Abstract
Objective
Clinical protocols may decrease unnecessary variation in care and improve compliance with desirable therapies. We evaluated whether highly protocolized intensive care units have superior patient outcomes compared with less highly protocolized intensive care units.
Design
Observational study in which participating intensive care units completed a general assessment and enrolled new patients one day each week.
Setting and Patients
6179 critically ill patients across 59 intensive care units in the United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study
Interventions: None
Measurements and Main Results
The primary exposure was the number of intensive care unit protocols; the primary outcome was hospital mortality. 5809 participants were followed prospectively and 5454 patients in 57 intensive care units had complete outcome data. The median number of protocols per intensive care unit was 19 (IQR 15 to 21.5). In single variable analyses, there were no differences in intensive care unit and hospital mortality, length of stay, use of mechanical ventilation, vasopressors, or continuous sedation among individuals in intensive care units with a high vs. low number of protocols. The lack of association was confirmed in adjusted multivariable analysis (p=0.70). Protocol compliance with two ventilator management protocols was moderate and did not differ between intensive care units with high vs. low numbers of protocols for lung protective ventilation in ARDS (47% vs. 52%; p=0.28) and for spontaneous breathing trials (55% vs. 51%; p=0.27).
Conclusions
Clinical protocols are highly prevalent in United States intensive care units. The presence of a greater number of protocols was not associated with protocol compliance or patient mortality.
Keywords: Protocol, intensive care unit, mortality
INTRODUCTION
Patients with life threatening illness are managed in critical care units with specialized monitoring and staffing requirements. The care of critically-ill patients remains challenging because of patient acuity, competing time demands of other seriously ill patients, in addition to large amounts of clinical, mechanical ventilation and laboratory information. In such an environment, it can be difficult to consistently provide desired care to each patient. Studies of patients with specific conditions such as sepsis and the acute respiratory distress syndrome suggest that many patients do not receive desired care1-3.
The use of clinical protocols that target specific clinical syndromes is one method to decrease unnecessary variation in care and improve compliance with desired therapies 4-6. Clinical protocols are prevalent in academic hospitals in the United States7, and have been shown to be associated with desired treatments in patients with acute lung injury, ventilator weaning and sedation management2,8-10. The use of clinical protocols in the intensive care unit (ICU) also appears to not adversely affect trainee knowledge11. However, the link between the number of protocols available in an ICU and patient outcomes is poorly understood.
The United States Critical Illness and Injury Trials Group-Critical Illness Outcomes Study (USCIITG-CIOS) is a multicenter observational cohort study trial designed to understand the association between ICU organization and structural characteristics on hospital mortality12. The primary hypothesis being tested was whether highly protocolized ICUs would have improved patient outcomes compared with less highly protocolized ICUs.
MATERIALS AND METHODS
Study setting
The United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study (USCIITG-CIOS) is a multicenter, prospective observational study of patients with critical illness treated in ICUs in the United States. The intent and content of the study has been previously described in detail13. All participating sites received IRB approval for data collection using a waiver of informed consent14.
Study design
In brief, participating investigators in 69 ICUs first completed a standardized questionnaire regarding patient and organizational characteristics of their intensive care unit, including use of clinical protocols13. Once this standardized questionnaire was completed and reviewed, participating sites were asked to enroll all newly admitted patients on alternating days of the week one day a week with 5 to 10 days between enrollments to allow for patient turnover. Thus,patients in the ICU who were present during previous study dates or who left prior to the next study dates were not enrolled.
The primary outcome measure was hospital mortality. Secondary outcome measures were ICU mortality, and ICU and hospital length of stay. The primary exposure variable was the number of protocols present within a single ICU. Protocols were defined prospectively prior to initiation of the study according to the MeSH term definition, as a precise and detailed plan for a regimen of therapy13. Protocols could be started by a separate physician order or included within standing admission orders13. We included 26 potential conditions that might be managed using protocols based on discussions by study investigators of common order sets and protocols within their own institutions (e.g., lung protective ventilation, ventilator liberation protocols). We analyzed protocols as both a categorical variable as well as our primary comparison of highly protocolized (≥19 protocols) versus less highly protocolized (<19 protocols) ICUs based on the median number of protocols of participating centers as previously reported13. USCIITG-CIOS was approved by the ethics review boards of all participating institutions.
