Abstract
Objective
Directly observed therapy is recommended worldwide for monitoring tuberculosis (TB) treatment; yet transportation and personnel requirements limit its use. We evaluated the feasibility and acceptability of “Video DOT” (VDOT), which allowed patients to record and transmit medication ingestion videos that were watched remotely by healthcare providers to document adherence.
Methods
We conducted a single-arm trial among TB patients in San Diego, CA (n=43) and Tijuana, B.C., Mexico (n=9) to represent high- and low-resources settings. Pre/post treatment interviews assessed participant characteristics and experiences. Adherence was defined as the proportion of observed doses to expected doses.
Results
Mean age was 34 years (range: 18–86), 54% were male, and 77% were non-Caucasian. Mean duration of VDOT use was 5.5 months (range: 1–11). Adherence was similar in San Diego (93%) and Tijuana (96%). Compared to time on in-person DOT, 92% preferred VDOT; 81% thought VDOT was more confidential; 89% never/rarely had problems recording videos; and 100% would recommend VDOT to others. Overall, 7 (13%) participants were returned to in-person DOT and 6 (12%) separate participants had their phone lost, broken or stolen.
Conclusions
VDOT was feasible and acceptable with high adherence in high- and low-resource settings. Efficacy and cost-effectiveness studies are needed.
Keywords: US-Mexico border, drug resistance, medication adherence, directly observed therapy, cellular phone, mHealth
INTRODUCTION
Tuberculosis (TB) is an airborne infectious disease that remains a global health threat affecting over two billion people—one third of the world’s population—and is the third leading cause of infectious disease deaths worldwide.1,2 Globally, there are 8.8 million new TB cases per year resulting in 1.4 million deaths.1,2 In 2013, the United States (US) had 9,582 new TB cases reported (3.0 cases per 100,000 population).3 While TB incidence in the US has declined, case rates in San Diego County were twice the national average with 206 active TB cases reported in 2013 (6.5 cases/100,000 pop.).4 Baja California, which shares the busiest land border-crossing in the worlds with California,5 has Mexico’s highest TB incidence (55.2 cases per 100,000 pop.); triple the national average.4 In San Diego County, 69% of cases in 2011 were foreign-born; 44% from Mexico.6
While TB is curable with antibiotics, poor compliance to treatment regimens lasting 6 months or more leads to increased mortality, ongoing disease transmission, and acquired drug resistance. The consequences to patients and the community of poor adherence are so great that the World Health Organization and the US Centers for Disease Control and Prevention recommend directly observed therapy (DOT) for administering TB treatment,7–9 because it is more effective than other interventions for achieving treatment completion.10 DOT is a patient-centered approach in which patients are observed ingesting each medication dose to ensure adherence and achieve completion. In the US, DOT workers typically travel to the patient, but in resource-constrained areas, including Mexico, DOT is largely clinic-based. Either way, DOT is labor-intensive, requires transportation, can restrict patients’ daily activities, and may be impractical for patients living far from health centers. The effectiveness of DOT compared with self-administered therapy has been questioned,11,12 possibly due to implementation barriers, suggest the need for improvement in DOT delivery. In low/middle income countries, resource constraints significantly limit DOT’s use.13 To reduce treatment monitoring costs and allow greater patient autonomy, mobile technology is increasingly used to improve patient care and treatment outcomes.14–16 Most mobile solutions involve for patient reminders and few actually document medication ingestion.15 We developed a flexible, low-burden method of providing remote DOT via smartphones called Video DOT (VDOT), which involves patients video-recording themselves taking their medications and securely transferring the videos to DOT workers for review. We then pilot tested VDOT for feasibility, acceptability, and potential efficacy in high (San Diego, California, USA) and low (Tijuana, Baja California, Mexico) resource settings.
METHODS
In 2010–2012, we conducted a single-arm trial among TB patients initially receiving treatment via in-person DOT. Informed by focus groups involving TB patients, care providers, and health officials in San Diego and Tijuana,17 we developed the VDOT System and then pilot-tested it in public health departments among newly diagnosed patients with drug-susceptible pulmonary TB.
