosomes in liquid biopsies of the diseased placenta in PE.
Acknowledgments
This work was supported by the South African National Research Foundation (grant 107626) and the University of KwaZulu-Natal College of Health Sciences.
Footnotes
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.American College of Obstetricians and Gynecologists Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina E, Ayala C, Callaghan W. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 3.Wagner LK. Diagnosis and management of pre-eclampsia. Am Fam Physician. 2004;70:2317–2324. [PubMed] [Google Scholar]
- 4.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 5.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 7.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 8.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007;76:61–67. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response: a review. Placenta. 2003;24:S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 10.Sargent IL, Germain SL, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 11.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia. the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 12.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One. 2011;6:e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol. 2017;119:98–106. doi: 10.1016/j.jri.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Tannetta D, Collett G, Vatish M, Redman C, Sargent I. Syncytiotrophoblast extracellular vesicles: circulating biopsies reflecting placental health. Placenta. 2017;52:134–138. doi: 10.1016/j.placenta.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Placenta. 2005;17:21–27. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 16.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29:S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13:680–686. doi: 10.1016/s1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- 18.Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and preeclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8:e56754. doi: 10.1371/journal.pone.0056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannetta DS, Hunt K, Jones CI, et al. Syncytiotrophoblast extracellular vesicles from pre-eclampsia placentas differentially affect platelet function. PLoS One. 2015;10:e0142538. doi: 10.1371/journal.pone.0142538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30:S1–S48. [Google Scholar]
- 22.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 23.Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia: statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Pregnancy Hypertens. 2013;3:44–47. doi: 10.1016/j.preghy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hypertension: management of hypertensive disorders of pregnancy according to international guidelines – a panel discussion (case 5: postpartum eclampsia) Am J Perinatol. 2015 Jun 5; No authors listed. Epub. [Google Scholar]
- 27.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 28.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416–441. doi: 10.1016/s1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 29.Scazzocchio E, Figueras F, Crispi F, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. Am J Obstet Gynecol. 2013;208:203.e1–203.e10. doi: 10.1016/j.ajog.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Park FJ, Leung CH, Poon LC, Williams PF, Rothwell SJ, Hyett JA. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust N Z J Obstet Gynaecol. 2013;53:532–539. doi: 10.1111/ajo.12126. [DOI] [PubMed] [Google Scholar]
- 31.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–818. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 32.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 33.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble Fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 35.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58.e1–e8. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 36.Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;112:43–52. doi: 10.1042/CS20110097. [DOI] [PubMed] [Google Scholar]
- 37.Stepan H, Herraiz I, Schlembach D, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol. 2015;45:241–246. doi: 10.1002/uog.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- 39.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 40.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 41.Verlohren S, Herraiz I, Lapaire O, et al. The routine measurement of the sFlt1/PlGF ratio allows differential diagnosis of hypertensive pregnancy disorders and has prognostic potential in preeclamptic patients. Pregnancy Hypertens. 2011;1:245–246. doi: 10.1016/j.preghy.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161.e1–161.e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174.e1–e5. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 44.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 45.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 46.Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble Fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 47.Martin A, Krishna I, Badell M, Samuel A. Can the quantity of cell-free fetal DNA predict preeclampsia? A systematic review. Prenat Diagn. 2014;34:686–691. doi: 10.1002/pd.4416. [DOI] [PubMed] [Google Scholar]