Biostatistical Methods
The primary aim was to determine if critically ill patients in highly protocolized ICUs had lower odds of hospital mortality than did those in less highly protocolized ICUs after adjusting for potential confounders. To test this hypothesis, we constructed a multivariable logistic regression model of hospital mortality as a function of a high vs. low number of protocols (≥19 vs. <19) and adjusted for a priori selected individual- and ICU-level variables. Individual-level variables included age, being male (vs. female), categories of admission source (vs. being in the Emergency Department) and admission diagnosis (indicator variables for Circulatory, Gastrointestinal, Nervous system, Respiratory, Infection, Endocrine and Trauma), APACHE II score, race (non-white vs. white), on mechanical ventilation, having sepsis and having ARDS. ICU-level variables included type of ICU (surgical vs. other), having a daily plan of care review (vs. not), bed-to-nurse ratio > 1.5:1 vs not, 1 and hospital volume (categorized as 25,000-39,999 and >40,000 vs. <25,000 admissions). Participants with missing data in either the outcome or any of the explanatory variables were excluded from multivariable analysis. Given that we enrolled more than one critically-ill patient per ICU and that the unit of analysis was an individual within ICU, we used generalized estimating equations with a compound symmetry matrix and a robust variance to account for ICU-level clustering15. We also conducted a similar analysis in which we treated the number of protocols as a continuous variable modeled using a natural cubic spline with one internal knot at 19.
A secondary aim was to determine compliance with two protocols: low tidal volume ventilation in patients with acute lung injury (i.e., tidal volume per kg of predicted body weight ≤6.5 ml/kg)16 and spontaneous breathing trials in patients with FiO2 ≤40% and PEEP≤5 cm H2O9,17. We also compared differences in compliance prevalence between highly protocolized vs. less highly protocolized ICUs. We conducted all analyses in R (www.r-project.org).
RESULTS
Participant characteristics
We enrolled 6179 critically ill patients across 59 ICUs (86% of all ICUs who completed the structure and process questionnaire), of which 3% (n=202) were missing information on race and 3% (n=168) were missing information on specific patient-centered outcomes (Figure 1). A total of 5809 participants (94%) were followed prospectively. Of these, 5454 (94%) in 57 ICUs had complete information for inclusion in multivariable analyses. In Table 1, we compared demographics and admission characteristics between the group of participants in ICUs with a high (≥_19) vs. low (<19) number of protocols. In unadjusted analyses, we found that individuals in less protocolized ICUs were younger and more likely to be white. In contrast, gender, admission source, admission type, type of ICU, hospital teaching status, severity scores (APACHE II and SOFA) and hospital case volume were similar in individuals in ICUs with a high vs. low number of protocols.
Figure 1.
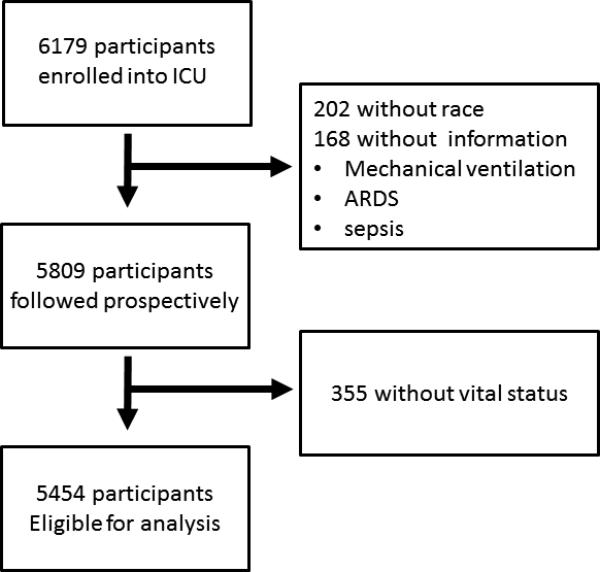
Study Flowchart
Table 1.
Characteristics in 6179 critically-ill patients enrolled into the United States Critical Illness and Injury Trials Group Clinical Illness Outcomes Study.
