Abstract
Introduction
Eicosanoids, including PGE-2 and 5-HETE, can increase levels of plasma vascular endothelial growth factor (VEGF). Overexpression of COX-2 or 5-LOX increases levels of PGE-2 and 5-HETE, respectively. Elevated levels of VEGF are common in patients with non-small cell lung cancer (NSCLC). We prospectively measured VEGF in serum collected from patients enrolled in Cancer and Leukemia Group B 30203, a randomized phase II study of eicosanoid modulation in addition to chemotherapy in patients with advanced NSCLC, to determine whether these levels had prognostic significance and whether they correlated with COX-2 expression and/or responded to inhibition of COX-2 or 5-LOX.
Methods
Pre- and post-treatment serum was collected from patients enrolled in CALGB 30203. Serum VEGF levels were determined using enzyme-linked immunosorbent assay methodology. Statistical analyses were performed to determine the correlation between pretreatment serum VEGF levels and time of overall survival. Pretreatment formalin fixed tissue was stained for 5-LOX and COX-2 by immunohistochemistry.
Results
The median baseline VEGF level was 502 pg/ml (range, 55–3453 pg/ml). Dichotomized serum VEGF levels at median inversely correlated with survival time (p = 0.008), as did VEGF levels as a continuous variable in multivariate analysis (p = 0.035). VEGF levels were significantly correlated neither with baseline COX-2 expression (Pearson r = 0.1524, p = 0.271) nor with 5-LOX expression. Treatment with COX-2 or 5-LOX inhibitors did not alter the levels.
Conclusion
These data indicate that elevated serum VEGF is a negative prognostic variable in NSCLC. VEGF levels are neither correlated with baseline tumor COX-2 expression nor do they respond to COX-2 and/or 5-LOX inhibition plus chemotherapy.
Keywords: Lung cancer, VEGF, Eicosanoid, Celecoxib
Angiogenesis plays a pivotal role in cancer development and progression. Central to angiogenesis is vascular endothelial growth factor (VEGF), the primary stimulus to the formation of new blood vessels.1 VEGF production may be induced through multiple pathways. Arachadonic acid metabolites (eicosanoids) produced through increased levels of COX-2 or 5-LOX expression are known to stimulate VEGF production. COX-2 metabolites, specifically PGE-2, have been documented to be associated with increased VEGF levels and their phenotypic manifestations such as microvessel density in a range of malignancies, including head and neck, colorectal, and lung cancer.2–4 Laboratory models have demonstrated that inhibition of COX-2 can decrease angiogenesis, as documented by diminished microvessel density.5 Arachadonic acid may also be metabolized through lipoxygenases including 5, 10, or 12 lipoxygenase. 5-LOX expression has also been associated with increased VEGF levels in mesothelioma and colorectal carcinoma.6,7
CALGB 30203 tested the concept of eicosanoid inhibition in advanced lung cancer.8 The hypothesis was that eicosanoid inhibition in addition to standard chemotherapy would potentially increase progression-free survival. Furthermore, the concept of single versus double pathway inhibition was tested with inhibitors of COX-2 and 5-LOX as both single agents and in combination. Based on preclinical observations that COX-2 and 5-LOX inhibitors might exert their effects through inhibition of VEGF; CALGB 150304 was an embedded companion study with the objective of exploring whether levels of VEGF correlated with COX-2 and 5-LOX expression and with outcome. To evaluate this hypothesis, serum specimens were requested before institution of therapy and after the first and second courses of treatment. An additional objective of this study, determining whether levels of cytokeratin fragments correlate with response and outcome, has been separately reported.9 This study complies with guidelines regarding the reporting of tumor markers.10 The study was approved by the Institutional Review Boards of all participating institutions, and all patients provided written informed consent.