Eligibility and recruitment
TB Control Program staff in both cities recruited individuals currently receiving treatment for confirmed or suspected pulmonary TB. Eligibility criteria included: 1) ability to speak English or Spanish; 2) age ≥18 years; 3) ≥1 month of treatment remaining; and 4) willing and able to provide informed consent. Minors, patients with confirmed or suspected drug-resistant TB, and patients with physical conditions that hindered smartphone use (i.e., severe arthritis, diminished vision) were excluded. Patients could be enrolled in the study any time after their providers determined that they were tolerating their medications (minimum of 2 weeks), prior to which patients received traditional in-person DOT. Patients who met the eligibility criteria were sequentially enrolled. Of the patients approached, two in San Diego and two in Tijuana refused to participate—three noting confidentiality concerns and one did not think he could keep the phone safe.
VDOT Procedures
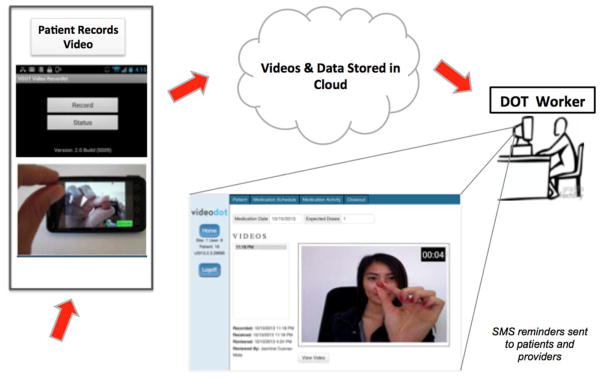
Participants used smartphones provided by the study to record videos of themselves taking each dose of TB medication. Uploaded videos were watched by DOT workers via a secure website to document whether the complete dose was ingested (Figure 1). The VDOT system includes a smartphone application for securely recording, transferring and storing videos; and a web-based client management system used by DOT workers to view and document each event. Daily text message reminders were sent to participants’ phones—one before doses were due and one after expected videos were not received. The smartphone application automatically sent encrypted, time/date-stamped videos to a secure server via cellular or Wi-Fi network. If service was unavailable, videos remained on the phone until connectivity was restored. For confidentiality, videos could not be viewed on the phone and were automatically deleted after videos were sent. The VDOT software application was developed at by the investigators exclusively for this study.
Figure 1.
Video Directly Observed Therapy (VDOT) flow diagram
All participants received in-person DOT for ≥2 weeks before switching to VDOT. DOT workers trained participants on VDOT procedures during routine DOT visits until the participant demonstrated competency (median=2 times; IQR=2–5 times). Participants continued on the same schedule as non-participants for routine clinic visits (i.e., monthly) for medication refills and health status monitoring. Participants were told they could use study smartphones for TB care purposes (e.g., call their doctor, find TB information, schedule appointments).
Data Collection
The primary outcome measure was adherence rate, calculated as the number of medication doses observed in videos divided by the number of doses expected during the treatment period. If missed or self-administered doses were added to the end of treatment, the number of doses expected was correspondingly increased. DOT workers in each city checked the VDOT website daily and documented whether: 1) a video was received, and 2) all tablets were swallowed. If ingestion was not visible or no video was received, participants were contacted to determine problems and retrained if necessary. Some videos were lost due to technical problems with the newly developed application. Since we could not confirm whether those doses were actually ingested, we treated lost videos as missing doses in calculating adherence rates.
Participant characteristics and perceptions of VDOT were obtained through brief (15–20 minute) structured interviews conducted by phone or in-person before (baseline) and after (follow-up) participants used VDOT. Baseline interviews included socio-demographics, comfort using smartphones, and concerns about privacy (i.e., TB-related stigma). Binational participants were those who spent ≥1 night during the study period across the border from their enrollment city. Alcohol and illicit drug use were also assessed. Follow-up interviews captured participant VDOT experiences, including days needed to learn VDOT process, problems recording videos, confidentiality and satisfaction with VDOT. Change in comfort using smartphones for phone calls, text messaging, taking photos/videos, and accessing the internet were assessed by asking participants to report their level of comfort with each on a 10-point scale (1=very uncomfortable to 10=very comfortable) on each interview.