| High number of protocols (≥ 19) | Low number of protocols (<19) | p-value | |
|---|---|---|---|
| Number of patients | 3116 | 3063 | |
| Median number of patients per ICU | 101 | 101 | |
| Age | 61.3 (17.4) | 57.8 (16.7) | 0.03 |
| Sex | 57% | 55% | 0.50 |
| Race | |||
| White (reference) | 79% | 61% | |
| Black | 16% | 29% | 0.02 |
| Other | 5% | 10% | 0.02 |
| Admission diagnosis | |||
| Cardiovascular only | 9% | 12% | 0.33 |
| Neurological only | 8% | 10% | 0.69 |
| Gastrointestinal only | 8% | 8% | 0.48 |
| Respiratory only | 12% | 15% | 0.14 |
| Infection only | 5% | 4% | 0.29 |
| Endocrine only | 1% | 2% | 0.34 |
| Trauma only | 3% | 3% | 0.56 |
| 2+ diagnoses (reference) | 41% | 39% | |
| Source of admission | |||
| Emergency Department (reference) | 46% | 43% | |
| Hospital Floor | 19% | 19% | 0.39 |
| Operating Room | 23% | 17% | 0.73 |
| Other Hospital | 12% | 14% | 0.57 |
| Other | 4% | 4% | 0.25 |
| Severity index | |||
| APACHE II, mean (SD) | 16.7 (7.0) | 16.6 (7.5) | 0.72 |
| SOFA, mean (SD) | 4.8 (3.6) | 4.9 (3.8) | 0.55 |
| Type of ICU | |||
| Surgical (reference) | 33% | 37% | |
| Medical | 49% | 37% | 0.45 |
| Mixed | 18% | 26% | 0.87 |
| Teaching status | |||
| Academic | 93% | 97% | 0.58 |
| Non-academic | 7% | 3% | |
| Annual number of hospital admissions | |||
| <25,000 (reference) | 19% | 30% | |
| 25,000 – 39,999 | 34% | 46% | 0.94 |
| ≥40,000 | 24% | 48% | 0.53 |
Number of protocols and hospital mortality
Participating ICUs had a high number of protocols (Figure 2). Specifically, no ICU had zero protocols and the median number of protocols in the 59 ICUs included in this analysis was 19 (IQR 15 to 21.5). In Table 2, we compared hospital mortality and other selected treatment and outcome variables between individuals in ICUs with a high vs. low number of protocols. We did not find differences in hospital or ICU mortality, hospital or ICU length of stay, in use of mechanical ventilation, vasopressors or continuous sedation or in withdrawal support among individuals in ICUs with a high vs. low number of protocols.
Figure 2.

Number of ICUs by number of protocols.
Table 2.
Selected treatment variables and clinical outcomes
| High number of protocols (≥ 19) | Low number of protocols (<19) | p-value | |
|---|---|---|---|
| Treatment | |||
| % mechanical ventilation | 43% | 38% | 0.23 |
| % on vasopressors | 20% | 16% | 0.21 |
| % on renal replacement therapy | 8% | 7% | 0.38 |
| % continuous sedation | 35% | 29% | 0.14 |
| Outcomes | |||
| ICU mortality, % | 12% | 13% | 0.64 |
| In-hospital mortality, % | 17% | 17% | 0.96 |
| ICU length of stay, days (SD) | 9.5 (14.9) | 9.7 (12.6) | 0.65 |
| Hospital length of stay, days (SD) | 18.0 (21.7) | 18.4 (21.2) | 0.59 |
| Withdrawal of support, % | 22% | 20% | 0.94 |
In multivariable analyses there was no significant association between a high vs. low number of protocols and hospital mortality (Table 3). We also did not find a dose-response relationship between the number of protocols and hospital mortality (Figure 3). In multivariable logistic regression in which individual patients were the unit of analysis, statistically significant risk factors for death included older age, higher illness severity (APACHE II score), receipt of mechanical ventilation, having sepsis or having ARDS.
Table 3.
Unadjusted and adjusted odds ratios for hospital mortality
| Single variable analysis, OR (95% CI) | p-value | Multivariable analysis, OR (95% CI) | p-value | |
|---|---|---|---|---|
| Age (for every 10 years) | 1.17 (1.12 to 1.23) | <0.001 | 1.07 (1.01 to 1.14) | 0.03 |
| Sex (being male) | 1.03 (0.90 to 1.18) | 0.66 | 0.98 (0.84 to 1.14) | 0.77 |
| Race (not white) | 1.05 (0.87 to 1.27) | 0.59 | 1.08 (0.87 to 1.33) | 0.48 |
| ICU type (vs. surgical) | ||||
