Abstract
Aripiprazole is a second-generation antipsychotic that is increasingly being prescribed to children and adolescents. Despite this trend, little preclinical research has been done on the neural and behavioral actions of aripiprazole during early development. In the present study, young male and female Sprague-Dawley rats were pretreated with vehicle, haloperidol (1 mg/kg), or aripiprazole (10 mg/kg) once daily on postnatal days (PD) 10–20. After one, four, or eight days (i.e., on PD 21, PD 24, or PD 28), amphetamine-induced locomotor activity and stereotypy, as well as dorsal striatal D2 receptor levels, were measured in separate groups of rats. Pretreating young rats with aripiprazole or haloperidol increased D2 binding sites in the dorsal striatum. Consistent with these results, dopamine supersensitivity was apparent when aripiprazole- and haloperidol-pretreated rats were given a test day injection of amphetamine (2 or 4 mg/kg). Increased D2 receptor levels and altered behavioral responding persisted for at least eight days after conclusion of the pretreatment regimen. Contrary to what has been reported in adults, repeated aripiprazole treatment caused D2 receptor up-regulation and persistent alterations of amphetamine-induced behavior in young rats. These findings are consistent with human clinical studies showing that children and adolescents are more prone than adults to aripiprazole-induced side-effects, including extrapyramidal symptoms.
Keywords: Aripiprazole, haloperidol, D-amphetamine, receptor up-regulation, supersensitivity, ontogeny
Introduction
Aripiprazole is a second-generation antipsychotic that is commonly used to treat schizophrenia in adult humans. Aripiprazole is purported to reduce both positive and negative symptoms, while exhibiting a good side-effect profile (for reviews, see Stip and Tourjman, 2010; Croxtall, 2012). Although aripiprazole is most typically categorized as a dopamine (DA) D2 partial agonist or as a functionally selective D2 ligand (Burris et al., 2002; Urban et al., 2007; Koener et al., 2012), this compound is also a partial agonist at serotonin 5-HT1A receptors (Jordan et al., 2002; Shapiro et al., 2003), a full antagonist at 5-HT2A receptors (Jordan et al., 2004), and it alters the expression of GABAergic binding sites and glutamatergic transporters (Segnitz et al., 2011; Peselmann et al., 2013). All of these actions have potential therapeutic impact because serotonergic, GABAergic, and glutamatergic dysfunction has been linked to schizophrenia (for reviews, see Benes and Berretta, 2001; Stone et al., 2007; Meltzer et al., 2011). Nonetheless, most of aripiprazole’s therapeutic effects have been ascribed to its ability to normalize dopaminergic functioning (Burris et al., 2002; Zocchi et al., 2005).
The low propensity of aripiprazole for inducing side-effects, including extrapyramidal motor movements, may be due to an absence of modifications at the DA D2 receptor [Table 1 near here]. Regardless of route of administration, treatment duration, or dose (see Table 1), researchers have reported that repeated aripiprazole treatment neither up-regulates D2 receptors in adult rats (Inoue et al., 1997; Seeman, 2008; Tadokoro et al., 2012) nor causes DA supersensitivity (Tadokoro et al., 2012). Instead, aripiprazole appears to normalize the D2 receptor up-regulation and behavioral supersensitivity caused by daily haloperidol administration (Tadokoro et al., 2012). The one exception to this general pattern was reported by Seeman (2008), who found that a one-week regimen of aripiprazole significantly increased the ratio of D2High receptors in adult rat striatum, an outcome that is often associated with DA supersensitivity (Seeman et al., 2005).
Table 1.
Effects of repeated aripiprazole treatment on D2 receptor densities in the dorsal striatum of adult rats
| Author and year of publication | Rat strain | Dose and Route of administration | Duration | Effects |
|---|---|---|---|---|
| Inoue et al., 1997 | Wistar | 12 or 100 mg/kg/day, oral | 21 days | Nonsignificant D2 receptor up-regulation |
| Seeman, 2008 | Sprague-Dawley | 1.5 mg/kg/day, subcutaneous | 7 days | No D2 receptor up-regulation Increased percentage of D2High receptors |
| Tadokoro et al., 2012 | Sprague-Dawley | 1.5 mg/kg/day, minipump | 14 days | No D2 receptor up-regulation No DA supersensitivity |
World-wide, aripiprazole is increasingly being administered to children and adolescents, with many of its prescribed uses being off-label (McKinney and Renk, 2011). Among pediatric populations, aripiprazole has received FDA approval for the treatment of Bipolar I disorder (ages 10–17) and schizophrenia (ages 13–17); however, aripiprazole is also reported to be of therapeutic benefit for the treatment of autism and other pervasive developmental disorders (Stachnik and Nunn-Thompson, 2007; Masi et al., 2009; Stigler et al., 2009), aggression and conduct disorder (Bastiaens, 2009; Findling et al., 2009a), as well as Tourette’s syndrome and chronic tic disorder (Seo et al., 2008; Murphy et al., 2009; Yoo et al., 2011). While there are an abundance of studies assessing the efficacy and side-effect profiles of aripiprazole in children and adolescent patients (Fraguas et al., 2011; Kirino, 2012), very few preclinical studies have examined the effects of repeated aripiprazole treatment using developmental animal models. Most notably, Der-Ghazarian et al. (2010) reported that an 11-day regimen of aripiprazole (10 mg/kg once daily) administered to rats on postnatal day (PD) 10–20 caused a short-term decline in the sensitivity of synthesis-modulating autoreceptors. This decrease in autoreceptor sensitivity was apparent one day, but not three days, after conclusion of the drug pretreatment regimen, and was similar to the pattern of effects produced by the D2 receptor antagonist haloperidol. When measured on PD 24 (i.e., three days after conclusion of the pretreatment regimen), repeated aripiprazole treatment produced a nonsignificant 35% elevation in dorsal striatal D2 binding sites when compared to control rats (Der-Ghazarian et al., 2010). To our knowledge, no studies have examined the effects of repeated aripiprazole treatment on the functioning of postsynaptic D2 receptors during early ontogeny.
The purpose of the present study was to determine whether repeated administration of aripiprazole causes DA supersensitivity in young rats. As mentioned above, Tadokoro et al. (2012) reported that aripiprazole does not cause supersensitivity in adult rats; however, it is well established that young and adult animals often respond in a qualitatively different manner after repeated treatment with DA-acting drugs (for reviews, see Spear, 1979; Tirelli et al., 2003; Andersen, 2005). For this reason, young rats were treated with aripiprazole (10 mg/kg) on PD 10–20 and behavioral responsiveness to a challenge injection of saline or amphetamine (2 or 4 mg/kg) was assessed one, four, or eight days later (i.e., on PD 21, PD 24, or PD 28). For comparison purposes, separate groups of young rats were pretreated with the D2 antagonist haloperidol on PD 10–20. Horizontal locomotor activity and stereotyped sniffing were quantified across a 120 min testing period. Sniffing was assessed because this discrete behavior is a very reliable measure of moderately intense stereotypy in young rats (Cortez et al., 2010; Charntikov et al., 2011); whereas, locomotor activity is typically viewed as a nonstereotyped behavior that, under certain circumstances, may include a transient low-intensity stereotypy component (Segal and Kuczenski, 1987; Mueller et al., 1989; for a review, see Arnt, 1987). Therefore, by quantifying sniffing and locomotor activity we were able to determine the impact of repeated aripiprazole administration on both a stereotyped and nonstereotyped behavior. To examine D2 receptor up-regulation, homogenate ligand assays were used to measure D2 receptor densities in the dorsal striatum of aripiprazole- and haloperidol-pretreated rats on PD 21, PD 24, and PD 28.
Methods and Materials
Subjects
Subjects were 306 male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA, USA) that were born and bred in the university vivarium. Litters were culled to ten pups at PD 3 (day of parturition is PD 0) and weaned at PD 25. Both preweanling and postweanling rats were housed on racks in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids. Food and water were freely available. The colony room was maintained at 22–24°C and kept under a 12/12 h light/dark cycle. Behavioral testing was conducted in a separate experimental room during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2010) under a research protocol approved by the university’s Institutional Animal Care and Use Committee.