- 48.Hahn S, Rusterholz C, Hösli I, Lapaire O. Cell-free nucleic acids as potential markers for preeclampsia. Placenta. 2011;32:S17–S20. doi: 10.1016/j.placenta.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Salvianti F, Inversetti A, Smid M, et al. Prospective evaluation of RASSF1A cell-free DNA as a biomarker of pre-eclampsia. Placenta. 2015;36:996–1001. doi: 10.1016/j.placenta.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Yu H, Shen Y, Ge Q, et al. Quantification of maternal serum cell-free fetal DNA in early-onset preeclampsia. Int J Mol Sci. 2013;14:7571–7582. doi: 10.3390/ijms14047571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papantoniou N, Bagiokos V, Agiannitopoulos K, Kolialexi A, Destouni A, Tounta G. RASSF1A in maternal plasma as a molecular marker of preeclampsia. Prenat Diagn. 2013;33:682–687. doi: 10.1002/pd.4093. [DOI] [PubMed] [Google Scholar]
- 52.Kolialexi A, Tounta G, Apostolou P, et al. Early non-invasive detection of fetal Y chromosome sequences in maternal plasma using multiplex PCR. Eur J Obstet Gynecol Reprod Biol. 2012;161:34–37. doi: 10.1016/j.ejogrb.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 53.Conka J, Konečná B, Lauková L, Vlková B, Celec P. Fetal DNA does not induce preeclampsia-like symptoms when delivered in late pregnancy in the mouse. Placenta. 2017;52:100–105. doi: 10.1016/j.placenta.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Sepulveda A, Torres MJ, Khoury M, Illanes SE. Innate immune system and preeclampsia. Front Immunol. 2014;5:244. doi: 10.3389/fimmu.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL-10, TNF-α & IFN-γ: potential early biomarkers for preeclampsia. Cell Immunol. 2013;283:70–74. doi: 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Opsjln SL, Wathen NC, Tingulstad S, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 57.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 58.Afshari JT, Ghomian N, Shameli A, et al. Determination of interleukin-6 and tumor necrosis factor-alpha concentrations in Iranian-Khorasanian patients with preeclampsia. BMC Pregnancy Childbirth. 2005;5:14. doi: 10.1186/1471-2393-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Q, Wang Y, Zhao M, Hyett J, Costa FS, Nie G. Serum levels of GDF15 are reduced in preeclampsia and the reduction is more profound in late-onset than early-onset cases. Cytokine. 2016;83:226–230. doi: 10.1016/j.cyto.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Nie G, Li Y, Hale K, et al. Serine peptidase HTRA3 is closely associated with human placental development and is elevated in pregnancy serum. Biol Reprod. 2006;74:366–374. doi: 10.1095/biolreprod.105.047324. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Puryer M, Lin E, et al. Placental HTRA3 is regulated by oxygen tension and serum levels are altered during early pregnancy in women destined to develop preeclampsia. J Clin Endocrinol Metab. 2011;96:403–411. doi: 10.1210/jc.2010-1405. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Li Y, Hyett J, Costa FS, Nie G. HTRA3 isoform-specific ELISAs for early detection of preeclampsia. J Biomol Screen. 2016 Dec 8; doi: 10.1177/1087057116682425. Epub. [DOI] [PubMed] [Google Scholar]
- 63.Peterson SE, Simhan HN. First-trimester pregnancy-associated plasma protein A and subsequent abnormalities of fetal growth. Am J Obstet Gynecol. 2008;198:e43–e45. doi: 10.1016/j.ajog.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zwahlen M, Gerber S, Bersinger NA. First trimester markers for pre-eclampsia: placental vs. non-placental protein serum levels. Gynecol Obstet Invest. 2007;63:15–21. doi: 10.1159/000094672. [DOI] [PubMed] [Google Scholar]
- 66.D’Anna R, Baviera G, Giordano D, Todarello G, Corrado F, Buemi M. Second trimester neutrophil gelatinase-associated lipocalin as a potential prediagnostic marker of preeclampsia. Acta Obstet Gynecol Scand. 2008;87:1370–1373. doi: 10.1080/00016340802464463. [DOI] [PubMed] [Google Scholar]
- 67.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bredaki FE, Mataliotakis M, Wright A, Wright D, Nicolaides KH. Maternal serum alpha-fetoprotein at 12, 22 and 32 weeks’ gestation in screening for pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47:466–471. doi: 10.1002/uog.15818. [DOI] [PubMed] [Google Scholar]
- 69.Hawfield A, Freedman BI. Pre-eclampsia: the pivotal role of the placenta in its pathophysiology and markers for early detection. Ther Adv Cardiovasc Dis. 2009;3:65–73. doi: 10.1177/1753944708097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Than NG, Balogh A, Romero R, et al. Placental protein 13 (PP13): a placental immunoregulatory galectin protecting pregnancy. Front Immunol. 2014;5:348. doi: 10.3389/fimmu.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternal-fetal interface. Trends Endocrinol Metab. 2012;23:23–31. doi: 10.1016/j.tem.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wortelboer EJ, Koster MP, Cuckle HS, Stoutenbeek PH, Schielen PC, Visser GH. First-trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early-onset pre-eclampsia. BJOG. 2010;117:1384–1389. doi: 10.1111/j.1471-0528.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 73.Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One. 2011;6:e20245. doi: 10.1371/journal.pone.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SM, Romero R, Lee YJ, Park IS, Park CW, Yoon BH. Systemic inflammatory stimulation by microparticles derived from hypoxic trophoblast as a model for inflammatory response in preeclampsia. Am J Obstet Gynecol. 2012;207(4):337.e1–e8. doi: 10.1016/j.ajog.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goswami D, Tannetta DS, Magee LA, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 76.Anand S, Young S, Esplin MS, et al. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. J Lipid Res. 2016;57:687–696. doi: 10.1194/jlr.P064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3:1–29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 79.Salomon C, Scholz-Romero K, Sarker S, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65:598–609. doi: 10.2337/db15-0966. [DOI] [PubMed] [Google Scholar]