| Medical | 2.42 (1.74 to 3.38) | <0.001 | 1.22 (0.87 to 1.69) | 0.25 |
| Mixed | 1.63 (1.06 to 2.51) | 0.03 | 1.16 (0.76 to 1.76) | 0.50 |
| Daily plan of care review | 0.88 (0.56 to 1.40) | 0.59 | 1.24 (0.86 to 1.78) | 0.25 |
| Bed:nurse ratio > 1.5:1 | 1.42 (1.03 to 1.96) | 0.03 | 0.88 (0.67 to 1.17) | 0.40 |
| On mechanical ventilation | 3.21 (2.67 to 3.87) | <0.001 | 1.55 (1.24 to 1.93) | <0.001 |
| Sepsis today | 2.91 (2.47 to 3.41) | <0.001 | 1.51 (1.28 to 1.79) | <0.001 |
| ARDS today | 3.04 (2.48 to 3.71) | <0.001 | 1.52 (1.19 to 1.95) | 0.001 |
| Hospital volume (vs. <25,000) | ||||
| 25,000 – 39,999 | 1.07 (0.72 to 1.64) | 0.72 | 1.02 (0.75 to 1.39) | 0.89 |
| ≥40,000 | 0.98 (0.63 to 1.51) | 0.92 | 0.72 (0.50 to 1.04) | 0.08 |
| Admission source (vs. Emergency Department) | ||||
| Hospital floor | 2.14 (1.71 to 2.69) | <0.001 | 1.89 (1.47 to 2.43) | <0.001 |
| Operating room | 0.50 (0.37 to 0.69) | <0.001 | 0.65 (0.44 to 0.96) | 0.03 |
| Other hospital | 1.27 (0.98 to 1.64) | 0.07 | 1.03 (0.76 to 1.40) | 0.86 |
| Other setting | 1.49 (1.02 to 2.15) | 0.03 | 1.52 (0.89 to 2.60) | 0.13 |
| Admission diagnosis | ||||
| Circulatory system (vs. other) | 1.44 (1.21 to 1.71) | <0.001 | 1.22 (1.01 to 1.46) | 0.03 |
| GI system (vs. other) | 1.48 (1.22 to 1.80) | <0.001 | 1.34 (1.06 to 1.69) | 0.01 |
| Nervous system (vs. other) | 1.42 (1.15 to 1.76) | 0.001 | 1.50 (1.21 to 1.85) | <0.001 |
| Respiratory system (vs. other) | 2.05 (1.76 to 2.39) | <0.001 | 1.30 (1.10 to 1.54) | 0.002 |
| Infection (vs. other) | 1.61 (1.36 to 1.92) | <0.001 | 0.89 (0.71 to 1.10) | 0.28 |
| Endocrine (vs. other) | 0.92 (0.64 to 1.31) | 0.63 | 0.72 (0.52 to 0.98) | 0.04 |
| Trauma (vs. other) | 0.76 (0.57 to 1.01) | 0.06 | 0.73 (0.49 to 1.10) | 0.14 |
| APACHE II (ten point increments) | 3.58 (3.09 to 4.16) | <0.001 | 2.81 (2.39 to 3.30) | <0.001 |
| Number of protocols ≥ 19 | 0.99 (0.71 to 1.39) | 0.97 | 0.94 (0.68 to 1.30) | 0.70 |
Figure 3.
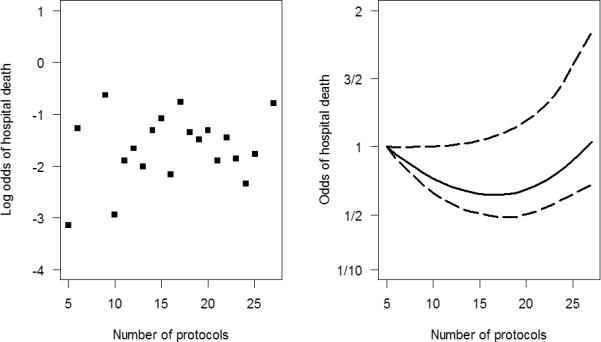
Unadjusted log odds of hospital mortality and protocols (panel A) and adjusted relationship between hospital mortality and protocols (panel B).
Protocol Compliance
To examine whether the total number of protocols in an ICU was associated with better compliance, we selected two common protocols based on patient and ICU characteristics. Overall compliance with two ventilator management protocols was found to be low. Of the 453 patients with ARDS under mechanical ventilation, 50% (n=227) of those with full ventilator parameters were deemed compliant by having ventilator tidal volumes ≤6.5 ml/kg predicted body weight. We found no difference in the prevalence of compliance with low tidal volume ventilation between individuals in ICUs with a high vs. low number of protocols (47% vs. 52%, p=0.28). Of the 1058 critically ill patients under mechanical ventilation who met criteria for weaning (FiO2≤40% and PEEP ≤5 cm H2O), only 53% (n=559) received a spontaneous breathing trial. There was no difference in the prevalence of compliance with a spontaneous breathing trial between individuals in ICUs with a high vs. low number of protocols (55% vs. 51%; p=0.27).
DISCUSSION
We conducted a multi-centered observational study of critically-ill patients from diverse hospitals in the United States to examine the relationship between hospital protocols and clinical outcomes and found that neither a highly protocolized ICU nor the absolute number of protocols was associated with superior risk-adjusted clinical outcomes. In addition, there was no dose-response relationship between protocols and mortality and compliance was modest for evaluated protocols. These findings were robust to sensitivity analyses testing the associations between specific protocol compliance and outcomes.