METHODS
Patients with advanced non-small cell lung cancer (NSCLC) (stage IIIb [pleural effusion]/IV) with performance status 0 to 2, and normal organ function were randomized to receive chemotherapy (carboplatin area under the curve =5.5, day 1 and gemcitabine 1000 mg/m2 day 1, 8 with one of three eicosanoid modulating regimens: zileuton 600 mg four times daily, celecoxib 400 mg twice daily, or both agents.8
Specimens were collected in 7 ml red top tubes, gently inverted five times to mix completely. After 30 minutes, the tubes were spun for 10 minutes at 1100 to 1300 g in swinging head rotor centrifuges. After centrifugation, serum was removed and placed in a polypropylene tube and frozen to at least −20°C or colder. Specimens were shipped within 30 days on dry ice by overnight express to the CALGB Pathology Coordinating Office at the Ohio State University. Aliquots were sent to the laboratory of one of the investigators (R.C.) on dry ice and kept at −80°C until analysis. The investigator was blinded to the identities, treatment allocation, and outcomes. VEGF was measured using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). All specimens were run on first thaw only and in duplicate. The lower limit for detection of VEGF was 9.0 pg/mL. The intra-assay precision was 6.7% at 53.7 pg/ml, 4.5% at 235 pg/ml, and 5.15% at 910 pg/ml. Interassay variation was 8.8% at 64.5 pg/ml, 7.0% at 250 pg/ml, and 6.2% at 1003 pg/ml.
Statistical Analysis
Patient registration and clinical data collection were managed by the CALGB Statistical Center. The CALGB Pathology Coordinating Office at the Ohio State University processed the specimens. Serum VEGF assays were conducted at the University of Maryland. Assays were conducted without knowledge of clinical outcomes. The statistical analyses were performed by CALGB statisticians using SAS version 9.1 (SAS, Cary, NC). The balance of demographic and clinical variables across study arms were tested by χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables. Kaplan-Meier curves were used to characterize survival. Median survival and its 95% confidence intervals were computed. Log-rank tests were used to test the survival difference between study arms.
To reduce the influence of potentially skewed data, serum VEGF values were natural-log-transformed at baseline, cycle 1, and cycle 2. The relationship to VEGF levels as continuous or binary variable was evaluated with Cox regression analysis in both univariate and multivariate models and the proportional hazards assumption was tested for all models. Logistic regression was used to evaluate the association between tumor response and VEGF levels. In multivariate regression analyses, stepwise variable selection was used to select significant predictive factors. The association with COX-2 and 5-LOX indices was computed for continuous VEGF levels using Pearson correlation coefficient and for dichotomized VEGF levels using Spearman rank correlation coefficient. All p values were two-sided and no adjustment for multiple comparisons was made.
RESULTS
CALGB 30203 enrolled 140 patients in under 1 year. Neither the population as a whole nor any of the three arms achieved the predetermined criterion for a successful outcome. These results have been previously reported.8 Blood specimens were received on 88 patients (63%). The characteristics of this patient population are presented in Table 1. The median baseline VEGF level was 502 pg/ml (range, 55–3453 pg/ml) (Table 2). Baseline VEGF levels were significantly associated with overall survival when dichotomized at the median (p = 0.008; Figure 1). Higher baseline VEGF was associated with inferior survival. After log transformation, higher baseline VEGF levels as a continuous variable were also significantly associated with worse overall survival both as a single predictor (p = 0.057) and when adjusted for age and performance status in multivariate analysis (p = 0.035; Table 3 and Figure 1). However, VEGF levels were not associated with failure-free survival. Additionally, the reduction in VEGF from baseline was not significantly associated with overall survival (p = 0.730), failure-free survival (p = 0.722; Table 3), or radiologic response (Table 4).
TABLE 1.