Apparatus
Behavioral testing was done in activity monitoring chambers (25.5 × 25.5 × 41 cm) that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Allentown, PA, USA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of horizontal locomotor activity). Photobeam resolution was 0.76 cm, with the position of each rat being determined every 100 ms.
Drugs
D-Amphetamine hemisulfate salt was dissolved in saline, whereas haloperidol was dissolved in a minimal amount of glacial acetic acid and diluted with saline. Aripiprazole was dissolved in (2-hydropropyl)-β-cyclodextrin solution (HBC, 45% (w/v) solution in water). All drugs were injected intraperitoneally (i.p.) at a volume of 2.5 ml/kg. With the exception of aripiprazole (Toronto Research Chemicals, Toronto, Canada), all nonlabeled ligands were purchased from Sigma-Aldrich (St. Louis, MO, USA). [3H]-spiperone (83.9 Ci/mmol) was purchased from PerkinElmer (Boston, MA, USA).
Behavioral Testing
On PD 10–20, young male and female rats (N = 216) were given daily pretreatment injections of saline vehicle, HBC vehicle, aripiprazole (10 mg/kg, i.p.), or haloperidol (1 mg/kg, i.p.). Behavioral testing occurred one, four, or eight days later (i.e., on PD 21, PD 24, or PD 28). Separate groups of rats were tested at each age to preclude sensitization effects. On the test day, rats (n = 8 per group) were injected with saline or D-amphetamine (2 or 4 mg/kg, i.p.) and immediately placed in activity monitoring chambers for 120 min (divided into six 20-min time blocks).
Distance traveled was assessed continuously across the testing session. In addition to this automated measure, behavior was recorded via ceiling-mounted hard disk cameras (JVC, model GZ-MG670) and data were later coded by observers blind to treatment conditions. Discrete behaviors (e.g., sniffing, vertical activity, and licking) were quantified using the fixed interval momentary time sampling method described by Cameron et al. (1988). Using this technique, the presence or absence of a particular behavior was determined at 20 s intervals during each 20-min time block. Behaviors of the saline- and HBC-treated rats did not differ, therefore data from the two vehicle groups were combined for presentation and statistical purposes.
D2 Homogenate Ligand Binding Assays
On PD 10–20, young male and female rats (N = 90) were given daily pretreatment injections of saline vehicle, HBC vehicle, aripiprazole (10 mg/kg, i.p.), or haloperidol (1 mg/kg, i.p.). After one, four, or eight days (i.e., on PD 21, PD 24, or PD 28), rats (n = 10 per group) were killed by rapid decapitation and dorsal striatal sections were dissected bilaterally on an ice-cold dissection plate and stored at –80°C. On the day of assay, tissue was thawed on ice and striatal samples were homogenized in 100 volumes of 50 mM Tris-HCl buffer (pH 7.4) for approximately 20 s using a Brinkmann Polytron. Homogenates were then centrifuged at 20,000 × g for 20 min. The pellet was resuspended in 100 volumes of the same buffer and centrifuged again at 20,000 × g for 20 min. The final pellet was suspended in approximately 30 volumes of buffer (pH 7.4). Protein concentrations for the final pellet were determined using the Bio-Rad Protein Assay.
Tissue suspensions were added to duplicate tubes containing 50 mM Tris, 2 mM NaCl2, 5 mM KCl, 1 mM MgSO4, and 2 mM CaCl2 (pH 7.4) at a final volume of 1 ml. The tubes also included [3H]-spiperone in concentrations ranging from 0.007 to 3.5 nM. Nonspecific binding was determined in the presence of 10 μM (–)-sulpiride. Incubation time for both assays was 30 min at 37°C. Incubation was terminated by vacuum filtration over glass fiber filters (Whatman GF/B, pretreated with 0.1% polyethylenimine). Filters were washed twice with ice-cold Tris-HCl buffer and radioactivity was measured by liquid scintillation spectrometry.
Data Analysis
Litter effects were minimized by assigning no more than one subject from each litter to a particular group (for a discussion of litter effects, see Zorrilla, 1997). When this procedure was not possible (i.e., analysis of body weights), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce, 1992; Zorrilla, 1997). Whenever possible, litter effects were also controlled through statistical procedures. Specifically, no more than one subject from each litter was assigned to a particular group and litter was used as the unit of analysis for statistical purposes (Zorrilla, 1997). With this statistical model each litter, rather than each rat, is treated as an independent observation (i.e., a within analysis using one value/condition/litter).
Body weight data on PD 21, PD 24, and PD 28 were analyzed using separate two-way (Pretreatment Condition × Sex) analyses of variance (ANOVA) at each test day. For the behavioral experiments, distance traveled and sniffing data were analyzed using three-way (Pretreatment Condition × Test Drug × Time Block) ANOVAs for each test day. Statistically significant three-way interactions were supplemented by separate two-way ANOVAs (Pretreatment Condition × Time Block) that were conducted for each test drug. For the ligand binding experiment, D2 binding sites (Bmax) and affinity (KD) were determined using nonlinear regression with Prism (GraphPad Software, San Diego, CA, USA). Bmax and KD values were analyzed using two-way (Pretreatment Condition × Recovery Interval) ANOVAs. When the assumption of sphericity was violated, as determined by Mauchly’s test of sphericity, the Huynh-Feldt epsilon statistic was used to adjust the degrees of freedom (Huynh and Feldt, 1976). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. Prepubescent rats, unlike young mice (Laviola et al., 1994), do not typically exhibit sex differences after DA agonist treatment (Frantz et al., 1996; Bowman et al., 1997; Snyder et al., 1998; Der-Ghazarian et al., 2012). Consistent with these past rat studies, distance traveled data did not differ according to sex, so this variable was not included in the final statistical analyses. Tukey tests (p < 0.05) were used for making post hoc comparisons.
Results
Body Weights
When measured on PD 21 and PD 24 (1 or 4 days after conclusion of the pretreatment regimen), body weights of aripiprazole- and haloperidol-pretreated rats were significantly reduced relative to the vehicle controls (Table 2) [Pretreatment Condition main effects, F(2,14)= 13.18, p < 0.01; F(2,14)= 8.89, p < 0.01, respectively]. Pretreatment regimen did not significantly affect body weights on PD 28 [Table 2 near here]. Overall, male rats weighed more than female rats on all three test days (i.e., PD 21, PD 24, and PD 28) [Sex main effects, F(1,7)= 10.25, p < 0.05; F(1,7)= 9.76, p < 0.05; F(1,7)= 44.51, p < 0.001, respectively]. At no age did the pretreatment variable interact with sex to affect body weight.
Table 2.
Mean body weights (g, +SEM) of male and female rats on PD 21, PD 24, and PD 28 (i.e., one, four, or eight days after conclusion of the 11-day drug pretreatment regimen
| Drug Pretreatment | Age
|
||||||
|---|---|---|---|---|---|---|---|
| PD 21
|
PD 24
|
PD 28
|
|||||
| Males | Females | Males | Females | Males | Females | ||
| Vehicle | 63.1 (+2.8) | 59.9 (+3.2) | 75.6 (+2.0) | 71.4 (+1.0) | 102.9 (+2.6) | 93.2 (+1.3) | |
| Aripiprazole (10 mg/kg) | 58.7 (+3.0)* | 54.5 (+3.4)* | 71.4 (+2.0)* | 66.1 (+1.7)* | 98.7 (+1.6) | 92.5 (+2.2) | |
| Haloperidol (1 mg/kg) | 57.2 (+3.0)* | 56.9 (+3.8)* | 72.2 (+2.2)* | 69.0 (+1.2)* | 100.5 (+1.9) | 93.6 (+1.6) | |
|
|
59.6 (+1.7) | 57.1 (+2.0)† | 73.0 (+1.2) | 68.8 (+0.9)† | 100.7 (+1.2) | 93.1 (+1.0)† | |
Significantly different from the vehicle-treated rats of the same age.
Significantly different from male rats of the same age.