The results from this study suggest that the number of protocols may not favorably influence hospital mortality or hospital length of stay in critically ill patients. Other studies have shown that protocols can influence process of care in critically ill patients, such as increasing the use of lung protective mechanical ventilation8. In addition, implementation of ARDS ventilation protocols has been shown to decrease mortality compared with historical controls18. In contrast, a multifaceted knowledge translation project was able to improve compliance with desired ICU therapies, although patient outcomes were not assessed19. It may be that any beneficial effects of protocol use are dependent upon better compliance, clinician education 1,3, ICU culture change20 , communication13 or other essential components of effective delivery of critical care, all of which may influence implementation of protocols. We found that the reported presence of a protocol was not necessarily an indicator that protocols were successfully implemented. In addition, our study evaluated protocols as a whole, and it may be that the effects of higher impact protocols outweigh the effects of lower impact protocols. Protocols in two specific areas of critical care, for example, have been shown in multiple randomized trials to improve outcomes. These include ventilator weaning protocols with spontaneous breathing trials as the centerpiece of the protocol, and sedation protocols that emphasize reductions in sedative exposure via daily interruption or targeted light sedation 9,10. In addition, educational efforts that have been included use of protocols and ordersets have improved processes of care and patient outcomes in patients with severe sepsis3, 21, Finally, it may be possible that this study included patients that could potentially be harmed by use of standardized protocols.
Our study has several important limitations. First, we collected ICU structural and organizational information from United States hospitals and primarily academic institutions. Our findings may therefore not be generalizable to ICUs in other locations or to community based ICUs. A recent survey of 1265 ICU's in 75 countries found an association between nurse staffing ratio and hospital death, but did not provide data on protocols.22 In addition, our study was observational with missing data for some covariates, and thus we cannot draw absolute conclusions about causality . In addition, we cannot rule out the possibility that our results can be explained by unmeasured confounders. Most ICUs participating in the study had a high number of protocols, and it is not known whether our findings would translate to ICUs with fewer protocols. The presence of protocols was self-reported, and we do not have data on how robust the protocol was or what was included in the protocol. We only tested ventilator protocols for compliance, so it is possible that the other protocols would have had a different relationship between number of protocols and compliance. We chose ventilator protocols for study since they are highly prevalent in ICU's, and the treatment effect for mechanical ventilation appears to be similar across different types of patients. 13, 23 Additionally, we cannot rule out the possibility that our results could be caused by unmeasured confounders. To minimize this possibility, we adjusted for factors individual and ICU level factors that could be associated with our primary outcome measure. Furthermore, our data does not allow for conclusions about whether protocols may have benefit in certain situations, such as baseline levels of care or staffing. The use of APACHE II has not been validated other than on the first day of hospital admission, or in trauma patients, despite its frequent use in these situations. Finally, we collected data once a week, which might have led to some misclassification. It is possible that daily collection would have provided different findings. We conducted analyses that address several possible limitations, including modeling protocols as both a continuous and a dichotomous variable. Despite these limitations, our study has significant strengths, including large sample size, geographically disperse multicenter design, and observational study with prospectively collected data.
While disease and syndrome mortality caused by critical illness have decreased in the past 20 years, 3, 24,25 several resource intensive efforts to decrease ICU mortality have not been successful26-29. Protocols may be an effective means to minimize variances in care, but the current data and that of others indicate that the presence of a protocol does not ensure its appropriate use30. In parallel to our findings, recent studies have shown that wide implementation of a surgical safety checklist did not decrease surgical complications30 and the inclusion of protocolized usual care for patients with severe sepsis and septic shock abrogates the effect of previously demonstrated targeted interventions.31
CONCLUSIONS
Clinical protocols are widely present in United States ICUs. A greater number of protocols in the ICU was not associated with greater protocol compliance or with improved outcomes such as length of stay or mortality. Methods to ensure appropriate protocol implementation and protocol compliance should be further examined, and other factors that promote culture and behavioral change may be necessary to improve patient outcomes with the use of clinical protocols in critically ill patients.
Acknowledgments
Sources of funding: Dr. Sevransky was supported in part by K23 GM071399. His institution received funding from Abbott Laboratories. Dr. Checkley was supported by a R00 HL096955. Dr. Brown is supported in part by K23GM094465. Dr. Girard was supported in part by K23 AG034257, and his institution received grant support from NIH. He also received honoraria and travel support from Hospira, Inc. Dr. Rice consulted for GlaxoSmithSkline, LLC and Asiva Pharma, LLC. Dr. Johnson consulted for Becton-Dickenson and Medimmune. He is employed by the American College of Surgeons and Banner Health. Dr. O'Brien serves as Chairman of Board of Sepsis Alliance and is the Vice-Chair of Quality Improvement Committee of ACCP (neither provide any financial reimbursement). Dr. Pastores consulted for Theravance and Pfizer (advisory board meeting participation). His institution received grant support from Spectral Diagnostics (Dr. Pastores is the Principal Investigator at MSKCC for a septic shock clinical trial) and Bayer Healthcare (Dr. Pastores is the Principal Investigator at MSKCC for a gram-negative pneumonia clinical trial) Dr. Park was supported in part by U01 HL108712 and U01 HL123031. She consulted for the National Board of Medical Examiners and her institution received grant support from NIH and Social & Scientific Systems, Inc.. Dr. Patil received support for travel from the FTCTS-Care Foundation and has stock options with Google, Apple, Microsoft, GE, Intel, Walmart and Twitter. Her institution received research grant support from NIH and from Canyon Pharmaceuticals. Dr. Shahul is supported by the Foundation for anesthesia research (FAER). Dr. Martin has board membership with Cumberland Pharmaceuticals and Pulsion Medical Systems (no money paid); has consulted for AstraZeneca and Agennix; and was supported in part by R01 FD003440,P50 AA013757, and UL1 TR000454.