Baseline Patient Characteristic (n = 88)
| Characteristics | Arm A (n = 22), n (%) | Arm B (n = 31), n (%) | Arm C (n = 35), n (%) | Total (n = 88), n (%) |
|---|---|---|---|---|
| Gender | ||||
| Male | 15 (68) | 18 (58) | 21 (60) | 54 (61) |
| Female | 7 (32) | 13 (42) | 14 (40) | 34 (39) |
| Age (yr) | ||||
| 40–49 | 1 (5) | 2 (6) | 2 (6) | 5 (6) |
| 50–59 | 4 (18) | 18 (58) | 13 (37) | 35 (40) |
| 60–69 | 11 (50) | 7 (23) | 12 (34) | 30 (34) |
| 70–79 | 5 (23) | 3 (10) | 6 (17) | 14 (16) |
| 80+ | 1 (5) | 1 (3) | 2 (6) | 4 (5) |
| Median (min, max) | 62 (41, 80) | 57 (47, 80) | 61 (48, 81) | 60 (41, 81) |
| Race | ||||
| White | 20 (91) | 27 (87) | 28 (80) | 75 (85) |
| Black | 1 (5) | 4 (13) | 5 (14) | 10 (11) |
| Other | 1 (5) | 0 (0) | 2 (6) | 3 (3) |
| Histology | ||||
| Adenocarcinoma | 11 (50) | 16 (52) | 17 (49) | 44 (50) |
| Squamous | 6 (27) | 6 (19) | 9 (26) | 21 (24) |
| Undifferentiated | 4 (18) | 7 (23) | 8 (23) | 19 (22) |
| Other | 1 (5) | 2 (6) | 1 (3) | 4 (5) |
| Performance status | ||||
| 0 | 4 (18) | 8 (26) | 15 (43) | 27 (31) |
| 1 | 17 (77) | 23 (54) | 15 (43) | 55 (63) |
| 2 | 1 (5) | 0 (0) | 5 (14) | 6 (7) |
| Stage | ||||
| IIIB | 2 (9) | 2 (6) | 3 (9) | 7 (8) |
| IV | 18 (82) | 27 (87) | 31 (89) | 76 (86) |
| Recurrent | 2 (9) | 2 (6) | 1 (3) | 5 (6) |
TABLE 2.
VEGF Levels (pg/ml)
| Markers | Arm A (n = 22) | Arm B (n = 31) | Arm C (n = 35) | Total (n = 88) |
|---|---|---|---|---|
| VEGF Baseline | 415, 457, (57, 1682) | 509, 695, (55, 2889) | 540, 794, (61, 3453) | 502, 675, (55, 3453) |
| VEGF Cycle 1 | 331, 504, (69, 1645) | 408, 578, (32, 2634) | 476, 751, (123, 2051) | 418, 629, (32, 2634) |
| VEGF Cycle 2 | 221, 246, (40, 694) | 299, 393, (34, 1135) | 335, 585, (92, 1538) | 305, 435, (34, 1538) |
| Log VEGF Baseline | 6.0, 5.9, (4.0, 7.4) | 6.2, 6.2, (4.0, 8.0) | 6.3, 6.4, (4.1, 8.1) | 6.2, 6.2, (4.0, 8.1) |
| Log VEGF Cycle 1 | 5.8, 5.9, (4.2, 7.4) | 6.0, 5.9, (3.5, 7.9) | 6.2, 6.3, (4.8, 7.6) | 6.0, 6.1, (3.5, 7.9) |
| Log VEGF Cycle 2 | 5.4, 5.3, (3.7, 6.5) | 5.7, 5.7, (3.5, 7.0) | 5.8, 6.0, (4.5, 7.3) | 5.6, 5.7, (3.5, 7.3) |
Values are expressed as Median, Mean, (minimum, maximum).
VEGF, vascular endothelial growth factor.
FIGURE 1.
