Abstract
Abnormalities in parasympathetic nervous system activity have been linked to depression, but less is known about processes underlying this relationship. The present study evaluated resting and stress-reactive respiratory sinus arrhythmia (RSA) to a laboratory stressor as predictors of daily interpersonal stress generation and depressive symptoms, whether stress generation mediated the relationship between RSA and depressive symptoms, and potential sex differences. A sample of formerly depressed 102 emerging adults (18–22 years; 78% female) completed a laboratory stressor and daily assessments of stressors and depressive symptoms over two weeks. Multilevel modeling revealed that: 1) lower resting RSA predicted daily depressive symptoms; 2) less RSA reactivity predicted interpersonal stress generation, 3) interpersonal dependent stressors mediated the relationship between RSA reactivity and daily depressive symptoms, and 4) sex differences occurred in the resting RSA-depression relationship. These findings highlight the importance of resting RSA and RSA reactivity in the examination of depression and interpersonal processes.
Keywords: respiratory sinus arrhythmia, depression, reactivity, stress generation, interpersonal
Depression is a severe disorder associated with significant individual and societal costs (Greenberg, Fournier, Sisitsky, Pike, & Kessler, 2015; Kessler et al., 2009). Beyond major depression, subthreshold depression is associated with a similar course and impairment (Kessler, Zhao, Blazer, & Swartz, 1997) and predicts the first onset of a major depressive episode (van Lang, Ferdinand, & Verhulst, 2007). Part of depression’s debilitating effect is due to its high rates of recurrence, with approximately 50% of individuals who recover from a depressive episode likely to have another episode in their life (American Psychiatric Association, 2000), and up to 80% of those with a history of two or more depressive episodes apt to have another recurrence (Burcusa & Iacono, 2007). Although many factors are implicated in depression onset and recurrence, the occurrence of stressful events is considered to be among the most robust predictors (Burcusa & Iacono, 2007; Monroe, Slavich, & Georgiadas, 2014, for a review), particularly stressors that involve interpersonal relationships (Kendler, Karkowski, & Prescott, 1999). However, given that most individuals encounter interpersonal stressors throughout their lifetime without developing depression, it is important to consider how individuals’ physiological response to stress may contribute to processes that predict depression risk and recurrence (for a review, see Hamilton & Alloy, 2016). In particular, we propose that individuals with atypical or maladaptive physiological stress responses may inadvertently contribute to the occurrence of stressful events in their interpersonal relationships (i.e., interpersonal stress generation), to which they are then less physiologically able to respond, thereby increasing the risk for depression. Thus, the current study sought to clarify and disentangle the relationship between these two important stress-related processes in depression by evaluating the psychophysiological stress response as an objective marker of interpersonal stress generation, a potent and modifiable mechanism of increased depression risk.
Psychophysiological Stress Response and Depression
According to research and theory, the brain receives input from the internal and external environment and transmits output to the sinoatrial node of the heart, the heart’s primary “pace maker” to adjust physiological arousal (Appelhans & Luecken, 2008; Berntson et al., 1997). Specifically, the parasympathetic nervous system (PNS) exerts its influence on the heart via the vagus nerve (i.e., tenth cranial nerve) and acts as a vagal “brake” to inhibit heart rate and sympathetic activation (Porges et al., 2007). A marker of PNS’ influence on the heart that has garnered significant attention in the depression literature is respiratory sinus arrhythmia (RSA), which is variability in the interbeat intervals in heart rate or heart rate variability (HRV) that occurs in the high-frequency range of respiration (HF-HRV; hereafter referred to as RSA). In this sense, higher levels of resting RSA reflect a greater capacity to apply the vagal “brake” and self-regulate (Beauchaine, 2001; Porges, 2007; Thayer & Lane, 2000). In response to environmental challenges or stress, the vagal “brake” is typically released, thereby decreasing control of the heart via the vagus nerve. Release of the vagal “brake” also facilitates heart rate acceleration to mobilize physiological and cognitive resources to effectively manage and cope with stress. Given that the PNS influences on the heart occur in milliseconds compared to the slower-acting sympathetic activation of the heart, flexibly and quickly responding to changing demands is more efficient. Therefore, greater RSA withdrawal is generally considered an adaptive stress response promoting coping responses and behaviors (Fabes & Eisenberg, 1997), whereas less RSA withdrawal or amplification (increases) of RSA is indicative of less efficient responses to stressors (Porges, 1995, 2003a). Although RSA is an indirect index of vagal tone (Berntson, Cacioppo, & Quigley, 1993), research indicates that resting RSA levels and RSA reactivity may serve as important indices of individual differences in the ability to self-regulate and react to stress in the environment (Thayer, Ahs, Fredrikson, Sollers, & Wager, 2012). Consistent with this, research has found that higher levels of resting RSA and RSA reactivity reflect better cognitive and attentional control, emotion regulation, executive functioning, and social engagement (Appelhans & Luecken, 2006; Hansen, Johnsen, & Thayer, 2003; Porges, 2003a; Thayer & Brosschot, 2005).
Given that depression is characterized by deficits in emotion regulation (Bylsma, Morris, & Rottenberg, 2008), cognitive control (Gotlib & Joormann, 2010), and interpersonal dysfunction (Hammen & Brennan, 2002; Hammen, 2005), it is not surprising that studies also link RSA levels with depression. Specifically, lower levels of RSA have been demonstrated among children, adolescents, and adults with current and past depression (for reviews, see Kemp et al., 2010; Koenig et al., 2016; Rottenberg, 2007). Surprisingly, fewer studies have focused on RSA reactivity to psychosocial stressors, such as speeches, mental arithmetic, or emotion-induction films, in depression. Those studies that have indicate that individuals with current depression exhibit less RSA withdrawal to laboratory stressors (e.g., Bylsma, Salomon, Taylor-Clift, Morris, & Rottenberg, 2014; Rottenberg, Clift, Bolden, & Salomon, 2007). However, studies of past depression yield mixed findings, with some studies finding that adults with past depression also exhibit blunted RSA stress reactivity (e.g., Yaroslavsky, Bylsma, Rottenberg, & Kovacs, 2013), whereas others suggest that adults with remitted depression exhibit similar reactivity to healthy controls (e.g., Bylsma et al., 2014). Importantly, recent research also indicates the importance of assessing the interactive effects of resting RSA and reactivity (Yaroslavsky, Rottenberg, & Kovacs, 2013; Yaroslavsky et al., 2014), finding that atypical RSA patterns, such as lower resting RSA and greater RSA withdrawal, increase the risk of depression (for a review of RSA reactivity and depression, see Hamilton & Alloy, 2016).
Although maladaptive RSA patterns have been documented in depression, less is known about the role of RSA in processes known to confer risk for the maintenance, recurrence, and onset of depression. In understanding potential mechanisms underlying the relationship between RSA reactivity and depression, it is important to consider that individuals with adaptive RSA reactivity (i.e., appropriate RSA withdrawal or reactivity) have the ability to flexibly respond to changing demands and modulate their emotional and behavioral responses, which facilitate effective coping strategies and more adaptive interpersonal behaviors (Fabes & Eisenberg, 1997; Geisler, Kubiak, Siewert, & Weber, 2013; Porges, 2003a). Interestingly, despite recent research demonstrating that the interpersonal environment influences RSA reactivity (e.g., McLaughlin, Alves, & Sheridan, 2014), fewer studies have evaluated whether RSA reactivity contributes to the interpersonal environment. However, it is possible that individuals with lower resting RSA or blunted RSA reactivity may have more difficulty with self- and stress-regulation, thereby contributing to more negative events in their lives, particularly in interpersonal relationships.