To minimize response bias, interviews were conducted by research assistants uninvolved in patient care. Participants received $25 USD in San Diego and 200.00 pesos ($15.25 USD) in Tijuana for completing each interview, but not for using VDOT. The University of California, San Diego Institutional Review Board and Tijuana General Hospital Bioethics Committee approved this study.
Data Analysis
We described the study sample using frequencies (categorical variables), means and ranges (continuous variables). Bivariate analyses were conducted using baseline and follow-up data to identify factors associated with adherence using t-tests or rank sum tests for continuous variables; categorical variables were examined using chi-square or Fisher’s exact test. Paired t-tests were used to assess changes in comfort with smartphones. Multivariable analyses were precluded due to the small sample size. Analyses were conducted using SAS 9.3. Patient comments provided during the follow-up interview are presented to contextualize quantitative findings.
RESULTS
We enrolled 43 participants in San Diego and nine in Tijuana. Overall, mean age was 37 years (range: 18–86); 50% were male, 88% were non-Caucasian and 51% were Hispanic (Table 1). There were 6 binational participants in San Diego and none in Tijuana. Participants used VDOT for a mean of 5.5 months (range: 1–11) following 2–22 weeks on in-person DOT in San Diego and 2–4 weeks on in-person DOT in Tijuana. Forty-five (87%) participants used VDOT until they completed treatment. Seven participants (13%) were returned to in-person DOT because: one had problems operating the smartphone, one who received assistance from a family member had to stop when that person moved out, two were non-compliant, one preferred in-person DOT, and two received baseline drug susceptibility test results revealing drug-resistant TB after enrollment rendering them ineligible. All participants returned to in-person DOT successfully completed treatment. Overall, 3 (6%) phones were stolen and 3 (6%) were lost or broken.
Table 1.
Baseline characteristics of tuberculosis patients enrolled in VDOT Pilot Study, 2010–2012
| Variables | Total (n=52) n (%) |
San Diego (n=43) n (%) |
Tijuana (n=9) n (%) |
|---|---|---|---|
| Spends time on both sides of border | 6 (11.5) | 6 (13.9) | 0 (0) |
| Age (n=50) | |||
| Mean (SD) | 37.3 (17.6) | 39.0 (17.6) | 28.1 (15.3) |
| Range | 18–86 | 18–86 | 18–65 |
| Gender | |||
| Male | 27 (51.9) | 23 (53.5) | 4 (44.4) |
| Female | 25 (48.1) | 20 (46.5) | 5 (55.6) |
| Race/Ethnicity (n=51) | |||
| Asian | 13 (25.5) | 13 (31.0) | 0 (0) |
| African American/Black | 3 ( 5.9) | 3 ( 7.1) | 0 (0) |
| Hispanic/Latino | 26 (51.0) | 17 (40.4) | 9 ( 100) |
| Pacific Islander/Native Hawaiian | 2 ( 3.9) | 2 ( 4.8) | 0 (0) |
| Caucasian/White | 6 (11.7) | 6 (14.3) | 0 (0) |
| Other/Mixed Race | 1 ( 2.0) | 1 ( 2.4) | 0 (0) |
| Highest Level of Education (n=49) | |||
| Primary school or below | 12 (24.5) | 7 (16.7) | 5 (71.4) |
| High school graduate | 9 (18.3) | 9 (21.4) | 0 (0) |
| Some college or above | 28 (57.2) | 26 (61.9) | 2 (28.6) |
| Housing Status (n=50) | |||
| Lives in own home | 35 (70.0) | 30 (71.4) | 5 (62.5) |
| Lives in parent’s home | 8 (16.0) | 7 (16.7) | 1 (12.5) |
| Lives in someone else’s home | 7 (14.0) | 5 (11.9) | 2 (25.0) |