Behavioral Effects of Aripiprazole and Haloperidol Pretreatment
Locomotor Activity
Regardless of test day (PD 21, PD 24, or PD 28), rats treated with 2 mg/kg D-amphetamine exhibited more locomotor activity than saline-treated rats, with 4 mg/kg D-amphetamine producing an intermediate level of locomotion that was significantly different from the other two groups [Test Drug main effects, F(2,14)= 124.16, p < 0.001; F(2,14)= 198.23, p < 0.001; F(2,14)= 98.07, p < 0.001, respectively].
When assessed one day after conclusion of the pretreatment phase (i.e., on PD 21), aripiprazole and haloperidol did not affect the locomotor activity of rats given saline or 2 mg/kg D-amphetamine (Figure 1) [Figure 1 near here]. When given a test day injection of 4 mg/kg D-amphetamine, haloperidol-pretreated rats exhibited less locomotor activity than vehicle controls on time blocks 2, 3, 5, and 6; whereas, aripiprazole-treated rats exhibited less locomotor activity than the vehicle group on time blocks 5 and 6 [aPretreatment Condition × Test Drug × Time Block interaction, F(16,113)= 1.78, p < 0.05; supplemented by aPretreatment Condition × Time Block interaction, F(10,67)= 6.30, p < 0.001; Pretreatment Condition main effect, F(2,14)= 5.32, p < 0.05].
Figure 1.
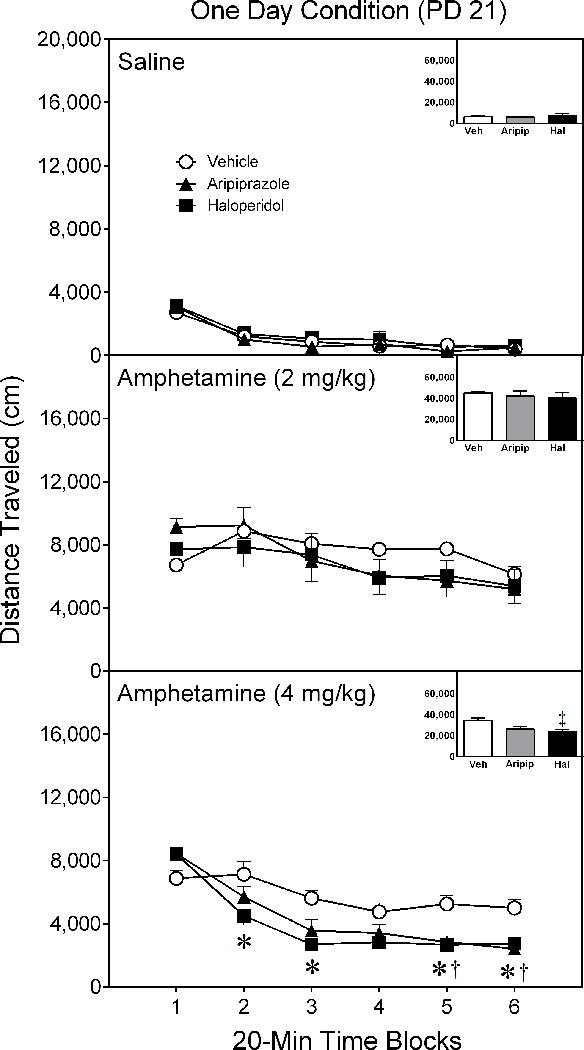
Mean distance traveled (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 21 (i.e., one day after conclusion of the drug pretreatment regimen). Rats had been pretreated with vehicle, 10 mg/kg aripiprazole, or 1 mg/kg haloperidol on PD 10–20. The testing session lasted 120 min (divided into six 20-min time blocks).
* Significant difference between the vehicle and haloperidol group on the same time block.
† Significant difference between the vehicle and aripiprazole group on the same time block.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6.
An almost identical pattern of behavior occurred when rats were tested four days after conclusion of the drug pretreatment regimen (i.e., on PD 24). Neither aripiprazole nor haloperidol pretreatment affected the test day performance of rats injected with saline or 2 mg/kg D-amphetamine (Figure 2) [Figure 2 near here]. On the other hand, haloperidol-pretreated rats given a test day injection of 4 mg/kg D-amphetamine exhibited less locomotor activity than vehicle controls on time blocks 2, 3, 5, and 6 [Pretreatment Condition × Test Drug × Time Block interaction, aF(14,95)= 3.58, p < 0.001; supplemented by aPretreatment Condition × Time Block interaction, F(4,30)= 6.54, p < 0.001; Pretreatment Condition main effect, F(2,14)= 5.71, p < 0.05]. On time blocks 5 and 6, aripiprazole-pretreated rats injected with 4 mg/kg D-amphetamine locomoted less than vehicle-pretreated rats given the identical dose of D-amphetamine.
Figure 2.
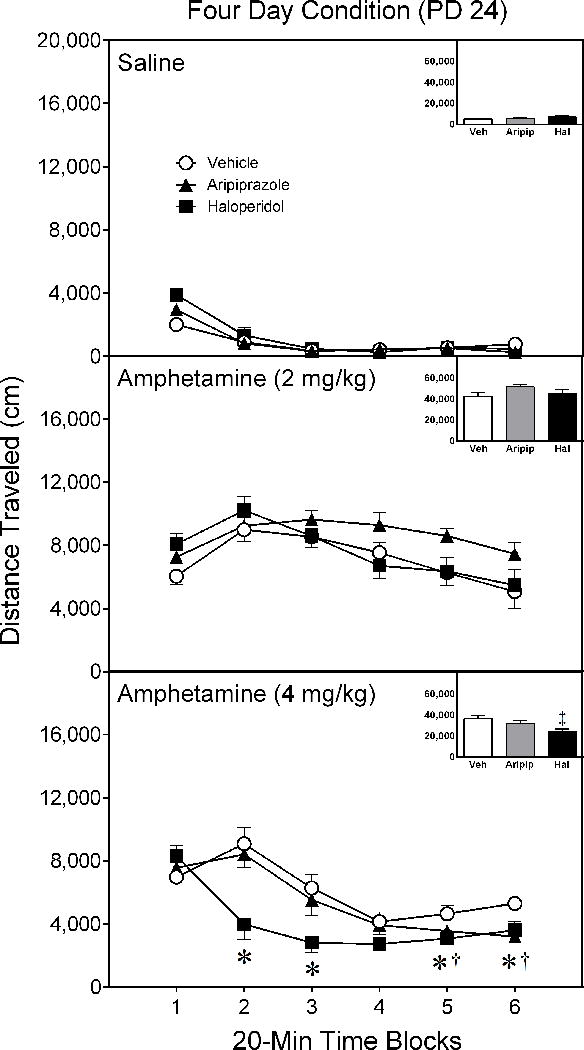
Mean distance traveled (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 24 (i.e., four days after conclusion of the drug pretreatment regimen). Rats had been pretreated with vehicle, 10 mg/kg aripiprazole, or 1 mg/kg haloperidol on PD 10–20. The testing session lasted 120 min (divided into six 20-min time blocks).
* Significant difference between the vehicle and haloperidol group on the same time block.
† Significant difference between the vehicle and aripiprazole group on the same time block.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6.
When assessed eight days after conclusion of the pretreatment phase (i.e., on PD 28), aripiprazole and haloperidol pretreatment did not differentially affect performance of rats given saline or 2 mg/kg D-amphetamine (Figure 3) [Figure 3 near here]. In contrast, aripiprazole- and haloperidol-pretreated rats given a test day injection of 4 mg/kg D-amphetamine exhibited less locomotor activity on time blocks 2–6 than vehicle controls injected with an identical dose of D-amphetamine [aPretreatment Condition × Test Drug × Time Block interaction, F(13,92)= 2.25, p < 0.05; supplemented by aPretreatment Condition × Time Block interaction, F(6,43)= 4.63, p < 0.001; Pretreatment Condition main effect, F(2,14)= 9.80, p < 0.01].
Figure 3.