Stress Generation and Depression
The notion that individuals shape their interpersonal environments and directly or indirectly contribute to stressful events dependent on their characteristics or behaviors is coined stress generation (Hammen, 1991). Stress generation theory has received considerable support over the past few decades, with research demonstrating that individuals with current, past, or subthreshold depression generate negative events in their interpersonal relationships (hereafter referred to as interpersonal dependent events) that are at least in part dependent on them (e.g., Liu & Alloy, 2010). However, this is specific to interpersonal dependent events and does not hold for events that are independent (i.e., fateful events to which we would typically not expect someone to contribute), such as parent losing a job or illness of a loved one. The types of stressors generated within the stress generation framework, specifically dependent stressors that occur within interpersonal relationships and are a result of an individual’s characteristics or behaviors (Hammen, 2005), are robust predictors of first onset and recurrent depression (Liu, 2013). More broadly, the occurrence of stressful events is one of the best predictors of depression, including first onset, relapse, and depressive symptoms (Burcusa & Iacono, 2007; Kendler et al., 1999; Stroud, Davila, & Moyer, 2008). Although major life events occur prior to 50% of first episodes of depression, there is also evidence of a “kindling” effect of stress, whereby less severe events or daily hassles precipitate depression onset among individuals with subthreshold or past history of depression (Monroe & Harkness, 2005; Stroud et al., 2008). Thus, identifying markers of stress generation is critical for identifying individuals at heightened vulnerability to experience interpersonal dependent stressors, a potent and malleable risk factor for depression onset and recurrence.
Although stress generation is documented among individuals with current and past depression, considerable research has extended the examination of the stress generation process to vulnerability factors for depression to better understand why certain individuals may contribute to negative events in their interpersonal relationships. These studies have found that individuals who possess certain vulnerabilities to depression, such as maladaptive cognitive and interpersonal styles (e.g., Eberhart & Hammen, 2009; Hamilton et al., 2013; Safford, Alloy, Abramson, & Crossfield, 2007), generate interpersonal dependent stressors beyond the effects of current depressed mood. Although a number of self-reported vulnerabilities for stress generation have been identified (Liu, 2013), less research has focused on biological markers for the generation of interpersonal dependent stressors, which may provide insight into the neurobehavioral processes that contribute to stressors and depression.
Psychophysiological Stress Response and Stress Generation
Although no known study has examined biological processes, such as RSA, in stress generation theory, it is possible that atypical RSA patterns may underlie many of the risk factors implicated in stress generation. Specifically, lower RSA and less RSA withdrawal is associated with negative interpersonal behaviors (Porges, 2003b), including poor social skills (Blair & Peters, 2003), reduced interpersonal warmth (Diamond & Cribbet, 2013), and social disengagement (Geisler et al., 2013), as well as maladaptive cognitive strategies, such as emotional suppression and rumination (Thayer & Brosschot, 2005; Yaroslavsky, Bylsma, et al., 2013). These maladaptive responses, in turn, have been linked to the occurrence of interpersonal dependent stressors. In particular, these negative traits and behaviors have been found to predict poor interpersonal relationships more generally, and specifically, interpersonal dependent stressors (i.e., interpersonal stress generation; for a review, see Liu, 2013). Thus, given that individuals with lower resting RSA or blunted RSA reactivity have more difficulty with self- and social-regulation and associated behaviors (Thayer & Lane, 2000; Thayer et al., 2012), and many of these maladaptive cognitive, emotional, and interpersonal styles are known risk factors for interpersonal stress generation, it is surprising that no known research to date has evaluated the role of RSA in interpersonal stress generation.
Although there is no direct evidence for this hypothesis, there is preliminary evidence from one study that demonstrates that lower RSA reactivity predicted more daily negative interactions among couples (Diamond, Hicks, & Otter-Henderson, 2011), thereby suggesting a potential relationship between blunted RSA reactivity and heightened daily interpersonal stressors. Thus, it is possible that individuals with less RSA stress reactivity would specifically experience the occurrence of more interpersonal dependent stressors, but not independent stressors. These stressors, in turn, may further contribute to their dysregulated physiological reactivity and inability to effectively cope, thereby leading to a cycle of increased risk for depression. Identifying resting RSA or RSA reactivity as a predictor of daily stressors might provide more objective markers of risk for interpersonal stress generation, and a more targeted point of intervention into a potent process for the first onset and recurrence of depression.
The Current Study
Recent research points to the importance of assessing daily stressors to gain insight into the everyday processes that confer risk for depression at the level of analysis at which they unfold (aan het Rot, Hogenelst, & Schoevers, 2012). Thus, micro-longitudinal or daily study designs are well-suited to explore the idiographic and complex relationships that may exist. The primary aim of the present study was to evaluate whether individual differences in RSA patterns predicted daily interpersonal stress generation and depressive symptoms among individuals with a history of clinical and subclinical depression (and therefore, at risk for subsequent depression). Specifically, we examined whether resting RSA, RSA reactivity, and the interactive effects of RSA resting and reactivity would predict higher levels of depressive symptoms and interpersonal dependent stressors, but not independent stressors, over two weeks of daily diary assessments. Although not primary to our hypotheses, we also explored the effects of RSA reactivity to anticipatory threat and RSA recovery from stress on interpersonal stress generation and depressive symptoms, particularly given the importance of RSA recovery in depression (Bylsma et al., 2014). In addition, our second aim was to examine whether interpersonal stress generation would mediate the relationship between RSA patterns and depressive symptoms. Finally, we examined potential sex differences in the relationship between RSA patterns and depression, given that women are at greater risk than men for both stress generation and depressive disorders (Hankin et al., 1998).
Method
Recruitment
The current sample of 102 emerging adults (ages 18–22) was recruited as part of the Stress and Emotion Study at Temple University to evaluate the predictive association between physiological reactivity to a laboratory stressor task and the occurrence of stressful events and depressive symptoms among individuals with a history of subthreshold or clinical depression. Participants were recruited from Temple University through flyers posted around campus, in-class announcements in psychology courses, and the Temple University Psychology Research Participation System (a HIPAA compliant online research management system). Individuals interested in the study were invited to complete an online screening, which included demographic questions, a self-report measure of current and lifetime depressive episodes (Inventory to Diagnose Depression- Lifetime; IDD-L; Zimmerman & Coryell, 1987), and measures of depressive symptoms (Beck Depression Inventory-II; BDI-II; Beck, Brown, & Steer 1996) and (hypo)manic symptoms (7 Up 7 Down Inventory; 7U7D; Youngstrom, Murray, Johnson, & Findling, 2013) to evaluate inclusion and exclusion criteria.
Eligible participants included individuals fluent in written and spoken English between the ages of 18–22 years old, which is a time during which individuals experience a wide variety of stressors (Compas, Wagner, Slavin, & Vannatta, 1986). In addition, eligible participants had to have a history of at least one major or minor (subthreshold) depressive episode, given that individuals with a history of depression are most vulnerable to future depressive episodes (Burcusa & Iacono, 2007). Subthreshold depression was categorized as: 1) having two or more symptoms (one of which must be depressed mood or anhedonia) nearly all day every day for 2 weeks or more, or 2) having five or more symptoms (one of which is depressed mood or anhedonia) most of the day every day for at least a week, and 3) functional impairment must be present. Depression diagnoses were confirmed with a diagnostic interview (see below). Including individuals with a full range of past depressive symptoms (both clinical and subclinical past depression) allowed us to examine depression on a continuum (Goldberg, 2011) and to examine these processes among individuals vulnerable to, but not currently experiencing, depression.