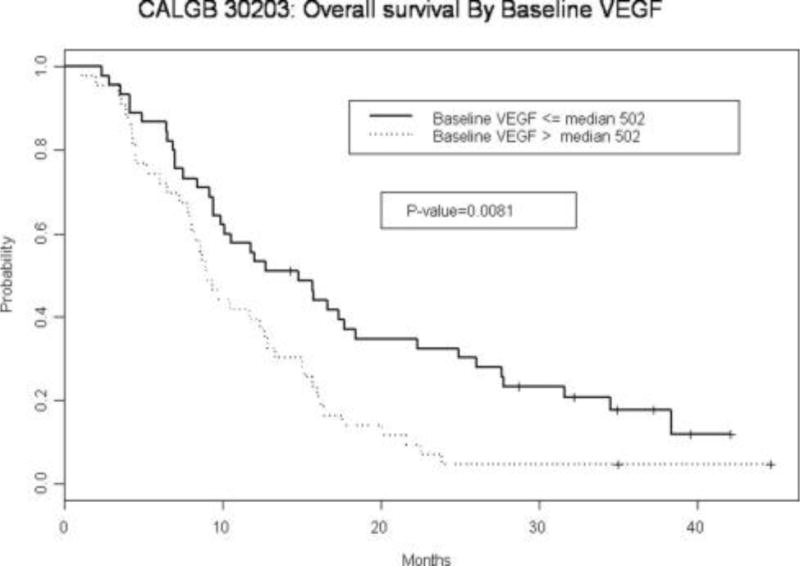
CALGB 30203: Overall survival by baseline vascular endothelial growth factor (VEGF).
TABLE 3.
Association of VEGF Level with Overall Survival and Failure-Free Survival
| Overall Survival | Failure-Free Survival | |||
|---|---|---|---|---|
| Hazard Ratio | Hazard Ratio | |||
| Parameter | p | 95% CI | p | 95% CI |
| Univariate Analysis | ||||
| Log (VEGF Baseline) | 0.057 | 1.32 (0.99, 1.77) | 0.491 | 0.91 (0.71,1.18) |
| Multivariate Analysisa | ||||
| Log (Baseline VEGF) | 0.035 | 1.42 (1.03,1.97) | 0.843 | 0.97 (0.72, 1.32) |
| Log (Baseline VEGF)-Log (VEGF Cycle 1) | 0.730 | 0.94 (0.64,1.37) | 0.722 | 0.93 (0.63 1.38) |
| Performance (1–2 Vs. 0) | 0.084 | 1.58 (0.94,2.66) | 0.695 | 1.11 (0.67, 1.84) |
| Age (≥65 Yr Vs. <65 Yr) | 0.077 | 1.59 (0.95,2.66) | 0.438 | 1.22 (0.74, 2.01) |
CI, confidence interval; VEGF, vascular endothelial growth factor.
Log baseline VEGF and Log (baseline VEGF)–Log (VEGF cycle 1) were forced into the final model with performance status, stage, age, treatment arm, sex, race, and histology as potential variables to be selected using stepwise algorithm with entry level of 0.10 and stay level of 0.10. Of 88 patients in the analysis, 78 patients were died; 85 failed (died or disease progressed).
TABLE 4.
Correlation of Radiologic Response and VEGF
| p | Odds Ratio (95% CI) | |
|---|---|---|
| Log (Baseline VEGF) | 0.2402 | 1.418 (0.792–2.542) |
| Log (Baseline VEGF) – Log (VEGF Cycle 1) | 0.2288 | 1.496 (0.776–2.882)a |
CI, confidence interval; VEGF, vascular endothelial growth factor.
If complete response or partial response, response = 1; else response = 0.
In the primary report of this trial, we presented data demonstrating that COX-2 expression was a negative prognostic factor but a positive predictive factor for benefit from celecoxib. Using immunohistochemistry of 80 tissue specimens, we demonstrated that patients with expression of COX-2 had a worse overall survival than those who did not express COX-2 or had minimal levels of COX-2 expression (hazards ratio [HR] for moderate expression (index of ≥4, HR = 2.68, p = 0.018). More importantly, analysis of patients receiving celecoxib (with or without zileuton) whose tumors expressed COX-2 (moderate to high expression, index ≥4, HR =0.420, p = 0.039) had a superior outcome compared with patients with expression who did not receive celecoxib. Furthermore, the higher the degree of COX-2 expression, the greater the degree of benefit from celecoxib.