Appendix
USCIITG-CIOS investigators
ARIZONA: University of Arizona Medical Center, Tucson, AZ, Terence O'Keeffe (PI), Coy Collins; Laurel Rokowski; CALIFORNIA: LA County-University of South California Hospital, Los Angeles, CA, Janice Liebler (PI), Ali Ahoui, Anahita Nersiseyan, Usman Shah, Hidenobu Shigemitsu, Nanditha Thaiyananthan; Stanford University Medical Center, Stanford, CA, Joe Hsu (PI), Lawrence Ho; VA Palo Alto Health Care System, Juliana Barr (PI); CONNECTICUT: Bridgeport Hospital, Bridgeport, CT; David Kaufman (PI) Yale University Hospital, New Haven, CT, Jonathan M. Siner (PI), Mark D. Siegel; GEORGIA: Emory University Hospital, Atlanta, GA, Greg S. Martin (PI), Craig Coopersmith, Micah Fisher, David Gutteridge, Mona Brown, Sang Lee, Apryl Smith; Emory University Midtown Hospital, Atlanta, GA, Greg S. Martin (PI), Kenneth Leeper, Mona Brown; Grady Memorial Hospital, Atlanta, GA ,Greg S. Martin (PI), Sushma Cribbs, Annette Esper, Mona Brown, David Gutteridge, Olufunmilayo Dosunmu; KANSAS: VA Medical Center, Wichita, KS, Zubair Hassan (PI), Jing Liu, Bart Ridder; ILLINOIS: Northwest Community Hospital, Arlington Heights, IL, Melanie Atkinson (PI), Aimee Draftz, Jackie Durgin, Yelena Rikhman, Jessica Scheckel, Mary Walthers; Saint Francis Hospital, Evanston, IL, Gerald Luger (PI), Carol Downer; University of Illinois Medical Center, Chicago, IL, Ruxana T. Sadikot (PI), Kamran Javaid, Daniel Rodgers, Vibhu Sharma; MARYLAND: Johns Hopkins University, Baltimore, MD, Jon Sevransky (PI), William Checkley, Romer Geocadin, David J. Murphy, Dale Needham, Adam Sapirstein, Steven Schwartz, Glenn Whitman, Brad Winters, Addisu Workneh, Sammy Zakaria; St. Agnes Hospital, Baltimore, MD, Anthony Martinez (PI), Fran Keith; University of Maryland Medical Center, Baltimore, MD, Steven Johnson (PI), Dan Herr, Giora Netzer, Carl Shanholtz, Arabela Sampaio, Jennifer Titus; NIH Clinical Center, Bethesda, MD; Michael Eberlein Suburban Hospital Bethesda, Bethesda, MD, Leo Rotello (PI), Jennifer Anderson; MASSACHUSETTS: Beth Israel Deaconess Medical Center, Boston, MA, Sajid Shahul (PI), Valerie Banner-Goodspeed, Michael Howell, Sabina Hunziker, Victoria Nielsen, Jennifer Stevens, Daniel Talmor; Brigham and Women's Hospital, Boston, MA, Namrata Patil (PI), Lisa Chin, Michael Myers, Stanthia Ryan; MICHIGAN: St Joseph Mercy Health System, Ann Arbor, MI, Joseph Bander, (PI) University of Michigan Health Systems, Ann Arbor, MI, Pauline K. Park (PI), James M. Blum, Vivek Arora, Kristin Brierley, Jessica DeVito, Julie Harris, Elizabeth Jewell, Deborah Rohner; Kathleen B. To, Sharon Dickinson; MINNESOTA: Mayo Clinic, Rochester, MN, Brian W. Pickering (PI), Jyothsna Giru, Rahul Kashyap, Naman Trivedi; MISSOURI: University of Missouri-Columbia Hospital, Columbia, Missouri; University of Kansas, Kansas City, MO, Timothy Dwyer (PI),Kyle Brownback; NEW JERSEY: University of Medicine and Dentistry of New Jersey, Newark, NJ, Steven Chang (PI), Zaza Cohen, Frank Italiano, Zeeshan Kahn, Amee Patrawalla; NEW MEXICO: Presbyterian Healthcare Services, Albuquerque, NM, Denise Gonzales (PI), Paul Campbell; NEW YORK: Columbia University Medical Center, New York, NY, David Chong (PI), Matthew Baldwin, Luke Benvenuto, Natalie Yip; Memorial Sloan Kettering Cancer Center, New York, NY; Steven M Pastores; University of Rochester Medical Center, Rochester, NY, Anthony Pietropaoli (PI), Kathleen Falkner, Timothy Bouck, Ann Marie Mattingly; NORTH CAROLINA: Wake Forest University Health Science, Winston-Salem, NC, Peter E. Morris (PI), Lori S. Flores; East Carolina University, Greenville, NC, Abid Butt (PI) , Mark Mazer, Kelly Jernigan; Moses Cone Health, Greensboro, NC, Patrick Wright (PI), Sarah Groce, Jeanette McLean, Arshena Overton; OHIO: Cleveland Clinic, Cleveland, OH, Jorge A. Guzman (PI), Mohammed Abou El Fadl, Tonya Frederick, Gustavo Cumbo-Nacheli, John Komara; The Ohio State Wexner University Medical Center, Columbus, OH, James M. O'Brien (PI), Naeem Ali, Matthew Exline; PENNSYLVANIA: Eastern Regional Medical Center Cancer Treatment Centers of America, Philadelphia, PA, Jeffrey Hoag (PI), Daniela Albu, Pat McLaughlin; Hahnemann University Hospital, Philadelphia, PA Jeffrey Hoag (PI); Emil Abramian, John Zeibeq; Hospital of the