Participants were excluded only if they met diagnostic criteria for current Major Depressive Disorder (MDD) based on the IDD-L or exhibited moderate or severe depressive symptoms currently on the BDI-II (indicated by a score of 20 or higher; Beck et al., 1996). Individuals currently experiencing MDD or with moderate to severe depressive symptoms were excluded given the importance of identifying markers of future risk that are distinct from the depression itself. Further, given our interest in depression, participants who scored greater than 3 for (hypo)mania symptoms (using the case scoring method) on the 7 Up scale of the 7U7D Inventory, which may indicate greater risk for bipolar spectrum disorders, also were excluded.
Of the 892 individuals who participated in the screener, 457 (51%) did not endorse a past major or subthreshold depressive episode, 111 (12.44%) met inclusion criteria, but endorsed moderate to severe depressive symptoms currently, and 44 (4.93%) met inclusion criteria, but endorsed current hypomanic symptoms. In addition, 159 (17.83%) were eligible and invited to participate in the study, but did not respond to invitations to complete the study. There were no significant differences on symptoms of depression (t = 1.33, p = .16; d = .16) or hypomania (t = 1.88, p = .06; d = .23), sex (χ2 = .06, p = .80; OR = 1.07), age (t = .27, p = .79; d = 1.09), or type of depressive episode (χ2 = .01, p = .92; OR = .96) between those who completed the study and those who were eligible and did not participate. An additional 19 students participated in the study, but did not meet criteria for a past subthreshold or major depressive episode based on the diagnostic interview, and therefore, were not included in the present analyses. Further, three additional participants reported that they had a cardiovascular condition. Given that this may influence heart rate variability (Licht et al., 2008), these participants were excluded from analyses. However, there were no differences between those who did and did not meet diagnostic criteria for depression on demographic or clinical variables (all ts and χ2s < 1.11, all ps >.29).
Procedure
Eligible individuals were invited to participate in the full micro-longitudinal study, which included a baseline assessment and two weeks of daily surveys. During the baseline assessment, individuals first provided written consent to participate in the full study. Participants then completed questionnaires, a diagnostic interview assessing current and past mood disorders, and the Trier Social Stress Test (TSST), during which participants were connected to an electrocardiogram (ECG) to measure heart rate variability. Participants then were asked to remotely complete 14 days of daily diaries to assess life events and depressive symptoms using any device connected with internet capability. This study was approved by the Temple University Institutional Review Board (IRB).
Participants
Participants (N = 102) in the final sample were 19.86 years old on average (SD = 1.17 years) and 78% were female. In addition, 70% self-identified as Caucasian, 7% as African American, 6% as Biracial, 15% as Asian and 2% as ‘Other.’ In our sample, 7% also identified as Hispanic. In terms of sexuality, 80% of participants identified as heterosexual, 12% as lesbian, gay, or bisexual, and 8% as ‘Something else’ or ‘Other.’ Although 79% of the sample currently lived in an urban area (e.g., Philadelphia), only 26% reported living in an urban area previously and the majority of participants previously lived in suburban (59%) or rural (15%) areas.
At the diagnostic assessment, 76 (74.5% of the sample) met criteria for past Major Depressive Disorder (MDD) and 26 (25.5%) for a past subthreshold depression. The mean age of first onset of MDD was 15.77 years (SD = 3.32 years) and 15.16 years (SD = 3.29 years) for a subthreshold episode. Of those with MDD, 46 (45.1%) had one MDD episode, 24 (23.5%) had two episodes, and 5.9% had 3 or 4 MDD episodes. Of those only with subthreshold episodes, 22 participants (21.6%) only had one episode. The average length of the most severe depressive episode ranged considerably, with 17% reporting that the depressive episode lasted for greater than a year, 18% for 6 to 12 months, 24% for 2 to 6 months, 16% for 1 to 2 months, 16% for 2 to 4 weeks, and 14% reporting a subthreshold depressive episode that lasted for 1–2 weeks.
Screener Measures
Inventory to Diagnose Depression-Lifetime version (IDD-L; Zimmerman & Coryell, 1987)
The IDD-L is a 22-item self-report measure that indexes the number of depressive symptoms that a person has experienced during their worst lifetime period of depression. Each item is rated on a 5-point scale (0 to 4; e.g., 0- “I did not lose interest in my usual activities” to 4 “I have lost interest in all of my usual activities.”) to assess the severity, duration, and impairment of clinically significant symptoms of depression. The IDD-L is scored using the criteria for the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV). As previously mentioned, individuals who endorsed subthreshold depression (e.g., two or more symptoms (one of which must be depressed mood or anhedonia) nearly all day every day for 2 weeks or more, or five or more symptoms (one of which is depressed mood or anhedonia) most of the day nearly every day for at least a week) were included. Functional impairment also must be present to meet for a diagnosis of any depressive disorder. This scale has excellent sensitivity and specificity for diagnoses made using structured diagnostic interviews (Zimmerman & Coryell, 1987). In the present study, the internal reliability was α = .94.
Beck Depression Inventory-II (BDI; Beck et al., 1996)
The BDI-II is a 21-item self-report questionnaire that assesses cognitive, affective, and somatic depressive symptoms experienced over the past 2 weeks. Items are scored on a Likert-scale (0–3), with higher scores indicative of more severe depression. The BDI-II has demonstrated strong psychometric support, including good internal consistency, test-retest reliability (r = .93), concurrent validity, and convergent validity (Beck et al., 1996; Beck, Steer, Ball, & Rainieri, 1996). In the current study, the BDI-II had good internal consistency (α = .90).
7 Up 7 Down Inventory (7U7D; Youngstrom et al., 2013)
The 7U7D is a 14-item measure of manic and depressive tendencies of an individual. Participants respond using a 4-point scale based on frequency of experience (1- Never or hardly ever to 4- Very often or almost constantly). Only the 7 Up subscale of the 7U7D was used in the present study to determine the presence of manic or hypomanic symptoms. Item scores of 3 or 4 were recoded as 1, and item scores of 1 or 2 were recoded as 0 (Youngstrom et al., 2013). All items then were summed for a total score, with scores ranging from 0–7. In the present study, individuals with scores greater than 3 were deemed ineligible, given findings that 3 or more on the 7U is associated with clinically significant (hypo)mania (Alloy et al., 2008).
Baseline Assessment
Diagnostic Interview
An expanded version of the Schedule for Affective Disorders and Schizophrenia - Lifetime (exp-SADS-L; Alloy et al., 2008; Endicott & Spitzer, 1978) is a semi-structured diagnostic interview used to assess past and current psychopathology. The present study utilized the depression and bipolar modules of the exp-SADS-L to determine whether individuals’ depressive symptoms met criteria for past major or minor/subthreshold depressive disorder according to Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition (DSM-IV) criteria (for inclusion criteria), and current MDD or bipolar disorder (for exclusion criteria). In the present study, subthreshold depression was defined as having five or more symptoms of depression nearly all day every day for one week (N = 15; 14% of full sample) or two or more symptoms all day nearly every day for 2 weeks (N = 12; 11%). Consistent with DSM-IV criteria, depressed mood or anhedonia and significant associated functional impairment must be present to meet criteria for any depressive disorder. In prior studies, the exp-SADS-L has demonstrated excellent inter-rater reliability, with κ > .90 for unipolar depression diagnoses based on 80 jointly rated interviews (Alloy et al., 2000). In the present study, interviews were audio-recorded for reliability coding and agreement on presence of symptoms and diagnosis were examined in a randomly selected 20 interviews. Reliability raters were blind to the outcome of the original interview. Our interviewers demonstrated excellent agreement (κ = .95, p < .001).