| Behavioral Risk Factors (n=50) | |||
| Frequency of alcohol use in past year | |||
| Never/Rarely | 34 (68.0) | 28 (66.7) | 6 (75.0) |
| 1–2 days/week | 12 (24.0) | 10 (23.8) | 2 (25.0) |
| 3–7 days/week | 4 ( 8.0) | 4 ( 9.5) | 0 (0) |
| Smoked cigarettes in past year | 7 (14.0) | 6 (14.3) | 1 (12.5) |
| Ever used marijuana | 13 (26.0) | 10 (23.8) | 3 (37.5) |
| Ever used illegal drugs | 4 ( 8.0) | 2 ( 4.8) | 2 (25.0) |
| Ever injected illegal drugs | 1 ( 2.0) | 1 ( 2.3) | 0 (0) |
| TB-Related Stigma Concerns (n=49) | |||
| Felt it is was embarrassing to have a DOT worker visit home or work | 17 (34.7) | 17 (41.5) | 0 (0) |
| Worried about family and close friends finding out about TB | 17 (34.7) | 15 (36.6) | 2 (25.0) |
| Worried about acquaintances, neighbors or coworkers finding out about TB | 21 (42.9) | 19 (46.3) | 2 (25.0) |
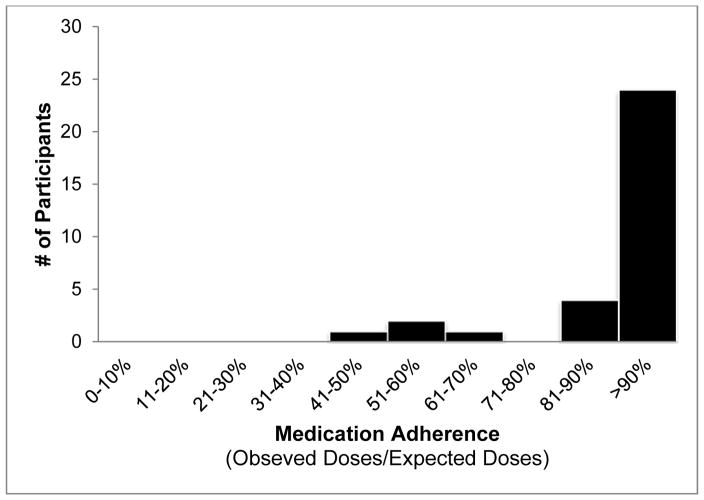
Mean adherence was 93% (51%–100%) in San Diego and 96% (88%–100%) in Tijuana (Figure 2). Overall, 5,626 videos were received, showing complete doses swallowed (96.0%), partial doses swallowed (0.4%), no pills swallowed (1.5%), and unable to tell if doses were swallowed (2.1%). No socio-demographic or behavioral factors were found to be associated with adherence (data not shown). Throughout treatment, daily text messages were sent to each participant as reminders to take their medication. DOT worker follow-up responses to missed doses included contacting participants to encourage adherence, provide re-training on VDOT procedures, and/or to troubleshoot technical problems with recording videos. Advanced age was not a barrier to using VDOT. Older participants in particular enjoyed learning to use a smartphone and reported feeling that VDOT allowed them to remain in control of their care rather than feeling like their provider did not trust them to take their medications.
Figure 2.
Tuberculosis medication adherence rates among participants in San Diego, CA and Tijuana, Baja California, Mexico (n=51)
Fifty (94%) participants completed follow-up interviews. Overall, 92% reported never/rarely having problems recording videos, 92% preferred VDOT over in-person DOT, 84% thought VDOT was more confidential and 100% said they would recommend VDOT to others (Table 2). Nearly two-thirds of participants thought text message reminders were useful. Open-ended questions revealed that participants in both cities thought VDOT was more confidential than in-person DOT. For example, a Tijuana participant said, “[with the VDOT system], I always had the confidence of confidentiality.” A San Diego participant said, “The phone is a great step for the person to find their own private space...” Additionally, participants valued the mobility that VDOT allowed and the convenience of taking medications on their own schedule. A San Diego participant stated that, “[VDOT] is a convenient, easy, good system...” Another San Diego participant said, “The phone was very convenient [for me]. I could take my pills on my own time instead of waiting for someone to watch me.” TB Control Program staff reported that patients had more autonomy and were grateful to have been able to use VDOT. They also reported that time and travel saved using VDOT allowed them to concentrate on less compliant patients.
Table 2.