University of Pennsylvania, Philadelphia, PA, Meeta Prasad (PI), Scott Zuick; TENNESSEE: Meharry Medical College Hospital, Nashville, TN, Richard D. Fremont (PI), Chinenye O. Emuwa, Victor C. Nwazue, Olufemi S. Owolabi; Vanderbilt University Medical Center, Nashville, TN, Bryan Cotton (PI), George Hart, Judy Jenkins; Vanderbilt University Medical Center, Nashville, TN, Todd W. Rice (PI), Timothy D. Girard, Margaret Hays, Susan Mogan; TEXAS: University of Texas-Houston Medical Center, Houston, TX; Imo P. Aisiku (PI); UTAH: Intermountain Medical Center, Murray, UT, Samuel Brown (PI), Colin Grissom, Russ Miller III, Anita Austin, Heather Gallo, Naresh Kumar; VIRGINIA: Inova Health Systems, Falls Church, VA, Maryann Putman (PI), Joanne Ondrush.
Copyright form disclosures: Dr. Pickering and his institution served as board member for, has patents with, received royalties from, and has stock in Ambient Clinical Analytics. Dr. Pickering and his institution received grant support from the Center For Medicare/Medicaid, Innovation funding, Grant # 1C1CMS330964-01-00. Dr. Barr disclosed government work. Dr. Brown served as a board member for Vecna Technologies, Inc. (Medical advisor to robotics/informatics company. <$1000/yr), lectured for the Society of Critical Care Medicine (SCCM) (Critical Care Ultrasound courses as faculty/co-chair), received royalties from Oxford University Press (For books on history and medical ethics), other support from SBP World Technologies (Dr. Brown is cofounder of an air pollution mitigation company), and received support for article research from the National Institutes of Health (NIH). His institution received grant support from NIGMS (K23 award), the Intermountain Research and Medical Foundation (Various investigator-initiated research studies), and the NHLBI (R21 ARDS Outcome Phenotypes). His institution has a patent with Intermountain Healthcare (Airway and catheter attachment devices assigned to Intermountain). Dr. Kaufman served as a board member for Critical Care Roundtable and received royalties from Up To Date. His institution received grant support and support for travel from the NIH/NHLBI. Dr. Girard lectured for Hospira, Inc. (Honoraria for non-promotional presentations) and received support for article research from the NIH. His institution received grant support from the NIH (AG034257). Dr. Kerlin's institution received grant support from the NIH/NHLBI (K08 Career Development Award). Dr. Liebler's institution received grant support from the St. Michaels Hospital, Toronto, Canada (For IOS Wean multicenter study). Dr. O'Brien disclosed lecturing on sepsis (Honoraria were donated to Sepsis Alliance. Travel and accommodation). Dr. Park's institution received grant support from the NIH. Dr. Pastores’ institution received grant support from Spectral Diagnostics (He is PI at MSKCC for a septic shock clinical trial and PI at MSKCC for a gram negative pneumonia clinical trial) and lectured for the American Physician Institute and the SCCM (Speaker at Board Review Courses). Dr. Patil has stock options in Google, Apple, GE, Intel, Microsoft, Twitter, Alibaba, AT& T, Walmart, TAL, and King (No direct relationship with the submitted manuscript) and received support for travel from the Foundation for Advanced Cardiovascular Thoracic Care (CME activity - Invited speaker). Her institution received grant support from the NIH/NIAID (This has no relationship with the submitted manuscript). Dr. Putman is employed by INOVA Fairfax hospital. Dr. Rice consulted for Avisa Pharma, LLCAvisa Pharma, LLC (Advisor) and GlaxoSmithKline, LLC (DSMB). Dr. Martin served as board member for Pulsion Medical Systems; consulted for Grifols (Medical advisory board), Cumberland Pharmaceuticals (Data safety monitoring board), and Vanderbilt University (Vanderbilt University) and received support for article research from the NIH. His institution received grant support from NIH, the FDA, Baxter Healthcare, and Abbott Laboratories. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 2.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RR, 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188(1):77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 7. 2000;132(5):373–383. doi: 10.7326/0003-4819-132-5-200003070-00007. [DOI] [PubMed] [Google Scholar]