Trier Social Stress Test (TSST)
The TSST is a valid, reliable, and widely used method to induce psychosocial stress and elicit an autonomic stress response (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). The TSST consists of socio-evaluative threat of a public speaking task and performance threat of mental arithmetic. First, participants were instructed to sit still and breathe normally for three minutes to establish baseline levels of HRV. To increase the socio-evaluative threat for individuals, after the baseline assessment of HRV, participants were informed that they would be asked to give a 3-minute speech about themselves in front a camera (where they would be seeing themselves on screen) and the video would be recorded and streamed live to a group of their peers who would rate how much they liked them and would want to spend time with them. In addition to participants viewing themselves on the screen, the experimenter was present and silently observed the participant while pretending to take notes on the participant’s speech and behavior. Prior to beginning the speech, participants were instructed to think about and prepare their speech (without writing anything down) for the next three minutes while the interviewer left the room. After three minutes elapsed, participants were instructed to give a speech for the full three-minutes. After three minutes, participants then completed a calculation task (subtracting increments of 13 from 2,083) for 60-seconds. The participants then were told to breathe normally for the next three minutes to monitor physiological recovery.
Heart Rate Variability (HRV). Electrocardiogram (ECG) data were measured using a BioPac BioHarness MP150 with AcqKnowledge v. 4.2 software, a system that monitors, analyzes, and records physiological parameters (Biopac Systems, Inc., Santa Barbara, CA). The BioHarness is a wireless device that is attached to individuals via a standard three-electrode setup sampled at 1000 Hz. Heart rate was monitored continuously during the TSST, with markers placed throughout the experiment to indicate the beginning and end of each component of the TSST. There were four distinct epochs in the present study: baseline, anticipatory threat, stressor task, and recovery. Data collected from Acknowledge then were imported into Kubios HRV software (version 2.2; Tarvainen, Niskanen, Lipponen, Ranta-Aho, & Karialanen, 2013), where it was visually inspected for artifacts and corrected manually. We used Kubios default parameters, which included piecewise cubic spline interpolation with the default rate of 4 Hz for RR interpolation rate and did not detrend the data. Power spectral analysis was conducted by integrating the power estimates over each frequency band with a fast Fourier transformation with a 256 second window with 50% overlap (Welch’s periodogram) for each phase of the TSST. Consistent with the recommendations by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996), the absolute power density (ms2) for each band of high-frequency (HF; .15 – .40 Hz) and low frequency (LF; .04 –.15), and Very Low Frequency (VLF; 0 – .04) was calculated and the average for each period was used. Only HF HRV was included in subsequent analyses because of our focus on HF-HRV (hereafter referred to as RSA). RSA variables for each portion of the task (baseline, anticipatory threat, full stressor [speech and math], and recovery) were log-transformed to normalize the distribution, which violated normality assumptions, prior to calculating variables (Bylsma et al., 2014; Yaroslavsky, Rottenberg et al., 2013). Because RSA may be influenced by respiration rates (RR), we controlled for respiration by regressing average RR on RSA for each task phase and used the unstandardized residuals (Bylsma et al. 2014; Ritz & Dahme, 2006). RSA reactivity was then calculated by subtracting the residualized RSA scores during the full stressor (speech and math) from RSA during the baseline period. To explore the full stress process, we also created variables of RSA anticipation and RSA recovery by subtracting the residualized RSA values of the anticipatory phase and recovery period from baseline, respectively. Unless noted otherwise, residualized RSA variables were used for study hypotheses.
PROMIS-Depression-Long Form (PROMIS-Depression-LF; Pilkonis et al., 2011)
The Patient Reported Outcomes Measurement Information System (PROMIS) network used Item Response Theory to develop a measure to assess depression as per the National Institutes of Health initiatives. The PROMIS-Depression-LF is a 27-item measure that assesses the severity of symptoms of depression. It measures the presence of depressive symptoms over the prior two weeks. The PROMIS-Depression-LF has been found to have better psychometric properties and be more sensitive to the dimensionality of depressive severity than other well known self-report scales of depression (Olino et al., 2012; 2013; Pilkonis et al., 2011). The PROMIS-Depression-LF was used as a covariate for analyses predicting to depressive symptoms over the subsequent two weeks using the short form of this measure (see below). In the present study, the internal reliability of the PROMIS-Depression-LF was α = .97.
Life Events Scale (LES) and Interview (LEI; Safford et al., 2007)
The LES asks individuals to indicate which events occurred to them over the past four weeks (specific dates and a calendar are provided to the participant). The LES includes 100 major and minor life events in a variety of domains, including school, work, finances, family, peer, and romantic relationships. Then, individuals were interviewed to validate that the events occurred during the given period and met a priori definitional criteria to reduce potential biases. The LES events were a priori rated on the extent to which they were independent (fateful) or dependent (i.e., an event to which an individual would be expected to contribute), and individually adjusted accordingly based on information provided by the participant (Safford et al., 2007). Dependent events also were categorized as interpersonal. The LEI does not consider the extent of independence or dependence of events, but considers whether the event was “fateful” or one that may have occurred within the realm of the individual’s control. For example, if an individual reported a conflict or fight with a friend, this event would qualify as interpersonal dependent, given that this event could have occurred as a result of their own actions (or inactions) or characteristics (regardless of the individual’s own perceptions). The a priori component of this prevents subjective bias from affecting the relative dependence and independence of these events. In this study, the LES and LEI were administered at baseline to control for the effects of prior stressors. Thus, only interpersonal dependent and independent stressors were included. The LES and LEI have demonstrated excellent reliability and validity (Safford et al., 2007).
Daily Diary
For the convenience of participants and feasibility, participants were emailed the daily survey at 6PM and instructed to complete the daily diary between the hours of 6PM–12AM. This time frame was designed to allow participants ample time to accommodate school and work obligations. Participants were instructed to report events that occurred since they last completed the diary the previous day. In the event that participants did not complete the survey the day prior, they were instructed to complete the survey based on the past 24 hours.
Daily Diary Events
To assess stressors that participants experienced each day, participants completed the Daily Diary, which included 26 events that are both major and minor negative events in the daily lives of emerging adults, including achievement (e.g., did poorly on a graded assignment) and social events (e.g., excluded or left out by group of friends). Interpersonal events also included an option for participants to endorse multiple parties (e.g., family, friend, significant other, coworker, or other). For instance, for the event “I got into a fight or argument with someone,” participants were able to select any individual to which the statement applied. This enabled us to more comprehensively assess events that could have occurred within one item. Thus, there were a total of 64 possible items that participants could have endorsed across the entire daily diary. At the end of the daily diary, participants were invited to report additional events that may have occurred but are not represented. Examples reported included: “My family and I attended a funeral today,” “I woke up with hives,” “My dog had a seizure today,” “My apartment flooded,” “I missed my train and was late for class.” All endorsed and added events were coded based on whether or not the event occurred that day (0 = no; 1 = yes). All events were categorized as dependent (i.e., a result of the individual’s behaviors or characteristics; Liu & Alloy, 2010) or independent (i.e., fateful events) based on the preexisting LEI event-specific criteria (Francis-Raniere, Alloy, & Abramson, 2006), which a priori defines any event that may have occurred directly or indirectly as a result of the individual’s behavior or characteristics as dependent (as noted above). In total, there were 38 dependent events and 26 independent events (e.g., “I had a minor illness or injury”), in addition to events added by the participant. Of these, 23 events were interpersonal dependent events (e.g., “I had a fight or conflict with a friend”). The present study focused on events that were interpersonal dependent and independent. On average, participants completed 12.20 diaries (87.14%; range 6–14; SD = 2.03) over the 14-day period.