Follow-up results among tuberculosis patients enrolled in VDOT Pilot Study, 2010–2012
| Variables | Total (n=52) n (%) |
San Diego (n=41)* n (%) |
Tijuana (n=9) n (%) |
|---|---|---|---|
| VDOT Outcomes | |||
| Time spent using VDOT (months) (n=52) | |||
| Mean (SD) | 5.5 (2.1) | 5.6 (2.2) | 5.2 (1.5) |
| Range | 1–11 | 1–11 | 2–7 |
| Number of missed doses (n=52)** | |||
| Mean (SD) | 2.7 (7.0) | 2.7 (7.7) | 1.9 (2.0) |
| Range | 0–41 | 0–41 | 0–5 |
| Returned to in-person DOT (n=52) | 7 (13.5) | 6 (14.0) | 1 (11.1) |
| Phone status at end of study (n=52) | |||
| Broken/Lost | 3 (5.8) | 2 (4.7) | 1 (11.1) |
| Stolen | 3 (5.8) | 1 (2.3) | 2 (22.2) |
| VDOT Training | |||
| Days practiced VDOT before recording videos alone (n=52) | |||
| 1 | 26 (50.0) | 25 (58.1) | 1 (11.1) |
| 2 | 6 (11.5) | 6 (13.9) | 0 ( 0) |
| 3 | 5 (9.6) | 2 (4.7) | 3 (33.3) |
| ≥4 | 12 (23.1) | 7 (16.3) | 5 (55.6) |
| Don’t Know/Missing | 3 (5.8) | 3 (7.0) | 0 ( 0) |
| Found VDOT training process helpful | |||
| Yes | 48 (96.0) | 39 (95.1) | 9 ( 100) |
| No | 2 (4.0) | 2 (4.9) | 0 ( 0) |
| Found VDOT training pamphlet helpful (n=47) | |||
| Yes | 37 (78.7) | 30 (79.0) | 7 (77.8) |
| No | 10 (21.3) | 8 (21.0) | 2 (22.2) |
| Used VDOT training pamphlet when alone (n=46) | |||
| Yes | 20 (43.5) | 16 (42.1) | 4 (50.0) |
| No | 26 (56.5) | 22 (57.0) | 4 (50.0) |
| VDOT Technical Issues | |||
| Frequency of video recording problems | |||
| ≥1/2 the time | 4 (8.0) | 3 (7.3) | 1 (11.1) |
| Rarely | 27 (54.0) | 22 (53.7) | 5 (55.6) |
| Never | 19 (38.0) | 16 (39.0) | 3 (33.3) |
| Frequency of problems sending videos | |||
| ≥1/2 the time | 7 (14.0) | 6 (14.6) | 1 (11.1) |
| Rarely | 34 (68.0) | 28 (68.3) | 6 (66.7) |
| Never | 9 (18.0) | 7 (17.1) | 2 (22.2) |
| Unable to send video due to poor reception | |||
| ≥1/2 the time | 2 (4.0) | 2 (4.9) | 0 ( 0) |
| Rarely | 17 (34.0) | 12 (29.2) | 5 (55.6) |
| Never | 31 (62.0) | 27 (65.9) | 4 (44.4) |
| Able to send videos when traveling to other cities (n=47) | |||
| Yes, always | 12 (25.5) | 10 (26.3) | 2 (22.2) |
| Yes, sometimes | 5 (10.6) | 4 (10.5) | 1 (11.1) |
| Never tried | 30 (63.8) | 24 (63.2) | 6 (66.7) |
| VDOT Confidentiality | |||
| Confidentiality of VDOT vs. in-person DOT (n=50) | |||
| More | 42 (84.0) | 35 (85.4) | 7 (77.8) |
| Less | 8 (16.0) | 6 (14.6) | 2 (22.2) |
| Failed to record videos because others might see | |||
| No | 49 (98.0) | 40 (97.6) | 9 (100) |
| Yes | 1 (2.0) | 1 (2.4) | 0 (0) |
| VDOT Satisfaction | |||
| If repeat TB treatment needed, would you choose VDOT or In-Person DOT? | |||
| VDOT | 46 (92.0) | 38 (92.7) | 8 (88.9) |
| No preference | 2 (4.0) | 2 (4.9) | 1 (11.1) |
| In-person | 2 (4.0) | 1 (2.4) | 0 (0) |
| Would you recommend VDOT to other TB patients? | |||
| No | 0 (0) | 0 (0) | 0 (0) |
| Yes | 50 (100) | 41 (100) | 9 ( 100) |
| Found text message reminders helpful (n=47) | |||
| Yes | 30 (63.8) | 24 (60.0) | 6 (85.7) |
| No | 17 (36.2) | 16 (40.0) | 1 (14.3) |
Two participants in San Diego did not complete the follow-up interview; thus, overall N=50 for interview questions unless otherwise noted.