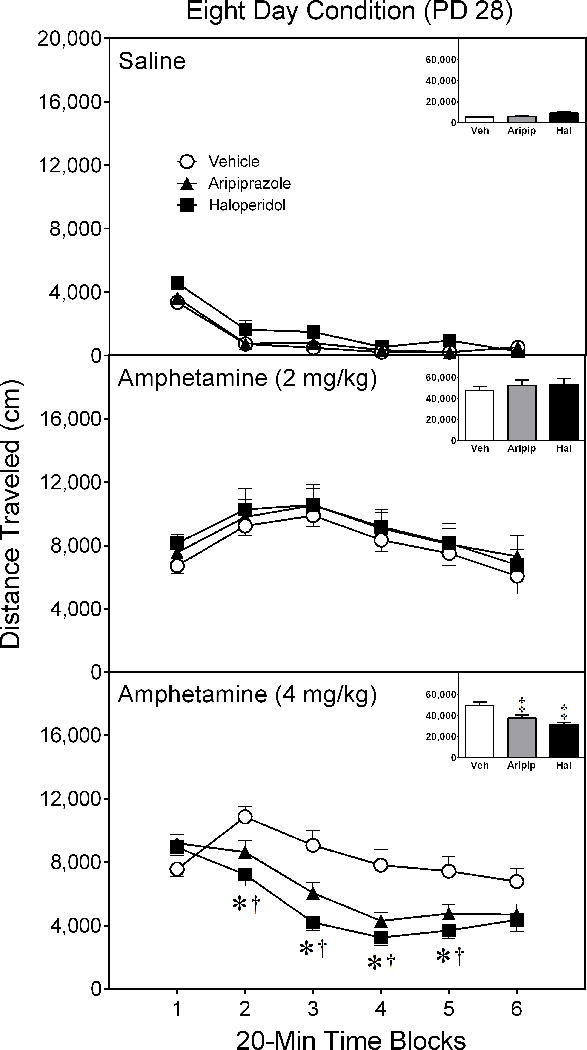
Mean distance traveled (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 28 (i.e., eight days after conclusion of the drug pretreatment regimen). Rats had been pretreated with vehicle, 10 mg/kg aripiprazole, or 1 mg/kg haloperidol on PD 10–20. The testing session lasted 120 min (divided into six 20-min time blocks).
* Significant difference between the vehicle and haloperidol group on the same time block.
† Significant difference between the vehicle and aripiprazole group on the same time block.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6.
Sniffing
On all test days (PD 21, PD 24, and PD 28), amphetamine caused a dose-dependent increase in stereotyped sniffing [Test Drug main effects, F(2,14)= 74.38, p < 0.001; F(2,14)= 108.00, p < 0.001; F(2,14)= 120.69, p < 0.001, respectively]. On PD 21 [Figure 4 near here], rats pretreated with either aripiprazole or haloperidol had significantly greater sniffing counts than rats exposed to vehicle (Figure 4) [Pretreatment Condition main effect, F(2,14)= 7.54, p < 0.01]. On PD 24 [Figure 5 near here], only haloperidol-pretreated rats had more sniffing counts than vehicle controls (Figure 5) [Pretreatment Condition main effect, F(2,14)= 5.09, p < 0.05].
Figure 4.
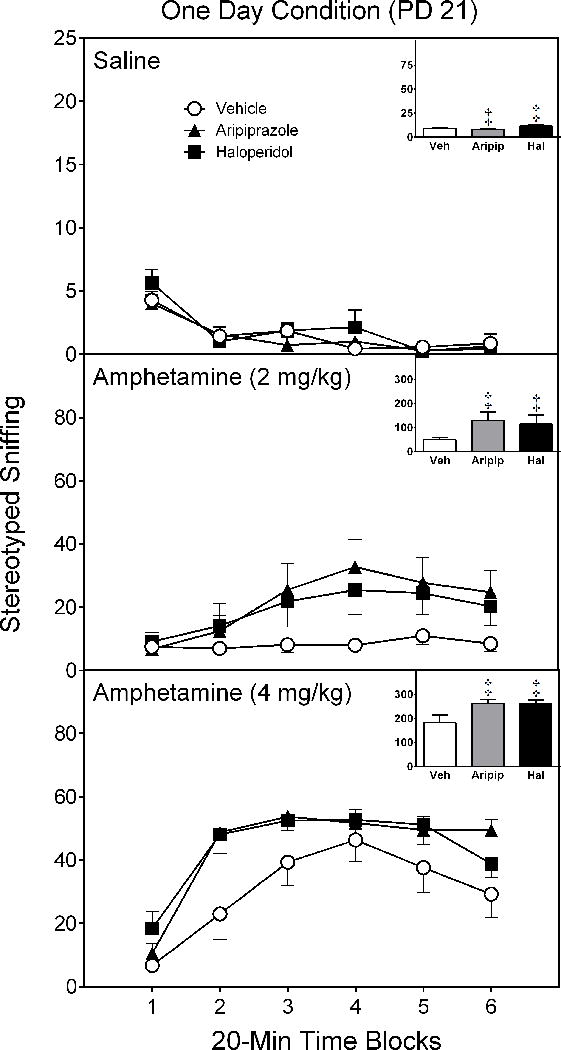
Mean sniffing counts (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 21 (i.e., one day after conclusion of the drug pretreatment regimen). These are the same rats as described in Figure 1.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6 (Pretreatment Condition main effect, p < 0.05).
Figure 5.
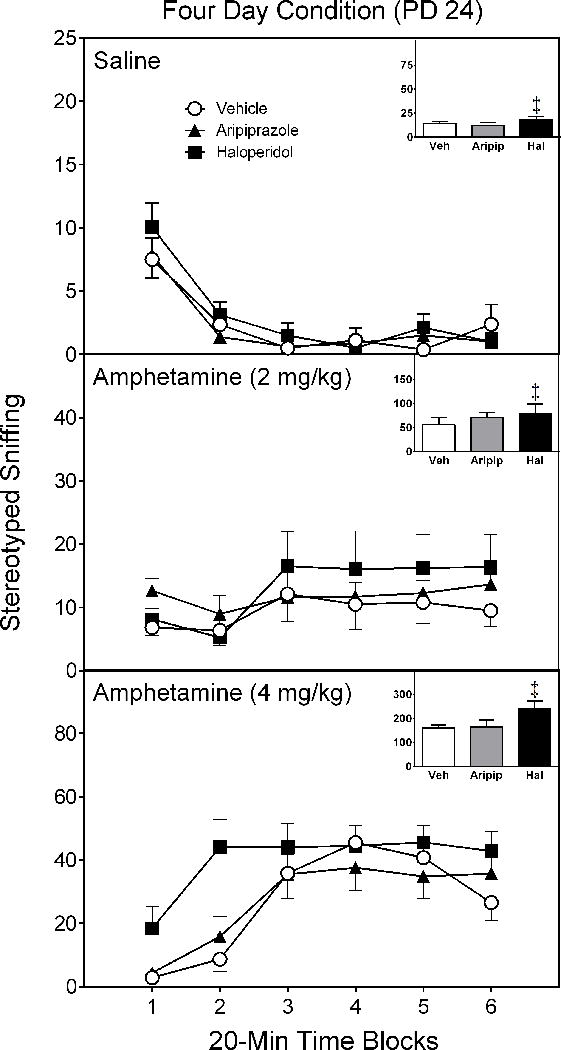
Mean sniffing counts (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 24 (i.e., four days after conclusion of the drug pretreatment regimen). These are the same rats as described in Figure 2.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6 (Pretreatment Condition main effect, p < 0.05).
When assessed eight days after conclusion of the pretreatment phase (i.e., on PD 28), pretreatment regimen did not affect the stereotyped sniffing of rats injected with saline or 2 mg/kg amphetamine (Figure 6) [Figure 6 near here]. In contrast, haloperidol-, but not aripiprazole-, pretreated rats exhibited significantly more stereotyped sniffing counts than vehicle controls when challenged with 4 mg/kg amphetamine [Pretreatment Condition × Test Drug interaction, F(4,28)= 6.54, p < 0.01; supplemented by Pretreatment Condition main effect, F(2,14)= 8.09, p < 0.01].
Figure 6.