PROMIS-depression-Short Form (PROMIS-Depression-SF; Pilkonis et al., 2011)
The PROMIS-Depression-SF is an 8-item measure that assesses the severity of symptoms of depression. Although it typically inquires about depressive symptoms over the past two weeks, the present study adapted it to assess depressive symptoms on a daily basis and was included on the daily diary measures. The PROMIS-Depression-SF also has been found to have sound psychometrics similar to the longer version (Olino et al., 2012; 2013; Pilkonis et al., 2011). In the present study, the internal reliability of the PROMIS-Depression-SF was α = .90.
Statistical Analyses
Preliminary Analyses
Preliminary analyses were conducted using SPSS Version 21.0 (IBM Corp., 2012). We used the unadjusted RSA values (not residualized) for these analyses. Specifically, t-tests examining differences in the primary variables of interest (RSA, average daily interpersonal dependent stressors, independent stressors, depressive symptoms) as a function of sex and depression history (MDD versus subclinical) were conducted. We also conducted paired-samples t-tests comparing HRV levels at each phase of the stressor task to ensure that the stressor task induced the expected physiological stress. Bivariate correlations between the primary variables of interest also were examined.
Primary Analyses
To determine whether RSA predicted daily stress generation and depressive symptoms, hypotheses were tested using multilevel modeling in Mplus 7.0 (Muthen & Muthen, 2007). This provided for more accurate and powerful tests than possible with a nomothetic approach. For our analyses, there were two levels of data, including the daily diary assessments (Level 1) nested within the individual (Level 2). Thus, data (e.g., stressors and depressive symptoms) from the 14 daily assessments are Level 1 variables and data from the Time 1 assessment (e.g., resting RSA and RSA reactivity) are Level 2 variables. Mplus also is advantageous because it uses Maximum Likelihood to estimate parameters for individuals with missing data (e.g. daily diary), which maximizes data use and prevents unnecessary exclusion of participants from analyses. All analyses were conducted covarying for history of major depressive disorder and sex, as well as depression medication use, age, and body mass index. We also covaried for Time 1 depressive symptoms and events (interpersonal dependent and independent events when each was examined as the outcome) that occurred in the prior four weeks to ensure that atypical RSA patterns predicted depressive symptoms and stressors controlling for previous effects. 1
Direct and Interactive Effects
We examined whether resting RSA or reactivity to the lab-induced stressor predicted depressive symptoms and interpersonal stress generation over the two weeks of daily diary assessments. For our first hypothesis, daily depressive symptoms were entered as the Level 1 outcome variable. For our second hypothesis, the total number of interpersonal dependent stressors from the daily diary assessments was entered as the Level 1 outcome variable, such that participants’ resting RSA and RSA reactivity predicted the intercept of depressive symptoms and interpersonal dependent stressors across the daily diary assessments. For our results to indicate stress generation, it is crucial that resting RSA and RSA reactivity only predict to interpersonal dependent stressors and not independent stressors. Thus, independent stressors also were examined to confirm the specificity of findings to the generation of dependent events.
To examine the interactive effects of resting RSA and RSA reactivity predicting interpersonal stress generation and depressive symptoms, we also centered and created an interaction term between resting RSA and RSA reactivity predicting stressful events and depressive symptoms. When there was evidence of a significant interaction, we probed the interaction at high and low levels (plus or minus 1 SD) of the moderator. We also conducted exploratory analyses to examine the full physiological stress process. Therefore, we also examined RSA reactivity to anticipatory threat and RSA recovery as predictors of depressive symptoms and interpersonal stress generation.
Mediational Hypotheses
To test our mediational hypotheses that stress generation would mediate the relationship between RSA and depressive symptoms, we conducted three mediational analyses within a 2-1-1 multilevel modeling framework with resting RSA and RSA reactivity. The number of daily interpersonal dependent stressors from the daily diary assessments was entered as the Level 1 mediating variable and daily depressive symptoms was entered as the Level 1 dependent variable, controlling for previous levels of depressive symptoms (lagged depressive symptoms). For all mediation analyses, we regressed the dependent and mediating variables on the level 2 predictor variables (RSA) and all covariates. We also regressed the dependent variable on the mediating variable and covariates. Thus, we examined each part of the mediation model with resting RSA and RSA reactivity predicting dependent stressors and depressive symptoms separately. We also estimated the slope of the within-person effects for interpersonal dependent stressors on depressive symptoms and constrained the slope of the between-person effects of interpersonal dependent stressors on depressive symptoms.
Further, we tested the indirect effect of resting RSA and RSA reactivity on daily depressive symptoms via stress generation. The indirect effect is calculated by estimating the interactive effects (a*b) of the independent to mediating variable (a) and mediating variable to dependent variable (b). Although direct effects are important for mediation in traditional mediation models (Baron & Kenny, 1986), more recent statistical approaches indicate that indirect effects may be present even without a significant direct effect between the independent and dependent variables (Hayes, 2009). Thus, we conducted our mediational analyses as planned regardless of whether there was a significant direct effect. Although bootstrapping is the recommended approach for tests of mediation, bootstrapping in multilevel models is a current area of research and multilevel modeling does not allow for bootstrapping.
Sex Differences
To investigate whether there were potential sex differences in these relationships, we conducted additional analyses using an interaction term between resting RSA and sex or RSA reactivity and sex predicting daily depressive symptoms and interpersonal stress generation (conducted separately). When there was evidence of a significant interaction, we probed the interaction for males and females separately to understand the direction of the effect. Thus, the full interaction term and the simple slopes for males and females are presented.
Results
Preliminary Analyses
First, we evaluated whether there were significant differences across each phase of the stressor task (Baseline, Anticipation, Stressor, Recovery) for the full sample for heart rate and unadjusted RSA levels (Table 1). These analyses revealed that participants experienced increases in heart rate across each phase of the stressor task, from baseline to anticipation (t = 10.78, p < .001; d = .58), anticipation to stressor (t = 13.34, p <. 001; d = .78), and baseline to stressor (t = 18.17, p < .001; d = 1.33), as well as decreases from stressor to recovery (t = −17.27, p <. 001; d = 1.31). In addition, participants experienced the expected decreases in RSA from baseline to stressor (t = 5.51, p <. 001; d = .54), anticipation to stressor (t = 4.88, p <. 001; d = .49) and increases in RSA from stressor to recovery (t = 3.98, p < .001; d = .38). However, there were no differences in RSA from baseline to anticipation (t = −1.15, p = .25; d = .11). These findings suggest that our stressor task induced physiological stress for most participants.
Table 1.
Descriptive Statistics and Physiological Characteristics Across Sample by Depression History
| Measure | Overall Sample (N = 102) | MDD (N = 76) | Subthreshold (N = 26) | Statistical Difference | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M (N) | SD (%) | M (N) | SD (%) | M (N) | SD (%) | t (X2) | |
| Demographic | |||||||
| Sex (Female) | 79 | 77.45% | 61 | 80.26% | 18 | 69.23% | 1.35 |
| Race (White) | 76 | 74.51% | 54 | 71.05% | 18 | 69.23% | .03 |
| Age | 19.86 | 1.17 | 19.91 | 1.16 | 19.73 | 1.22 | .67 |
| BMI | 23.75 | 3.82 | 23.98 | 4.04 | 23.04 | 3.06 | 1.08 |
| RSA | |||||||
| Baseline RSA | 6.49 | 1.14 | 6.57 | 1.10 | 6.28 | 1.22 | 1.09 |
| Anticipatory RSA | 6.41 | 1.15 | 6.41 | 1.19 | 6.43 | 1.05 | .07 |
| Stressor RSA | 5.93 | 1.02 | 5.93 | 1.02 | 5.92 | 1.03 | .03 |
| Recovery RSA | 6.31 | 1.13 | 6.35 | 1.17 | 6.19 | 1.02 | .62 |
| HR | |||||||
| Baseline HR | 75.43 | 10.16 | 75.20 | 10.06 | 76.10 | 10.65 | .39 |
| Anticipatory HR | 81.61 | 11.38 | 81.77 | 12.11 | 81.13 | 9.10 | .24 |
| Stressor HR | 91.33 | 14.00 | 91.34 | 14.53 | 91.28 | 12.60 | .02 |
| Recovery HR | 75.60 | 10.44 | 75.30 | 10.44 | 76.51 | 10.64 | .49 |
| Daily Diary | |||||||
| PROMIS-Dep | 10.89 | 2.95 | 11.28 | 3.20 | 9.77 | 1.61 | 3.12** |
| Int Dep Stress | 1.49 | .51 | 1.55 | .59 | 1.35 | .30 | 2.20* |
| Indep Stress | .36 | .34 | .38 | .36 | .31 | .27 | .97 |
Note.
p <. 05;
p < .01;
p < .001.