Missed doses included failure to take medication and technical problems that made some videos not viewable.
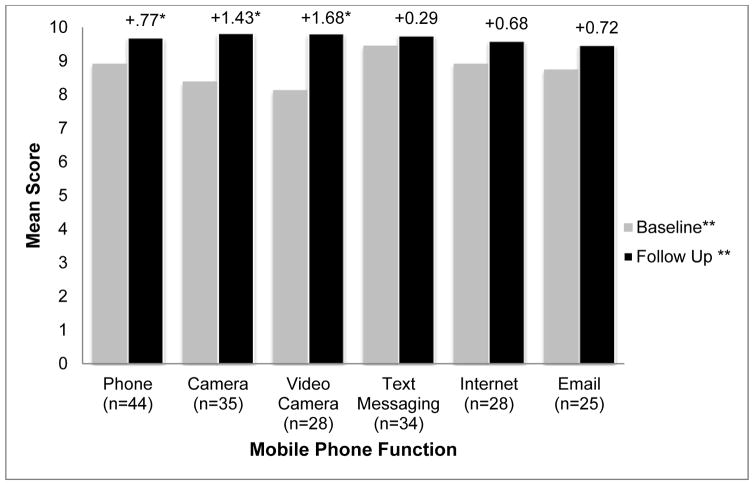
While comfort using smartphone functions were generally high at baseline (Figure 3), further increases were reported after using VDOT. These increases were statistically significant for making phone calls (+0.77, p=0.008), taking pictures (+1.43, p=0.006), and recording videos (+1.68, p=0.009).
Figure 3.
Level of comfort using smartphone functions before and after having tuberculosis treatment monitored using the VDOT System. *Change in comfort score from baseline to follow-up was statistically significant (p≤0.05). ** Measured on a 10 point scale.
DISCUSSION
VDOT was highly feasible and acceptable among TB patients and providers in both high- and low-resource settings. Most participants preferred VDOT over in-person DOT. Documented observation of nearly all expected doses (mean 93% in San Diego and 96% in Tijuana) was achieved using VDOT. VDOT provided a convenient alternative to in-person DOT without sacrificing treatment adherence because participants could ingest their medications at the time and location of their choosing without needing to coordinate with a DOT worker. Furthermore, DOT workers could observe the videos during normal business hours. Adherence was conceivably increased using VDOT because it had fewer barriers to medication-taking than in-person DOT. While findings from this pilot study might not be generalized to all TB patients, we enrolled 43 (21%) of San Diego’s 206 annually-reported cases and obtained similar results in Tijuana where 9 (1%) of that city’s approximately 1,000 cases were enrolled. Further studies with fewer eligibility criteria in more healthcare settings are needed to determine which patients are best suited for VDOT.
Inability to monitor medication taking while patients are out of the health department’s jurisdiction can prolong treatment. VDOT allows observation of all doses, even among mobile patients. For example, six San Diego participants spent part of the week in Mexico making it impractical for San Diego DOT workers to observe these doses in-person. VDOT enabled DOT workers to remotely observe the doses taken while participants were in Mexico. Hence, participants reached the number of observed doses needed to complete treatment sooner than they would have with in-person DOT.