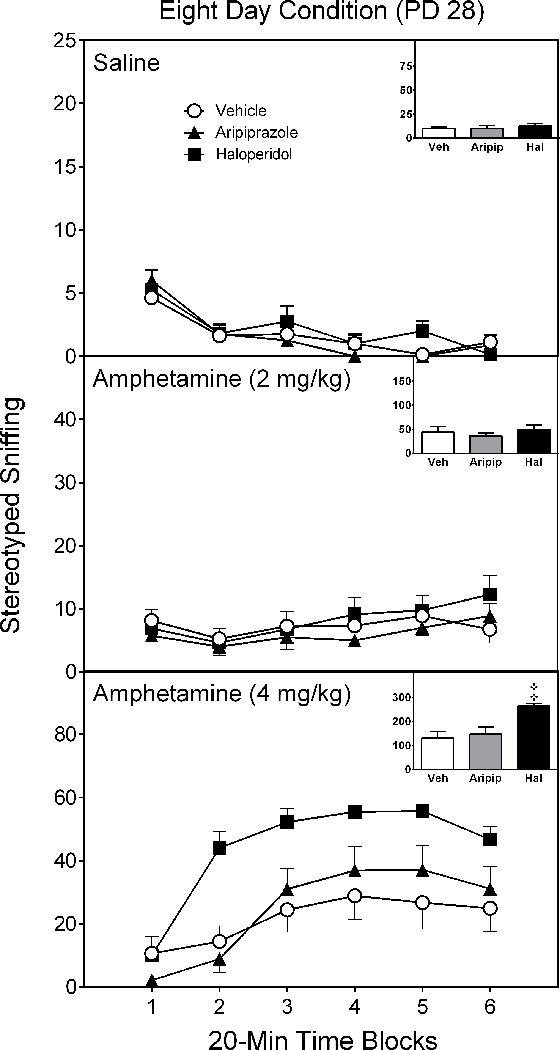
Mean sniffing counts (±SEM) of saline- and amphetamine-treated rats tested on postnatal day (PD) 28 (i.e., eight days after conclusion of the drug pretreatment regimen). These are the same rats as described in Figure 3.
‡ Significantly different from the vehicle group when collapsed across time blocks 1–6 (Pretreatment Condition × Test Drug interaction, p < 0.05).
D2 Receptor Binding in the Dorsal Striatum
Overall, both aripiprazole and haloperidol increased D2 binding site densities (i.e., Bmax values) in the dorsal striatum (Table 3) [Pretreatment Condition main effect, F(2,54)= 7.54, p < 0.001]. This effect was not restricted to a single day, but was evident when collapsed over the three assessment periods [Table 3 near here]. D2 receptor densities were significantly greater on PD 28 (eight days after conclusion of the drug pretreatment regimen) than on PD 21 or PD 24 [Test Day main effect, F(2,27)= 4.46, p < 0.05]. D2 receptor affinity (KD) did not differ according to drug pretreatment regimen; however, KD values of rats tested on PD 21 ( , SEM = +.028) were significantly greater than on PD 24 ( , SEM = +.008) or PD 28 ( , SEM = +.010) [Test Day main effect, F(2,27)= 13.09, p < 0.001].
Table 3.
Mean D2 receptor densities of young rats (n = 10 per group) measured one, four, or eight days after conclusion of the drug pretreatment regimen that occurred on PD 10–20. Bmax values are expressed as fmol/mg protein (+SEM).
| Drug Pretreatment | Days After Pretreatment Regimen
|
|||
|---|---|---|---|---|
| One | Four | Eight |
|
|
| Vehicle | 255.34 (+31.6) | 235.11 (+20.5) | 350.51 (+23.1) | 280.32 (+17.0) |
| Aripiprazole (10 mg/kg) | 334.39 (+33.4) | 298.56 (+30.1) | 370.35 (+26.5) | 334.43 (+17.7)* |
| Haloperidol (1 mg/kg) | 326.11 (+30.1) | 320.73 (+36.2) | 398.16 (+22.0) | 348.33 (+18.0)* |
Significantly different from the vehicle group.
Discussion
Although having different mechanisms of action, repeated treatment with either the partial D2 agonist aripiprazole or the full D2 antagonist haloperidol caused a supersensitive behavioral response in young rats challenged with a high dose of D-amphetamine. The pattern of behavioral supersensitivity varied depending on when D-amphetamine was administered. If assessed one day after conclusion of the drug pretreatment regimen, rats given aripiprazole or haloperidol exhibited both a potentiated sniffing response and an attenuated locomotor response after a challenge injection of D-amphetamine. If measured four or eight days after the drug pretreatment regimen, both aripiprazole and haloperidol partially attenuated the locomotor activating effects of 4 mg/kg D-amphetamine. In contrast, only haloperidol was able to potentiate D-amphetamine-induced stereotyped sniffing on PD 24 and PD 28. This pattern of results suggests that repeated haloperidol treatment produced a more robust supersensitive response than aripiprazole. A simplistic explanation is that the doses of aripiprazole (10 mg/kg) and haloperidol (1 mg/kg) were not equivalent, thus causing haloperidol to have a more pronounced behavioral effect. Differences in D2 receptor affinity and intrinsic efficacy may play a more important role (Lawler et al., 1999; Burris et al., 2002). Alternatively, the divergent effects of aripiprazole and haloperidol may be a consequence of nonDA-mediated processes. For example, the partial agonist activities of aripiprazole at the 5-HT1A receptor may moderate or partially compensate for actions at the D2 receptor.
Bordi et al. (1989) have proposed that DA-mediated behaviors are on a continuum: locomotor activity and rearing are induced by relatively small doses of a DA agonist (e.g., apomorphine or amphetamine); low- and moderate-intensity stereotypies (e.g., stereotyped sniffing) predominate after medium doses; and high-intensity stereotypies (e.g., vacuous oral movements) are observed after larger doses. Importantly, as the dose of psychostimulant increases “behavioral competition” can occur, in which stereotypy competes with, and partially masks, the locomotor response (Morelli et al., 1980; Bordi et al., 1989; Mestlin and McDougall, 1993). In terms of this conceptual framework, we believe that evidence for behavioral supersensitivity is provided by both the increased stereotyped sniffing and the decreased locomotion exhibited by aripiprazole- and haloperidol-pretreated rats. In some cases, locomotor activity was the more sensitive measure for detecting drug-induced effects. This was probably a consequence of stereotypy being represented by a single behavior (i.e., various manifestations of stereotypy competed against the locomotor response).
DA receptor up-regulation and associated neuronal changes may have been responsible for behavioral supersensitivity, because rats given a pretreatment regimen of aripiprazole or haloperidol on PD 10–20 had significantly more dorsal striatal D2 receptors than vehicle-treated controls. Although basal levels of striatal D2 receptors increased from PD 21 to PD 28, D2 receptor up-regulation was evident across all three test days. In contrast to these results, Tadokoro et al. (2012) reported that adult rats do not exhibit behavioral supersensitivity or D2 receptor up-regulation when tested seven days after conclusion of a 14-day aripiprazole pretreatment regimen. Repeated haloperidol treatment, on the other hand, did cause both behavioral supersensitivity and D2 receptor up-regulation in adult rats (Tadokoro et al., 2012). A similar pattern of neurochemical effects was observed after a 21-day drug regimen, as repeated haloperidol treatment increased dorsal striatal D2 receptor numbers in adult rats, while aripiprazole was without significant effect (Inoue et al., 1997). Interestingly, Seeman (2008) also reported that a pretreatment regimen of aripiprazole (1.5 mg/kg/day) failed to increase D2 receptor levels in the dorsal striatum of adult rats; however, the percentage of receptors in the D2High state nearly doubled. The elevated number of D2High receptors is potentially important, because these high affinity receptors have been linked to DA supersensitivity (Seeman et al., 2005).