MDD = Major Depressive Disorder (Coded as 0 = None; 1 = Present); BMI = Body Mass Index; RSA = Respiratory Sinus Arrhythmia (unadjusted); HR = Heart Rate; Int Dep = Interpersonal Dependent; Indep = Independent; Dep Sx = Depressive Symptoms.
For our bivariate correlations of primary study variables, higher resting RSA levels were significantly associated with greater RSA stressor reactivity (r = .57, p < .001), reactivity to anticipatory threat (r = .29, p < .01), and RSA recovery from stress (r = .35, p < .001). RSA reactivity also was associated with anticipatory threat (r = .41, p < .001) and RSA recovery (r = .79, p < .001). RSA reactivity to anticipatory threat was not associated with RSA recovery (r = .04, p = .71). Depressive symptoms were significantly associated with interpersonal dependent stressors (r = .21, p < .05). However, RSA variables were not associated with stressors or depressive symptoms across the daily assessments (r’s < .20, p’s > .05). Interestingly, interpersonal dependent and independent stressors also were not correlated (r = .17, p >. 05). We also compared individuals by sex on mean levels of primary study variables and found no significant sex differences (ts < 1.80, p’s > .05).
Clinical versus Subclinical Depression
Demographic characteristics and primary study variables are displayed in Table 1 for the overall sample and by depression history. Although the main hypotheses were conducted with both clinical and subclinical depression, we examined the study variables by depression history to better understand the characteristics of our sample and potential physiological differences among those with clinical versus subclinical depression. Overall, there were no significant differences between participants with clinical and subclinical depression history on demographic (age, race, sex) or clinical variables (e.g., age of first onset, depression symptoms, depression medication). In terms of physiological differences, there were no significant differences in levels of RSA or HR between those with past MDD and subthreshold depression across the stressor task phases (t’s < 1.22, p’s > .05), or RSA reactivity (t = 1.19, p = .24), reactivity to anticipatory threat (t = 1.86, p = .07), or RSA recovery from the stressor (t = .78, p = .44). In addition, individuals with a history of MDD reported greater depressive symptoms on average during the daily diaries than those with subclinical depression history, as well as greater interpersonal dependent stressors (Table 1).
Primary Analyses
Daily Depressive Symptoms
In partial support of our hypotheses, only resting RSA predicted depressive symptoms over the two weeks of daily diary assessments (Table 2). This relationship was such that lower resting RSA levels predicted more subsequent depressive symptoms over the two-week period (intercept), controlling for MDD history, sex, age, BMI, depression medication, and Time 1 depressive symptoms. However, there was no effect of RSA reactivity on depressive symptoms over the daily diary assessments (Table 2). In addition, contrary to prior literature, there was no significant interaction between resting RSA and RSA reactivity predicting depressive symptoms (t = .13, p = .90). Further, there were no direct effects of RSA anticipatory threat (t = −.16, SE = .36, p = .88) or RSA recovery (t = .10, SE = .46, p = .83) on depressive symptoms, indicating that the effects were specific to resting RSA.
Table 2.
Main Effects of Resting RSA and RSA Reactivity on Depressive Symptoms
| Variable | Depressive Symptoms | ||
|---|---|---|---|
|
| |||
| B | SE | t | |
| Between-level | |||
| Intercept (Dep Sx) | 8.01 | 4.60 | 1.74 |
| MDD | 1.07 | .55 | 1.96* |
| Sex | .89 | .60 | 1.53 |
| Past Dep Med | .59 | .59 | 1.00 |
| BMI | .07 | .01 | 1.04 |
| Age | .02 | .21 | .09 |
| T1 Dep Sx | .07 | .01 | 6.11*** |
| Resting RSA | −.46 | .22 | −2.13* |
| RSA Rx | .16 | .25 | .63 |
| Random Effects | |||
| Intercept (Dep Sx) | 4.34 | .76 | 5.73*** |
Note.
p <. 05;
p < .01;
p < .001.
Dep Sx = Depressive Symptoms; MDD = Major Depressive Disorder (Coded as 0 = None; 1 = Present); Dep Med = Depressive Medication Use; BMI = Body Mass Index; T1 = Time 1; RSA = Respiratory Sinus Arrhythmia; Rx = Reactivity. RSA levels are adjusted for respiration rates.
Daily Stress Generation
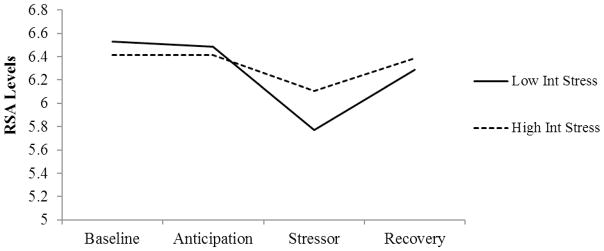
Consistent with our hypotheses, there was a main effect of RSA reactivity on daily interpersonal dependent stressors (Table 3; Figure 1), controlling for MDD history, sex, age, BMI, depression medication, and Time 1 interpersonal dependent stressors that occurred in the previous four weeks. However, there was no direct effect of resting RSA predicting interpersonal dependent stressors. Further, consistent with our hypotheses and stress generation literature, there were no direct effects of resting RSA or RSA reactivity predicting independent stressors (Table 3). In addition, our analyses examining the combined effects of resting RSA and RSA reactivity indicated that there was no significant interactive effect on daily interpersonal dependent stressors (t = −.44, p = .66). Further, there were no direct effects of RSA anticipatory threat (t = .11, SE = .07, p = .91) or RSA recovery (t = −.94 SE = .10, p = .35) on interpersonal dependent stressors symptoms.
Table 3.
Main Effects of Resting RSA and RSA Reactivity on Stressors
| Variable | Interpersonal Dependent Stressors | ||
|---|---|---|---|
|
| |||
| B | SE | t | |
| Between-level | |||
| Intercept (Int dep Stress) | 2.40 | .92 | 2.61** |
| MDD | .13 | .11 | 1.15 |
| Sex | .11 | .12 | .83 |
| Past Dep Med | .06 | .12 | .49 |
| BMI | .02 | .01 | 1.59 |
| Age | .10 | .04 | 2.44* |
| T1 Dep Sx | <.01 | <.01 | .16 |
| T1 Neg Int Dep | .04 | .02 | 2.07* |
| Resting RSA | .04 | .04 | 1.02 |
| RSA Rx | −.10 | .05 | −2.09* |
| Random Effects | |||
| Intercept (Int dep Stress) | .17 | .03 | 5.57*** |
| Variable | Independent Stressors | ||
|---|---|---|---|
|
| |||
| B | SE | t | |
| Fixed Effects | |||
| Intercept (Indep Stress) | .03 | .63 | .05 |
| MDD | .03 | .07 | .47 |
| Sex | .07 | .08 | .82 |
| Age | .01 | .03 | .45 |
| Dep Med | −.07 | .08 | −.87 |
| BMI | .03 | .01 | 3.60*** |
| T1 Dep Sx | <.01 | <.01 | .15 |
| T1 Indep Stress | .06 | .02 | 3.21*** |
| Resting RSA | −.03 | .03 | −1.02 |
| RSA Rx | −.03 | .03 | −.88 |
| Random Effects | |||
| Intercept (Indep Stress) | .06 | .01 | 4.82*** |
Note.
p <. 05;
p < .01;
p < .001.