Given the above results, we explored whether there was any correlation between VEGF levels or change in levels and COX-2 expression. We also evaluated whether there was any relationship to 5-LOX expression. In addition, we wished to determine whether celecoxib had any influence on VEGF levels, including an analysis as to whether there was any relationship with COX-2 expression in the tumor. These analyses are limited by small numbers and incomplete overlap (n = 54) of those who had both tissue specimens adequate for COX-2 or 5-LOX expression (n = 83) and adequate serum samples (n = 88). There was a statistically insignificant trend for a positive correlation between baseline VEGF and COX-2 expression. The Pearson correlation coefficients between baseline VEGF and COX-2 was 0.1524 (p = 0.271), and the Spearman correlation coefficient between baseline VEGF and COX-2 as ≥4 versus less than 4 was also not significant at 0.1260 (p = 0.364).
A possible mechanism for COX-2 and/or 5-LOX inhibition is suppression of VEGF. We did not find any indication of a relationship between baseline tumor expression of either COX-2 or 5-LOX and VEGF levels or response to the inhibitor, Table 5. We found that the degree of decline in VEGF levels (baseline-cycle 1) was not significantly related to COX-2 index with a Pearson correlation efficient of −0.1443 (p = 0.298).
TABLE 5.
Correlation of VEGF Levels with COX-2 and 5-LOX
| VEGF and COX-2 (n = 54) | VEGF and 5-LOX | |||
|---|---|---|---|---|
| Correlation | Cox-2 Indexa | High Cox-2 vs. Low (≥4 vs. <4)b | 5-LOX Indexa | High 5-LOX vs. Low (>6 vs. ≤6)b |
| Baseline VEGF | 0.1127 (p = 0.417) | 0.1260 (p = 0.364) | 0.1534 (p = 0.268) | 0.2178 (p = 0.114) |
| Log (baseline VEGF) | 0.1524 (p = 0.271) | 0.1260 (p = 0.364) | 0.2049 (p = 0.137) | 0.2178 (p = 0.114) |
| Decrease of VEGF from baseline to cycle 1 | −0.1232 (p = 0.375) | −0.1210 (p = 0.384) | −0.1179 (p = 0.396) | −0.1156 (p = 0.405) |
| Diff_Log baseline cycle 1 | −0.1443 (p = 0.298) | −0.1235 (p = 0.374) | −0.1469 (p = 0.289) | −0.1378 (p = 0.320) |
VEGF, vascular endothelial growth factor.
Pearson correlation coefficients.
Spearman correlation coefficients.
Similarly, the Spearman rank correlation coefficient was −0.1235 (p = 0.374) for VEGF decline and COX-2 ≥4 versus less than 4, indicating again no significant association between COX-2 index and the decline in VEGF. In addition, comparing patients who received celecoxib (arms B, C) with those who did not (arm A), there was no significant difference in VEGF decline (p = 0.422). The relationship between baseline COX-2 expression, treatment with celecoxib and change in VEGF levels was also evaluated. For patients with COX-2 ≥4 (n = 18), no significant differences in changes of VEGF levels were observed between patients who did or did not receive celecoxib (p = 0.863). Similar results were found for 5-LOX.
DISCUSSION
Inhibition of angiogenesis has now been validated as an effective anticancer strategy in general and in lung cancer specifically. The use of the anti-VEGF antibody bevacizumab has been demonstrated to improve outcome in terms of progression-free survival in two trials and overall survival in one study.11,12 Small molecule inhibitors of the VEGF receptor have also demonstrated some activity, though the results of randomized studies have been mixed.13
Another approach to angiogenesis inhibition is the use of agents that decrease VEGF production directly or indirectly. There is preclinical evidence that eicosanoid modulators reduce VEGF production.14 Others have found that chemotherapy alone was associated with a decrease in VEGF levels.15 We did not demonstrate that eicosanoid inhibition in addition to chemotherapy suppressed VEGF levels, even in patients with response or with high COX-2 expression. This finding does not exclude an interrelationship of COX-2 and VEGF as in some models it is VEGF that stimulates the production of COX-2.16 However, we did demonstrate the negative prognostic value of baseline serum VEGF levels, which has been seen in both the early (i.e., preoperative) and metastatic settings.17 This finding is somewhat different from the results of the Eastern Cooperative Oncology Group, which demonstrated a correlation between baseline VEGF levels and failure free but not overall survival.18 In the Eastern Cooperative Oncology Group trial, there was a trend toward poorer overall survival with elevated VEGF levels.