When the latter results are considered together, it appears that repeated aripiprazole treatment causes D2 receptor up-regulation in the dorsal striatum of young rats (present study), whereas a similar aripiprazole regimen does not increase D2 receptor numbers in adult rats (Inoue et al., 1997; Seeman, 2008; Tadokoro et al., 2012). In comparison, haloperidol up-regulates dorsal striatal D2 receptors in both age groups. An obvious possibility is that the differential actions of aripiprazole are a function of age. Needless to say, many dopaminergic elements (e.g., DA content, VMAT2 transporters, plasma membrane DA transporters) exhibit maturational changes across ontogeny (Giorgi et al., 1987; Broaddus and Bennett, 1990; Truong et al., 2005; Kuperstein et al., 2008). Perhaps of greatest relevance, dorsal striatal D1 and D2 receptor sites increase in number from birth through the preweanling period (Rao et al., 1991; Kuperstein et al., 2008), are dramatically overproduced during adolescence (Teicher et al., 1995; Andersen et al., 1997), and then gradually decline until adult-like levels are reached (for reviews, see Andersen and Teicher, 2000; Andersen, 2003). To our knowledge, no systematic experiments involving multiple age groups have been conducted to determine whether D2 receptor up-regulation varies across early ontogeny; however, it is possible that the brains of younger, still developing rats are more plastic than adults and, as a consequence, are more prone to aripiprazole-induced receptor up-regulation.
Alternatively, there are many procedural differences between these studies, hence other factors might be responsible for the occurrence (or nonoccurrence) of aripiprazole-induced receptor up-regulation. An obvious candidate is the aripiprazole dosing schedule; however, young rats exhibited D2 receptor up-regulation after daily injections of 10 mg/kg aripiprazole, whereas adult rats showed an absence of receptor up-regulation after daily treatments with a broad dose range (1.5–100 mg/kg) of aripiprazole (Inoue et al., 1997; Seeman, 2008; Tadokoro et al., 2012). Length of the drug pretreatment regimens varied greatly between studies but overlapped with our procedure, as we administered aripiprazole for 11 days, while adult rats were given aripiprazole for 7 (Seeman, 2008), 14 (Tadokoro et al., 2012), or 21 (Inoue et al., 1997) days. In terms of assessing DA supersensitivity, the biggest difference between studies was that we measured locomotor activity and stereotypy after challenge injections of either a moderate or high dose of D-amphetamine, whereas Tadokoro et al. (2012) assessed locomotor activity after treatment with a relatively low dose of methamphetamine (1 mg/kg). In our study, behavioral supersensitivity was most prominent when a high dose of D-amphetamine (4 mg/kg) was administered. Considering the latter result, it remains possible that aripiprazole-pretreated adult rats might exhibit a supersensitive behavioral response if stereotyped behaviors were assessed and a high dose of psychostimulant was administered on the test day.
In the present study, various stereotyped behaviors were quantified and it was apparent that sniffing was the predominant form of stereotypy. Young rats and mice typically exhibit less intense stereotypies than adults (Adriani and Laviola, 2000; but see Abrams and Bruno, 1992), with stereotyped sniffing rather than vacuous oral movements predominating in young rats given high-dose DA agonist treatment (e.g., Charntikov et al., 2008, 2011; Cortez et al., 2010). A different pattern of effects was evident when behavior was assessed one day after conclusion of the drug pretreatment regimen (i.e., on PD 21). Specifically, aripiprazole- and haloperidol-pretreated rats exhibited substantial amounts of stereotyped licking (a vacuous oral movement) when challenged with 4 mg/kg D-amphetamine (data not shown). Stereotyped licking was not observed in vehicle-treated rats or when testing occurred four or eight days after conclusion of the drug pretreatment regimen. Thus, it appears that the most intense stereotypy occurred one day after the final haloperidol or aripiprazole injection.
When assessed one or four days after conclusion of the drug pretreatment regimen, both aripiprazole and haloperidol significantly reduced the body weight gain of our male and female rats (similar trends were reported in a previous study; Der-Ghazarian et al., 2012). Unfortunately, studies involving adult rats provide inconsistent results, because repeated haloperidol treatment (3–12 weeks) has been reported to reduce (von Wilmsdorff et al., 2010, 2013), not affect (Minet-Ringuet et al., 2005; Lin et al., 2006; Han et al., 2008), or enhance (Fell et al., 2004, 2005) body weight gain. In the latter case, females were more likely than males to exhibit haloperidol-induced weight gain (Baptista et al., 1987; Pouzet et al., 2003). Although studied less intensively, long-term treatment with a high dose of aripiprazole (40 mg/kg) may cause a relative decline in the body weights of adult rats (Segnitz et al., 2009), while a 12-week exposure to a low dose of aripiprazole (2.25 mg/kg) was without effect (Han et al., 2008). The reason why aripiprazole and haloperidol decreased the body weights of our young rats is uncertain, but it could be a consequence of elevated levels of circulating insulin (Lin et al., 2006; von Wilmsdorff et al., 2013). It is also possible that the drugs interfered with the ability to suckle, since body weight differences between the aripiprazole and vehicle groups were apparent by PD 13 (data not shown).
In clinical terms, adult humans respond well to aripiprazole, as it has an excellent side-effect profile (for reviews, see Stip and Tourjman, 2010; Croxtall, 2012). Even so, the response of younger patients to any pharmacotherapy cannot be deduced from observing adults, especially since children and adolescents are more prone to tolerability issues (Correll and Carlson, 2006). Moreover, safety concerns increase in prominence if drugs are prescribed for long durations (Fraguas et al., 2011), and available evidence suggests that children and adolescents are typically maintained on second-generation antipsychotics for 30 months or more (Kalverdijk et al., 2008). All of that being said, there are enough studies involving pediatric populations to tentatively conclude that aripiprazole has a good side-effect profile in younger age groups as well as adults (Greenaway and Elbe, 2009; Doey, 2012; Kirino, 2012; but see Rugino and Janvier, 2005; Fraguas et al., 2011; McKinney and Renk, 2011). When compared to second-generation antipsychotics, aripiprazole causes less weight gain in children, less sedation, and does not increase serum prolactin levels (Ardizzone et al., 2010; Fraguas et al., 2011; Zuddas et al., 2011). Neuromotor side-effects are more problematic. Although superior to first-generation antipsychotics (e.g., haloperidol), aripiprazole is prone to akathisia and extrapyramidal symptoms, such as tremor and Parkinsonism (Findling et al., 2008, 2009b; Zuddas et al., 2011). Not surprisingly, higher doses (e.g., 30 mg/day) of aripiprazole are more likely to produce neuromotor side-effects in children and adolescents than are lower doses (10 mg/day) (Findling et al., 2008, 2009b; Ardizzone et al., 2010).
With regard to translational relevance, the dose of aripiprazole used in the present study (10 mg/kg/day) is substantially greater than the dose range typically administered to children and adolescents (10–30 mg/day). These dosing parameters can be explained by drug metabolism pharmacokinetics, as the elimination half-life of aripiprazole is approximately 47–75 hr in humans (Mallikaarjun et al., 2004; Greenaway and Elbe, 2009) and 1.9–2.2 hr in rats (Shimokawa et al., 2005). When coupled with the different lengths of drug exposure (days for young rats vs. months to years for young humans), it is possible that the aripiprazole- and haloperidol-induced neural changes reported in the present study conservatively reflect receptor alterations that might be occurring in children and adolescents. As mentioned above, aripiprazole has a good side-effect profile, although younger patients may exhibit mild-to-moderate extrapyramidal symptoms and akathisia. Based on the present results, it is possible that some of these motoric effects may be a consequence of DA supersensitivity and associated D2 receptor changes in the dorsal striatum (for further discussion, see Schröder et al., 1998; Tenback et al., 2010).
In terms of limitations and constraining factors, the most notable issue is that only a single dose of aripiprazole was used and neither serum nor brain levels of this compound were determined. Aripiprazole doses ranging from 10–12 mg/kg are commonly administered to rodents (Semba et al., 1995; Li et al., 2005; Cheng et al., 2008; Segnitz et al., 2009), but a broader dose range would have made interpretation easier. The overarching purpose of this study was to determine whether administering aripiprazole to young rats would cause D2 receptor up-regulation and DA supersensitivity. Because rodent development is so rapid (for a review, see Andersen, 2003), the drug pretreatment regimen was necessarily short (11 days). To test the same experimental hypotheses using a substantially longer pretreatment regimen would require a species with a more prolonged developmental trajectory. It is also important to recognize that we used “normal” rats and not an animal model designed to mimic one or more aspects of a human disorder (for the advantages and disadvantages of using these animal models, see Lipska and Weinberger, 2000; McGonigle, 2013). This decision was partially based on the realization that aripiprazole is used to treat a wide variety of developmental disorders (e.g., bipolar I disorder, schizophrenia, autism and other pervasive developmental disorders, aggression, conduct disorder, Tourette’s syndrome and chronic tic disorder); thus, the use of a standard rat strain was considered appropriate for this exploratory study. Lastly, since aripiprazole affects DA functioning, as well as serotonergic, GABAergic, and glutamatergic systems, future research should be aimed at parsing out whether nondopaminergic systems interact with DA systems to mediate or influence aripiprazole-induced behavioral supersensitivity and D2 receptor up-regulation.