Asynchronous (“store-and-forward”) VDOT used in this study may have advantages over synchronous (“video conferencing”) forms of remote DOT. For example, in 2004, the San Diego County TB Control Branch pilot tested video-conferencing over landline phones to reduce costs and improve efficiency of DOT. On weekdays, a DOT worker called patients using a video-phone and watched them ingest their pills. Among 33 patients over the nine-month trial, the county saved 27,840 miles in travel and 795 staff hours.18 More recently, health departments have attempted to replicate this process using mobile devices to overcome the problem of tying patients to their homes and the growing trend toward switching from landline to mobile phones. However, patients could still only be observed during business hours and when there was a consistent phone connection. Using asynchronous VDOT, patients can take their medications whenever and wherever they choose, and DOT workers can manage more patients in less time during business hours.
Attention should also be given to those who did not complete treatment on VDOT. Of the seven participants who returned to in-person DOT, two began treatment presumptively for drug-sensitive TB, but were later found to have drug-resistant TB once drug susceptibility testing was complete. Two participants, both college students, repeatedly failed to record videos; their adherence improved after returning to in-person DOT. Only one participant requested to return to in-person DOT. After 2 weeks on VDOT, she explained that as a parent of three young children, having a DOT worker visit her home was easier than self-managing her treatment. Another patient with mild cognitive deficits initially performed VDOT with the assistance of a family member, but when that person moved out, the participant was not comfortable recording videos himself. One 83 year-old participant had difficulty using the phone’s touchscreen for VDOT because he did not “feel” a button being pushed. Despite retraining attempts, the participant was unable to consistently use the application correctly and was returned to in-person DOT. Conversely, an 86 year-old participant used VDOT successfully for 7.6 months with 99% adherence, suggesting that age alone should not disqualify individuals from using VDOT. These findings suggest that VDOT should complement, rather than replace, in-person DOT. Further research is needed to identify patient characteristics for determining which method best suits each patient, and what criteria should be used to withdrawal a patient from VDOT.
Some study limitations must be considered. First, the VDOT application was developed specifically to assess whether this concept was feasible and acceptable; therefore, some early participants had lower documented adherence because videos that were lost due to software error were treated as missed doses in calculating adherence rates. Consequently, our adherence estimates were conservative. Second, since we were uncertain about how VDOT would work in practice, patients with drug-resistant TB or a history of poor adherence on in-person DOT were ineligible. Thus, patient selection could have been biased toward adherent patients limiting generalizability of adherence rates to all TB patients; however, such bias is unlikely to have affected satisfaction with VDOT. Notably, after gaining experience implementing VDOT for this study, TB Control Program staff reported feeling comfortable expanding eligibility to include patients with drug resistant TB and some patients who had low adherence using in-person DOT. Third, this study lacked a control group and we were unable to compare adherence between VDOT and in-person DOT. Since participants were allowed personal use of the study smartphones, providing smartphones could have had an intervention effect; however, providing enablers is not uncommonly practiced by health departments for TB.
CONCLUSIONS
We found VDOT feasible to implement and highly acceptable to patients and providers in both high- and low-resource settings. VDOT effectively captured medication-taking behavior allowing DOT workers to observe ingestion of nearly all expected doses. VDOT provides a promising, low-burden alternative to in-person DOT for monitoring TB treatment adherence. The reduced burden on patients and providers using VDOT could also make DOT feasible in resource-limited settings where in-person DOT is impractical, as well as allow TB program staff more time to support to less-adherent patients. VDOT may also be used to monitor other health conditions where strict medication adherence is essential. Larger, controlled trials are needed to evaluate the efficacy and cost-effectiveness of VDOT for improving and maintaining adherence throughout treatment. Studies in high-burden, low-resource settings are also needed to evaluate VDOT’s generalizability. As mobile technology plays an increasingly important role in healthcare, VDOT has great potential to expand the coverage of TB treatment monitoring to more patients worldwide by reducing the burden on both patients and providers, resulting in higher treatment completion rates, fewer new cases of TB, and prevention of acquired drug resistant TB.
Acknowledgments
The authors wish to thank Deborah McIntosh, Gabriela Escalante, Cristhian Colin, Dr. José Guadalupe Bustamante, Dr. Rafael Laniado-Laborin, and Maureen Clark for their efforts and for sharing insights that were crucial for developing the VDOT System. We greatly appreciate Michelle Bulterys’ expert editorial review of the manuscript. The authors also thank the study participants for their willingness to use the VDOT System and for the feedback they provided to help improve the system.