In summary, this study confirms the negative prognostic value of serum VEGF in patients with advanced non-small cell lung cancer. However, it failed to demonstrate that eicosanoid modulation in addition to chemotherapy could reduce VEGF production. In addition, there was no correlation demonstrated between VEGF levels and COX-2 expression. Although these results are limited by the small numbers of patients, it seems likely that the beneficial results of COX-2 inhibition in addition to chemotherapy demonstrated in the subset of patients with high levels of COX-2 expression was mediated by a process other than VEGF suppression.
Acknowledgments
The research for CALGB 15034 was supported, in part, by grants from the National Cancer Institute (U10CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli, MD, Chair) and to the CALGB Statistical Center (Daniel J. Sargent, PhD, U10CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Hicklin DJ, Ellis LM. Role of vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 2.Gallo, Franchi A, Magnelli L, et al. Cyclooxygenase-2 pathway correlated with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–1347. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 4.Marrogi AJ, Travis WD, Welsh JA, et al. Nitric oxide synthase, cyclooxygenase2 and vascular endothelial growth factor in the angiogenesis of non-small cell lung cancer. Clinical Cancer Res. 2000;6:4739–4744. [PubMed] [Google Scholar]
- 5.Soo RA, Wu J, Aggarawal A, et al. Celecoxib reduces microvessel density in patients treated with nasopharyngeal carcinoma and induces changes in gene expression. Ann Onc. 2006;17:1625–1630. doi: 10.1093/annonc/mdl283. [DOI] [PubMed] [Google Scholar]
- 6.Romano M, Catalano A, Nutini M, et al. 5-lipoxygenase regulates malignant mesothelial cell survival: involvement of vascular endothelial growth factor. FASEB J. 2001;15:2326–2336. doi: 10.1096/fj.01-0150com. [DOI] [PubMed] [Google Scholar]
- 7.Ye YN, Liu ES, Shin VY, et al. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004;203:179–188. doi: 10.1016/j.tox.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Edelman MJ, Watson D, Xiaofei W, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy–Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt PY, Edelman MJ, Christenson RH, et al. CYFRA 21–1 (CYFRA) as a prognostic and predictive marker in advanced non-small cell lung cancer (NSCLC): CALGB 150304. J Clin Oncol. 2009;27(suppl):562. doi: 10.1097/JTO.0b013e31824a8db0. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 11.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. Erratum in N Engl J Med, 356 (2007), p. 318. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. Erratum in J Clin Oncol 27 (2009), p. 2415. [DOI] [PubMed] [Google Scholar]
- 13.Horn L, Sandler A. Epidermal growth factor receptor inhibitors and antiangiogenic agents for the treatment of non-small cell lung cancer. Clin Cancer Res. 2009;15:5040–5048. doi: 10.1158/1078-0432.CCR-09-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2 suppl 7):2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Kido Y. Vascular endothelial growth factor (VEGF) serum concentration changes during chemotherapy in patients with lung cancer. Kurume Med J. 2001;48:43–47. doi: 10.2739/kurumemedj.48.43. [DOI] [PubMed] [Google Scholar]
- 16.Chien MH, Ku CC, Johansson G, et al. Vascular endothelial growth factor-c (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis. 2009;30:2005–2013. doi: 10.1093/carcin/bgp244. [DOI] [PubMed] [Google Scholar]
- 17.Brattstrom D, Bergvist M, Hesselius P, et al. Elevated preoperative serum levels of angiogenic cytokines correlate to larger primary tumors and poorer survival in non-small cell lung cancer patients. Lung Cancer. 2002;37:57–63. doi: 10.1016/s0169-5002(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 18.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]