Acknowledgments
We are grateful to Ana Veliz for help with testing the animals.
Funding
This work was supported by the National Institute of General Medical Sciences [grant number GM083883]; and the National Institute of Drug Abuse [grant number DA025319].
Footnotes
Conflict of interest
The Authors declare that there is no conflict of interest.
References
- Abrams DR, Bruno JP. Ontogeny of apomorphine-induced stereotypy and its D1 and D2 receptor mediation in rats depleted of dopamine as neonates. Dev Psychobiol. 1992;25:475–495. doi: 10.1002/dev.420250703. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral responsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, et al. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Ardizzone I, Nardecchia F, Marconi A, et al. Antipsychotic medication in adolescents suffering from schizophrenia: a meta-analysis of randomized controlled trials. Psychopharmacol Bull. 2010;43:45–66. [PubMed] [Google Scholar]
- Arnt J. Behavioral studies of dopamine receptors: evidence for regional selectivity and receptor multiplicity. In: Creese I, Fraser CM, editors. Receptor biochemistry and methodology. Vol. 8. Dopamine receptors; Liss, New York: 1987. [Google Scholar]
- Baptista T, Parada M, Hernandez L. Long term administration of some antipsychotic drugs increases body weight and feeding in rats. Are D2 dopamine receptors involved? Pharmacol Biochem Behav. 1987;27:399–405. doi: 10.1016/0091-3057(87)90340-6. [DOI] [PubMed] [Google Scholar]
- Bastiaens L. A non-randomized, open study with aripiprazole and ziprasidone for the treatment of aggressive behavior in youth in a community clinic. Community Ment Health J. 2009;45:73–77. doi: 10.1007/s10597-008-9154-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Blatt B, Kuhn CM. Ontogeny of the behavioral response to dopamine agonists after chronic cocaine. Psychopharmacology (Berl) 1997;129:121–127. doi: 10.1007/s002130050171. [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP. Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Dev Brain Res. 1990;52:265–271. doi: 10.1016/0165-3806(90)90244-s. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Crosbie J, Crocker AD. A fixed interval momentary sampling method for assessing on-going behaviours induced by dopamine receptor agonists. Prog Neuro-Psychopharmacol Biol Psychiatry. 1988;12:595–606. doi: 10.1016/0278-5846(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Charntikov S, Halladay LR, Herbert MS, et al. Effects of dorsal striatal infusions of R(–)-propylnorapomorphine (NPA) on κ-opioid-mediated locomotor activity in the young rat: possible role of the indirect pathway. Neuroscience. 2008;155:603–612. doi: 10.1016/j.neuroscience.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Der-Ghazarian T, Herbert MS, et al. Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats. Neuroscience. 2011;183:121–133. doi: 10.1016/j.neuroscience.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MC, Liao DL, Hsiung CA, et al. Chronic treatment with aripiprazole induces differential gene expression in the rat frontal cortex. Int J Neuropsychopharmacol. 2008;11:207–216. doi: 10.1017/S1461145707008048. [DOI] [PubMed] [Google Scholar]
- Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Cortez AM, Charntikov S, Der-Ghazarian T, et al. Age-dependent effects of κ-opioid receptor stimulation on cocaine-induced stereotyped behaviors and dopamine overflow in the caudate-putamen: an in vivo microdialysis study. Neuroscience. 2010;169:203–213. doi: 10.1016/j.neuroscience.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD. Aripiprazole: a review of its use in the management of schizophrenia in adults. CNS Drugs. 2012;26:155–183. doi: 10.2165/11208400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Der-Ghazarian T, Charntikov S, Varela FA, et al. Effects of repeated and acute aripiprazole or haloperidol treatment on dopamine synthesis in the dorsal striatum of young rats: comparison to adult rats. J Neural Transm. 2010;117:573–583. doi: 10.1007/s00702-010-0396-5. [DOI] [PubMed] [Google Scholar]
- Der-Ghazarian T, Gutierrez A, Varela FA, et al. Dopamine receptor inactivation in the caudate-putamen differentially affects the behavior of preweanling and adult rats. Neuroscience. 2012;226:427–440. doi: 10.1016/j.neuroscience.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doey T. Aripiprazole in pediatric psychosis and bipolar disorder: a clinical review. J Affect Disord. 2012;138(Suppl):S15–S21. doi: 10.1016/j.jad.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Neill JC, Marshall KM. Effects of the classical antipsychotic haloperidol and atypical antipsychotic risperidone on weight gain, the oestrous cycle and uterine weight in female rats. Eur Neuropsychopharmacol. 2004;14:385–392. doi: 10.1016/j.euroneuro.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Neill JC, Rao C, et al. Effects of sub-chronic antipsychotic drug treatment on body weight and reproductive function in juvenile female rats. Psychopharmacology (Berl) 2005;182:499–507. doi: 10.1007/s00213-005-0131-3. [DOI] [PubMed] [Google Scholar]
- Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- Findling RL, Kauffman R, Sallee FR, et al. An open-label study of aripiprazole: pharmacokinetics, tolerability, and effectiveness in children and adolescents with conduct disorder. J Child Adolesc Psychopharmacol. 2009a;19:431–439. doi: 10.1089/cap.2008.0111. [DOI] [PubMed] [Google Scholar]
- Findling RL, Nyilas M, Forbes RA, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2009b;70:1441–1451. doi: 10.4088/JCP.09m05164yel. [DOI] [PubMed] [Google Scholar]
- Fraguas D, Correll CU, Merchán-Naranjo J, et al. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol. 2011;21:621–645. doi: 10.1016/j.euroneuro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Frantz K, Babcock D, Van Hartesveldt C. The locomotor effects of a putative dopamine D3 receptor agonist in developing rats. Eur J Pharmacol. 1996;302:1–6. doi: 10.1016/0014-2999(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, et al. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Dev Brain Res. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Greenaway M, Elbe D. Focus on aripiprazole: a review of its use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2009;18:250–260. [PMC free article] [PubMed] [Google Scholar]
- Han M, Deng C, Burne TH, et al. Short- and long-term effects of antipsychotic drug treatment on weight gain and H1 receptor expression. Psychoneuroendocrinology. 2008;33:569–580. doi: 10.1016/j.psyneuen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Inoue A, Miki S, Seto M, et al. Aripiprazole, a novel antipsychotic drug, inhibits quinpirole-evoked GTPase activity but does not up-regulate dopamine D2 receptor following repeated treatment in the rat striatum. Eur J Pharmacol. 1997;321:105–111. doi: 10.1016/s0014-2999(96)00920-x. [DOI] [PubMed] [Google Scholar]
- Jordan S, Koprivica V, Chen R, et al. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- Jordan S, Koprivica V, Dunn R, et al. In vivo effects of aripiprazole on cortical and striatal dopaminergic and serotonergic function. Eur J Pharmacol. 2004;483:45–53. doi: 10.1016/j.ejphar.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Kalverdijk LJ, Tobi H, van den Berg PB, et al. Use of antipsychotic drugs among Dutch youths between 1997 and 2005. Psychiatr Serv. 2008;59:554–560. doi: 10.1176/ps.2008.59.5.554. [DOI] [PubMed] [Google Scholar]
- Kirino E. Efficacy and safety of aripiprazole in child and adolescent patients. Eur Child Adolesc Psychiatry. 2012;21:361–368. doi: 10.1007/s00787-012-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koener B, Focant MC, Bosier B, et al. Increasing the density of the D2L receptor and manipulating the receptor environment are required to evidence the partial agonist properties of aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:60–70. doi: 10.1016/j.pnpbp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem. 2008;106:662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Dell’Omo G, Chiarotti F, et al. d-Amphetamine conditioned place preference in developing mice: relations with changes in activity and stereotypies. Behav Neurosci. 1994;108:514–524. doi: 10.1037//0735-7044.108.3.514. [DOI] [PubMed] [Google Scholar]