Int dep = Interpersonal dependent; MDD = Major Depressive Disorder (Coded as 0 = None; 1 = Present); Dep Med = Depressive Medication Use; BMI = Body Mass Index; T1 = Time 1; Dep Sx = Depressive Symptoms; Neg = Negative; RSA = Respiratory Sinus Arrhythmia; Rx = Reactivity; Indep = Independent. RSA levels are adjusted for respiration rates.
Figure 1.
RSA Levels Across the Stressor Task by Interpersonal Stress Generation
Note. To illustrate changes in RSA levels across the stressor task by interpersonal stress generation, we plotted RSA levels for each phase (baseline, anticipation, stressor, recovery) for individuals high and low on interpersonal stress generation (+/− 1 SD of the mean across the daily diaries). RSA = Respiratory Sinus Arrhythmia; Int Stress = Interpersonal Stress Generation.
Stress Generation as Mediator of RSA Reactivity and Depressive Symptoms
When examining the relationship between our mediating and dependent variables, there was a direct relationship from daily interpersonal dependent stressors to depressive symptoms (B = .70; SE = .15; t = 4.59, p < .001), even controlling for prior day depressive symptoms. For our first round of mediation analyses, although there was no evidence of a significant direct effect from RSA reactivity to depressive symptoms, there was a significant indirect effect of RSA reactivity on depressive symptoms via interpersonal stress generation (B = −.09, SE = .05, t = −1.98, p < .05; CI [95%]= −.19 - −.001). Specifically, these findings indicated that less RSA reactivity (or less RSA withdrawal) predicted greater interpersonal dependent stressors, which, in turn, predicted to greater depressive symptoms. To confirm that this effect was specific to interpersonal dependent stressors, we evaluated the effect of RSA reactivity to depressive symptoms via independent stressors and found there was no significant indirect effect (B = −.03, SE = .04, t = −.78, p = .43). In addition, although there was a direct effect from lower resting RSA to depressive symptoms, our results indicated that this was not mediated by interpersonal stress generation (i.e., no significant indirect effect; B = .04, SE = .05, t = .94, p = .35). Further, there was no indirect effect from the combined indices of resting RSA and RSA reactivity to depressive symptoms via interpersonal dependent stressors (B = −.01, SE = .03, t = −.24, p = .81).
Sex Differences
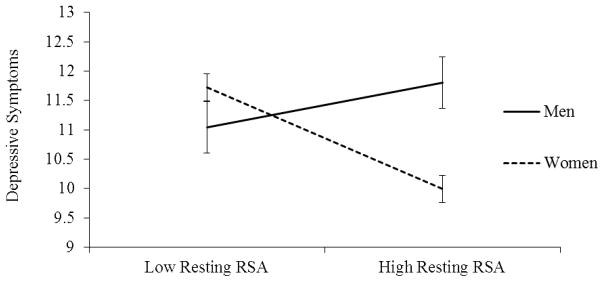
First, there was a significant interaction between resting RSA and sex predicting depressive symptoms (t = 2.53, SE = .49, p = .01; Figure 2). The nature of this interaction was such that the association between resting RSA and subsequent depressive symptoms was significant only for females (B = −.73, SE = .23; t = −3.14, p < .01), but not for males (B = .51, SE = .44; t = 1.17, p = .24). Specifically, women with lower resting RSA were more likely to report depressive symptoms compared to those with greater resting RSA, who had the lowest depressive symptoms. However, there was no relationship between resting RSA and depressive symptoms for male emerging adults. Also, there was no interaction between RSA reactivity and sex in predicting to depressive symptoms (B = −.49, SE = .74, t = −.66, p = .51). Finally, there were no sex differences predicting interpersonal dependent stressors for resting RSA (B = −.11, SE = .10; t = −1.04, p = .30) or reactivity (B = .07, SE = .15; t = .45, p = .66).
Figure 2.
Sex Differences in the Effects of Resting RSA on Depressive Symptoms
Note. RSA = Respiratory Sinus Arrhythmia; Error bars represent standard error of the mean.
Discussion
Considerable research has documented the atypical patterns of resting RSA and RSA reactivity exhibited among individuals with depression (Bylsma et al., 2014; Rottenberg, 2007). However, less research has moved beyond this to examine the role of RSA, particularly RSA reactivity, in processes known to confer risk for depression. In particular, stress generation is a robust predictor of depression onset and recurrence, and vulnerabilities to depression have been found to predict the occurrence of stressors (Liu, 2013). Given the importance of RSA and stressful events in depression, it is surprising that no known study has examined resting and fluctuating RSA levels in the process of interpersonal stress generation. Thus, the aim of the present study was to examine resting RSA and RSA reactivity (and their interactive effects) as prospective predictors of interpersonal stress generation and depressive symptoms among individuals with a history of depression, as well as potential sex differences in these relationships. Further, the second aim of our study was to examine whether interpersonal stress generation mediated the relationship between RSA and depressive symptoms, increasing the risk for recurrence.
First, our preliminary results suggest that our stressor, the Trier Social Stress Test, induced physiological stress among most individuals, as the majority of individuals exhibited the expected heart rate increase and RSA withdrawal across the phases of the socio-evaluative stressor, including increases in HR and decreases in RSA during the anticipatory threat and stressor phases, as well as decreases in HR and increases in RSA during the recovery phase. Second, individuals with a history of MDD did not differ from those with a subclinical depression history on resting RSA and RSA reactivity. Specifically, individuals with subclinical depression exhibited similar levels of resting RSA and RSA fluctuation to the stressor (as well as anticipatory threat and recovery from the stressor) as those with past MDD. Although we were unable to compare these groups to healthy controls to determine whether they have unique RSA patterns compared to never-depressed individuals, this finding represents an important step in evaluating the heterogeneity of depression in psychophysiological processes. According to our findings, there may not be meaningful differences between individuals with past clinical and subclinical depression on RSA levels (including baseline, stressor task, and recovery), which suggests that these individuals may demonstrate similar levels of adaptive (and maladaptive) physiological self- and stress-regulation following depressive episodes. However, it is important to note that there may still be differences in RSA patterns between individuals with current major and subthreshold depression, which were not examined in the present study.
One of the main findings of the present study was that lower resting RSA, but not RSA reactivity, prospectively predicted depressive symptoms over two weeks of daily diary assessments, controlling for depressive symptoms at the initial assessment, history of MDD, and a number of variables associated with RSA (depression medication, age, BMI). Specifically, formerly depressed individuals with lower resting RSA experienced greater daily depressive symptoms on average than those with higher levels of resting RSA. This finding is consistent with prior research indicating that lower RSA is associated with greater depressive symptoms one year later (Vasquez et al., 2016), but highlights the impact of resting RSA on daily depressive symptoms and extends these findings to a sample of emerging adults with a major or subclinical depression history. To our knowledge, this is the first study to document that individual differences in RSA predict subsequent depressive symptoms among individuals with remitted depression, which is notable given that individuals with a history of depression typically exhibit lower levels of RSA than never-depressed individuals (Yaroslavsky et al., 2014). These results suggest that formerly depressed individuals with lower resting RSA may be at greater risk for depression recurrence than individuals with a history of depression and higher resting RSA, and therefore, RSA levels could serve as a potential indicator of future depression risk.