- 80.Salomon C, Torres MJ, Kobayashi M, et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9:e98667. doi: 10.1371/journal.pone.0098667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarker S, Scholz-Romero K, Perez A, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. doi: 10.1186/1479-5876-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191:5515–5523. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- 83.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 84.Carayon K, Chaoui K, Ronzier E, et al. Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem. 2011;286:34426–34439. doi: 10.1074/jbc.M111.257444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and early and late onset pre-eclamptic pregnancies. Placenta. 2016;46:18–25. doi: 10.1016/j.placenta.2016.08.078. [DOI] [PubMed] [Google Scholar]
- 86.Dragovic RA, Collett GP, Hole P, et al. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence nanoparticle tracking analysis. Methods. 2015;87:64–74. doi: 10.1016/j.ymeth.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7:780–788. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8:e56754. doi: 10.1371/journal.pone.0056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams JL, Gatson NN, Smith KM, Almad A, McTigue DM, Whitacre CC. Serum exosomes in pregnancy-associated immune modulation and neuroprotection during CNS autoimmunity. Clin Immunol. 2013;149:236–243. doi: 10.1016/j.clim.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salomon C, Ryan J, Sobrevia L, et al. Exosome signalling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frängsmyr L, Baranov V, Nagaeva O, et al. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005;11:35–41. doi: 10.1093/molehr/gah129. [DOI] [PubMed] [Google Scholar]
- 92.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10(1):55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 93.Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982–990. doi: 10.1016/j.placenta.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salomon C, Rice GE. Role of exosomes in placental homeostasis and pregnancy disorders. Prog Mol Biol Transl Sci. 2017;145:163–179. doi: 10.1016/bs.pmbts.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hedlund M, Stenqvist AC, Nagaeva O, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 97.Vargas A, Zhou S, Ethier-Chiasson M, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with pre-eclampsia. FASEB J. 2014;28:3703–3719. doi: 10.1096/fj.13-239053. [DOI] [PubMed] [Google Scholar]
- 98.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 99.Makiya R, Stigbrand T. Placental alkaline phosphatase is related to human IgG internalization in HEp2 cells. Biochem Biophys Res Commun. 1992;182:624–630. doi: 10.1016/0006-291x(92)91778-o. [DOI] [PubMed] [Google Scholar]
- 100.Goetzl L, Darbinian N, Goetzl EJ. Novel window on early human neurodevelopment via fetal exosomes in maternal blood. Ann Clin Transl Neurol. 2016;3:381–385. doi: 10.1002/acn3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomasetti M, Lee W, Santarelli L, Neuzil J. Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med. 2017;49:e285. doi: 10.1038/emm.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou S, Abdouh M, Arena V, Arena M, Arena GO. Reprogramming malignant cancer cells toward a benign phenotype following exposure to human embryonic stem cell microenvironment. PLoS One. 2017;12:e0169899. doi: 10.1371/journal.pone.0169899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bruno S, Porta S, Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol. 2016;790:83–91. doi: 10.1016/j.ejphar.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 105.Amarnath S, Foley JE, Farthing DE, et al. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33:1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lasser C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert Opin Biol Ther. 2015;15:103–117. doi: 10.1517/14712598.2015.977250. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells. 2016;8:297–305. doi: 10.4252/wjsc.v8.i9.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsao CR, Liao MF, Wang MH, Cheng CM, Chen CH. Mesenchymal stem cell derived exosomes: a new hope for the treatment of cardiovascular disease? Acta Cardiol Sin. 2014;30:395–400. [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Adult stem cells as a tool for kidney regeneration. World J Nephrol. 2016;5:43–52. doi: 10.5527/wjn.v5.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan L, Wu H, Liu Y, Zhao M, Li D, Lu Q. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity. 2016;49:357–365. doi: 10.1080/08916934.2016.1191477. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kalani A, Tyagi N. Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res. 2015;10:1565–1567. doi: 10.4103/1673-5374.165305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12:19–22. doi: 10.4103/1673-5374.198966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolialexi A, Gourgiotis D, Daskalakis G, et al. Validation of serum biomarkers derived from proteomic analysis for the early screening of preeclampsia. Dis Markers. 2015;2015:121848. doi: 10.1155/2015/121848. [DOI] [PMC free article] [PubMed] [Google Scholar]