- Li M, Budin R, Fleming AS, et al. Effects of novel antipsychotics, amisulpiride and aripiprazole, on maternal behavior in rats. Psychopharmacology (Berl) 2005;181:600–610. doi: 10.1007/s00213-005-0091-7. [DOI] [PubMed] [Google Scholar]
- Lin EJ, Lee NJ, Slack K, et al. Distinct endocrine effects of chronic haloperidol or risperidone administration in male rats. Neuropharmacology. 2006;51:1129–1136. doi: 10.1016/j.neuropharm.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44:179–187. doi: 10.1177/0091270003261901. [DOI] [PubMed] [Google Scholar]
- Masi G, Cosenza A, Millepiedi S, et al. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: a retrospective study. CNS Drugs. 2009;23:511–521. doi: 10.2165/00023210-200923060-00005. [DOI] [PubMed] [Google Scholar]
- McGonigle P. Animal models of CNS disorders. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.06.016. http://dx.doi.org/10.1016/j.bcp.2013.06.016. [DOI] [PubMed]
- McKinney C, Renk K. Atypical antipsychotic medications in the management of disruptive behaviors in children: safety guidelines and recommendations. Clin Psychol Rev. 2011;31:465–471. doi: 10.1016/j.cpr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology (Berl) 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- Mestlin M, McDougall SA. Ontogenetic differences in the effects of EEDQ on dopamine mediated behaviors. Pharmacol Biochem Behav. 1993;45:797–802. doi: 10.1016/0091-3057(93)90123-b. [DOI] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Guesdon B, et al. Effects of chronic neuroleptic treatments on nutrient selection, body weight, and body composition in the male rat under dietary self-selection. Behav Brain Res. 2005;163:204–211. doi: 10.1016/j.bbr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Morelli M, Porceddu ML, Di Chiara G. Lesions of substantia nigra by kainic acid: effects on apomorphine-induced stereotyped behaviour. Brain Res. 1980;191:67–78. doi: 10.1016/0006-8993(80)90315-7. [DOI] [PubMed] [Google Scholar]
- Mueller K, Hollingsworth EM, Cross DR. Another look at amphetamine-induced stereotyped locomotor activity in rats using a new statistic to measure locomotor stereotypy. Psychopharmacology (Berl) 1989;97:74–79. doi: 10.1007/BF00443416. [DOI] [PubMed] [Google Scholar]
- Murphy TK, Mutch PJ, Reid JM, et al. Open label aripiprazole in the treatment of youth with tic disorders. J Child Adolesc Psychopharmacol. 2009;19:441–447. doi: 10.1089/cap.2008.0149. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8th. National Academies Press; Washington: 2010. [Google Scholar]
- Peselmann N, Schmitt A, Gebicke-Haerter PJ, et al. Aripiprazole differentially regulates the expression of Gad67 and γ-aminobutyric acid transporters in rat brain. Eur Arch Psychiatry Clin Neurosci. 2013;263:285–297. doi: 10.1007/s00406-012-0367-y. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Mow T, Kreilgaard M, et al. Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacol Biochem Behav. 2003;75:133–140. doi: 10.1016/s0091-3057(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: quantitative autoradiographic study. Dev Brain Res. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Rugino TA, Janvier YM. Aripiprazole in children and adolescents: clinical experience. J Child Neurol. 2005;20:603–610. doi: 10.1177/08830738050200071301. [DOI] [PubMed] [Google Scholar]
- Schröder J, Silvestri S, Bubeck B, et al. D2 dopamine receptor up-regulation, treatment response, neurological soft signs, and extrapyramidal side effects in schizophrenia: a follow-up study with 123I-iodobenzamide single photon emission computed tomography in the drug-naive state and after neuroleptic treatment. Biol Psychiatry. 1998;43:660–665. doi: 10.1016/s0006-3223(97)00442-3. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2(High) receptors moderately elevated by bifeprunox and aripiprazole. Synapse. 2008;62:902–908. doi: 10.1002/syn.20557. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Segnitz N, Schmitt A, Gebicke-Härter PJ, et al. Differential expression of glutamate transporter genes after chronic oral treatment with aripiprazole in rats. Neurochem Int. 2009;55:619–628. doi: 10.1016/j.neuint.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Segnitz N, Ferbert T, Schmitt A, et al. Effects of chronic oral treatment with aripiprazole on the expression of NMDA receptor subunits and binding sites in rat brain. Psychopharmacology (Berl) 2011;217:127–142. doi: 10.1007/s00213-011-2262-z. [DOI] [PubMed] [Google Scholar]
- Semba J, Watanabe A, Kito S, et al. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34:785–791. doi: 10.1016/0028-3908(95)00059-f. [DOI] [PubMed] [Google Scholar]
- Seo WS, Sung HM, Sea HS, et al. Aripiprazole treatment of children and adolescents with Tourette disorder or chronic tic disorder. J Child Adolesc Psychopharmacol. 2008;18:197–205. doi: 10.1089/cap.2007.0064. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Shimokawa Y, Akiyama H, Kashiyama E, et al. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: application to the pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:8–14. doi: 10.1016/j.jchromb.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The use of psychopharmacological procedures to analyse the ontogeny of learning and retention: issues and concerns. In: Spear NE, Campbell BA, editors. Ontogeny of learning and memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1979. [Google Scholar]
- Stachnik JM, Nunn-Thompson C. Use of atypical antipsychotics in the treatment of autistic disorder. Ann Pharmacother. 2007;41:626–634. doi: 10.1345/aph.1H527. [DOI] [PubMed] [Google Scholar]
- Stigler KA, Diener JT, Kohn AE, et al. Aripiprazole in pervasive developmental disorder not otherwise specified and Asperger’s disorder: a 14-week, prospective, open-label study. J Child Adolesc Psychopharmacol. 2009;19:265–274. doi: 10.1089/cap.2008.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stip E, Tourjman V. Aripiprazole in schizophrenia and schizoaffective disorder: a review. Clin Ther. 2010;32(Suppl 1):S3–S20. doi: 10.1016/j.clinthera.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia – a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38:1012–1020. doi: 10.1093/schbul/sbr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tenback DE, van Harten PN, Slooff CJ, et al. Incidence and persistence of tardive dyskinesia and extrapyramidal symptoms in schizophrenia. J Psychopharmacol. 2010;24:1031–1035. doi: 10.1177/0269881109106306. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, et al. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. J Pharmacol Exp Ther. 2005;314:1087–1092. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Urban JD, Vargas GA, von Zastrow M, et al. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. 2007;32:67–77. doi: 10.1038/sj.npp.1301071. [DOI] [PubMed] [Google Scholar]
- von Wilmsdorff M, Bouvier ML, Henning U, et al. The impact of antipsychotic drugs on food intake and body weight and on leptin levels in blood and hypothalamic ob-r leptin receptor expression in wistar rats. Clinics. 2010;65:885–894. doi: 10.1590/S1807-59322010000900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wilmsdorff M, Bouvier ML, Henning U, et al. The sex-dependent impact of chronic clozapine and haloperidol treatment on characteristics of the metabolic syndrome in a rat model. Pharmacopsychiatry. 2013;46:1–9. doi: 10.1055/s-0032-1321907. [DOI] [PubMed] [Google Scholar]
- Yoo HK, Lee J-S, Paik K-W, et al. Open-label study comparing the efficacy and tolerability of aripiprazole and haloperidol in the treatment of pediatric tic disorders. Eur Child Adolesc Psychiatry. 2011;20:127–135. doi: 10.1007/s00787-010-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21:600–620. doi: 10.1016/j.euroneuro.2011.04.001. [DOI] [PubMed] [Google Scholar]