This study was funded by grants from the National Institutes of Health (R21-AI088326) and the Alliance Healthcare Foundation. FM was supported by NIDA grant R01-DA031074-03S1. TCR was supported by NIAID grant K01-AI083784. JLB was also supported by NIMH grant K01-MH095680. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.World Health Organization. [Accessed March 25, 2015];Tuberculosis Report 2013 Fact Sheet No 104. 2013 http://www.who.int/mediacentre/factsheets/fs104/en/
- 2.Curry International Tuberculosis Center. [Accessed March 25, 2015];Drug-Resistant Tuberculosis: A Survival Guide for Clinicians, 2011. (2). 2011 http://www.currytbcenter.ucsf.edu/drtb/docs/MDRTB_book_2011.pdf.
- 3.Centers for Disease Control and Prevention. [Accessed March 25, 2015];Trends in Tuberculosis, 2013 Factsheet. 2013 http://www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm.
- 4.Secretaria de Salud Jurisdiccion Sanitaria No. 2 Tijuana. Programa de Microbacteriosis TB & Lepra 2013. Plan de Accion para el manejo y acceso universal al dignostico y tratamiento de la Tuberculosis y TB-MFR. 2013 [Google Scholar]
- 5.U.S. General Services Administration. [Accessed March 25, 2015];San Ysidro Land Port of Entry. 2014 http://www.gsa.gov/portal/content/104872.
- 6.County of San Diego Health and Human Services Agency. [Accessed March 25, 2015];Tuberculosis and Refugee Health. 2013 http://www.sdcounty.ca.gov/hhsa/programs/phs/documents/Factsheet_2013-final_3-13-14.pdf.
- 7.World Health Organization. [Accessed March 25, 2015];Global Tuberculosis Report 2013. 2013 http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf?ua=1.
- 8.Centers for Disease Control and Prevention. Ten great public health achievements--worldwide, 2001–2010. MMWR Morbidity and Mortality Weekly Report. 2011;60(24):814–818. [PubMed] [Google Scholar]
- 9.World Health Organization. [Accessed March 25, 2015];The Stop TB Strategy: Building on and Enhancing DOTS to Meet the TB-Related Millenium Development Goals. 2006 http://whqlibdoc.who.int/hq/2006/WHO_HTM_STB_2006.368_eng.pdf.
- 10.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus Statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279(12):943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 11.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57(1):21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;(4):CD003343. doi: 10.1002/14651858.CD003343.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Floyd K, Pantoja A. Financial resources required for tuberculosis control to achieve global targets set for 2015. Bull World Health Organ. 2008;86(7):568–576. doi: 10.2471/BLT.07.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. MHealth: New horizons for health through mobile technologies. [Accessed March 25, 2015];WHO Global Observatory for eHealth Series. 2011 3 http://whqlibdoc.who.int/publications/2011/9789241564250_eng.pdf?ua=1. [Google Scholar]
- 15.Hoffman JA, Cunningham JR, Suleh AJ, et al. Mobile direct observation treatment for tuberculosis patients: a technical feasibility pilot using mobile phones in Nairobi, Kenya. Am J Prev Med. 2010;39(1):78–80. doi: 10.1016/j.amepre.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Denkinger CM, Grenier J, Stratis AK, Akkihal A, Pant-Pai N, Pai M. Mobile health to improve tuberculosis care and control: a call worth making [Review article] Int J Tuberc Lung Dis. 2013;17(6):719–727. doi: 10.5588/ijtld.12.0638. [DOI] [PubMed] [Google Scholar]
- 17.Garfein RS, Patrick K, Moser K, et al. Mobile phone-based video directly observed therapy for tuberculosis. Paper presented at: mHealth Summit; November 8–10, 2010; Washington, DC. [Google Scholar]
- 18.DeMaio J, Schwartz L, Cooley P, Tice A. The application of telemedicine technology to a directly observed therapy program for tuberculosis: a pilot project. Clin Infect Dis. 2001;33(12):2082–2084. doi: 10.1086/324506. [DOI] [PubMed] [Google Scholar]