- 5.Morris AH. Rational use of computerized protocols in the intensive care unit. Crit Care. 2001;5(5):249–254. doi: 10.1186/cc1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blagev DP, Hirshberg EL, Sward K, et al. The evolution of eProtocols that enable reproducible clinical research and care methods. J Clin Monit Comput. 2012;26(4):305–317. doi: 10.1007/s10877-012-9356-y. [DOI] [PubMed] [Google Scholar]
- 7.Prasad M, Christie JD, Bellamy SL, Rubenfeld GD, Kahn JM. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25(4):610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umoh NJ, Fan E, Mendez-Tellez PA, et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36(5):1463–1468. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 10.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 11.Prasad M, Holmboe ES, Lipner RS, et al. Clinical protocols and trainee knowledge about mechanical ventilation. JAMA. 2011;306(9):935–941. doi: 10.1001/jama.2011.1226. [DOI] [PubMed] [Google Scholar]
- 12.Ali NA, Shahul S, Checkley W, Sevransky J, Martin GS. Critical Illness Outcome Study: an observational study or protocols and mortality in intensive care units. Open Access J Clin Trials. 2011;3:55–65. doi: 10.2147/OAJCT.S24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checkley W, Martin GS, Brown SM, et al. Structure, Process, and Annual ICU Mortality Across 69 Centers: United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2014;42(2):344–56. doi: 10.1097/CCM.0b013e3182a275d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polito CC, Cribbs SK, Martin GS, et al. Navigating the Institutional Review Board Approval Process in a Multicenter Observational Critical Care Study. Crit Care Med. 2014;42(5):1105–9. doi: 10.1097/CCM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang KY, Zeger SL. Regression analysis for correlated data. Ann Rev Pub Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 16.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 18.Kallet RH, Jasmer RM, Pittet JF, et al. Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med. 2005;33(5):925–929. doi: 10.1097/01.ccm.0000162382.59289.9c. [DOI] [PubMed] [Google Scholar]
- 19.Scales DC, Dainty K, Hales B, et al. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA. 2011;305(4):363–372. doi: 10.1001/jama.2010.2000. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman JE, Shortell SM, Rousseau DM, et al. Improving intensive care: observations based on organizational case studies in nine intensive care units: a prospective, multicenter study. Crit Care Med. 1993;21(10):1443–1451. [PubMed] [Google Scholar]
- 21.Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 22.Sakr Y, Moreira CL, Rhodes A, et al. The Impact of Hospital and ICU Organizational Factors on Outcome in Critically Ill Patients: Results From the Extended Prevalence of Infection in Intensive Care Study. Crit Care Med. 2015;43:519–26. doi: 10.1097/CCM.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 23.Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Resp Crit Care Med. 2001:231–6. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 24.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Resp Crit Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 25.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 26.Kerlin MP, Small DS, Cooney E, et al. A randomized trial of nighttime physician staffing in an intensive care unit. N Engl J Med. 2013;368(23):2201–2209. doi: 10.1056/NEJMoa1302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366(22):2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas EJ, Lucke JF, Wueste L, Weavind L, Patel B. Association of telemedicine for remote monitoring of intensive care patients with mortality, complications, and length of stay. JAMA. 2009;302(24):2671–2678. doi: 10.1001/jama.2009.1902. [DOI] [PubMed] [Google Scholar]
- 29.Kahn JM. The use and misuse of ICU telemedicine. JAMA. 2011;305(21):2227–2228. doi: 10.1001/jama.2011.716. [DOI] [PubMed] [Google Scholar]
- 30.Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med. 2014;370(11):1029–1038. doi: 10.1056/NEJMsa1308261. [DOI] [PubMed] [Google Scholar]
- 31.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]