Consistent with our hypotheses, our second main finding was that only RSA reactivity significantly predicted more interpersonal dependent stressors over the next two weeks, controlling for prior interpersonal dependent stressors, depression history, current depressive symptoms, and cardiac-related variables (BMI, depression medication use). Further, these results were specific to interpersonal dependent stressors, which is consistent with the stress generation theory that individuals’ characteristics or behaviors would only be expected to contribute to dependent events (Hammen, 1991). These findings suggest that individuals with less RSA withdrawal prospectively contributed to subsequent interpersonal dependent stressors, but not independent stressors, controlling for RSA-related variables and stressors that occurred in the prior four-week period. Thus, it is possible that individuals who are not able to effectively and adequately mobilize resources to respond to changing or stressful environmental demands may inadvertently generate more negative stressful events, especially in their interpersonal relationships. Specifically, individuals with less RSA withdrawal may have certain negative interpersonal behaviors, such as lack of interpersonal warmth, social disengagement, and maladaptive affective responses (Diamond & Cribbett, 2013; Porges, 2003a), that reduce interpersonal functioning and elicit more negative daily interactions or stressors with friends, family, romantic partners, and coworkers, among others. Further, given the proximity of the vagus nerve to facial nerves (Porges, 2003a; Porges, 2007), individuals with lower RSA withdrawal also may have contextually-inappropriate facial expressions and microexpressions that contribute to difficulties with social communication and interpersonal relationships (Porges, 2003a). Thus, this study identified RSA reactivity as an objective marker of interpersonal stress generation, which may help in identifying those at risk for greater self-generated exposure to interpersonal stressors to which they are subsequently less physiologically able to respond. It is particularly notable that these findings are documented among individuals with a history of depression, who already are at risk for stress generation (Liu & Alloy, 2010). Of note, it is possible that atypical RSA patterns may serve as the biological underpinnings of other known cognitive, interpersonal, and affective risk factors for interpersonal stress generation. However, this study did not directly examine this possibility, which should be explored in future research.
Notably, we did not find direct effects of RSA reactivity on depressive symptoms. However, we did find evidence of an indirect effect for less RSA reactivity on depressive symptoms via interpersonal stress generation, controlling for prior day depressive symptoms. Specifically, individuals with less RSA withdrawal experienced more average daily interpersonal stressors, which, in turn, predicted higher levels of daily depressive symptoms. This suggests that maladaptive physiological stress responses may predict interpersonal stress generation and subsequent depressive symptoms among individuals who are formerly depressed, and potentially distinguish those who are at greater risk for interpersonal dysfunction and depression recurrence. We also did not find main effects of resting RSA on interpersonal dependent stressors. This suggests that only resting RSA may directly predict depression symptoms, but RSA reactivity contributes to maladaptive interpersonal processes (i.e., stress generation) that predict greater levels of depressive symptoms. These findings suggest that there may be something specific about RSA reactivity’s role in interpersonal stress generation, and resting RSA’s role as a predictor of depressive symptoms. Specifically, it could be that resting RSA is a better index of depressive symptoms and affect dysregulation, whereas RSA reactivity may be associated more strongly with stress-specific and social regulation and maladaptive responses to stress, which elicits interpersonal stress generation. Our lack of support for a mediational effect of resting RSA to depressive symptoms via stress generation supports this, and suggests that there may be other mechanisms through which resting RSA contributes to depression.
Further, we did not find any significant interactions between resting RSA and RSA reactivity on interpersonal stress generation or depressive symptoms, which is in contrast to prior research on atypical RSA patterns and depression (Yaroslavsky, Rottenberg et al., 2013; Yaroslavsky et al., 2014). One possibility for these discrepant findings may be that the present study utilized a socio-evaluative stressor for our stressor task, whereas these prior studies (Yaroslavsky et al., 2014) used an emotion-induction task, which may elicit different patterns of RSA response (Panaite et al., 2016). It is also possible that the short-term nature of our study design precluded our ability to detect effects of RSA patterns over longer follow-up intervals. In addition, we did not find significant effects of RSA reactivity to anticipatory threat or RSA recovery from the stressor on interpersonal stress generation or depressive symptoms. Interestingly, the threat of stress did not sufficiently induce changes in RSA from baseline in the present study, which may indicate the inability to adequately prepare for an impending stressor among formerly depressed individuals in the present study. Most individuals did experience significant increases in RSA following the stressor task (i.e., RSA recovery). However, individual differences in RSA reactivity to anticipatory threat and RSA recovery did not predict depressive symptoms or interpersonal stress generation. The latter was particularly surprising given research indicating that poorer RSA recovery is associated with major depression (Bylsma et al., 2014). One possibility is that poorer RSA recovery may better distinguish between those with and without depression rather than serve as a marker of risk among individuals with a history of depression. In this sense, only baseline and stress-reactive RSA may have unique effects on interpersonal stress generation and depressive symptoms among those with a history of depression. However, it is also possible that a longer recovery period may better highlight individual differences in recovery post-stressor, which may better reflect the effects of poor recovery in real-world contexts.
In contrast to several studies of current depression (Chambers & Allen, 2007; Thayer et al., 1998), we did not find any sex differences in resting RSA or RSA reactivity among individuals with past depression. Although there weren’t main differences by sex in resting RSA, we did find sex differences in the predictive association between resting RSA and daily depressive symptoms. Specifically, women with lower resting RSA were significantly more likely to have daily depressive symptoms than women with higher levels of resting RSA, but there was no effect of resting RSA on depression for men. Interestingly, there were no sex differences in the effects of lower resting RSA on depression between men and women. There also were no significant sex differences in the effects of resting RSA or RSA reactivity on interpersonal stress generation, or RSA reactivity on depressive symptoms. Thus, it appears that there is something specific about higher resting RSA that is protective for women with a history of depression, whereas women with lower resting RSA may be more vulnerable to depressive symptoms, thereby heightening the risk for future depression. One possible explanation for this sex difference is that affective regulation may be more important for women, given that women experience greater emotional intensity and arousal than men (Kelly, Tyrka, Anderson, Price, & Carpenter, 2008). However, it is also possible that the smaller number of men in the study may have limited the variance of our variables (RSA, depression, stressors) and ability to detect effects among men. Research should further examine potential sex differences in the relationship between RSA and depressive symptoms with a sample more evenly distributed by sex.
Although our study has a number of strengths, including a micro-longitudinal design with laboratory and daily assessments and a sample with past depression, there are also several weaknesses that should be addressed to enhance future research. First, we only included a sample of individuals who experienced past major or subclinical depression, but did not have groups of healthy controls or individuals currently depressed. Including these additional groups with this design would provide more information about the unique and common elements of resting RSA and RSA reactivity that confer risk for depression and depression-related processes. In addition, although we examined sex differences, we had relatively few men in our sample due to the greater prevalence of depression among women (Hankin et al., 1998), which may have limited our power to detect effects. Thus, it is important that future research replicates this work with more equal sample sizes, which would allow for more statistical power to detect sex differences. Further, we only evaluated a socio-evaluative stressor task, whereas some research with RSA has used an emotion-induction task. Although a socio-evaluative task has been demonstrated to elicit significant RSA withdrawal, subsequent studies should conduct research on RSA fluctuations using both socio-evaluative stressor and emotion-induction tasks to allow for comparability of study effects, which also may provide information about the influences of active (speaking) versus passive (watching emotional clip) stressors. In addition, our daily diary assessments of daily stressors did not include an interview portion to evaluate the dependency of the stressors. Although we utilized the a priori coding scheme employed in stress generation studies, future research should utilize the accompanying life events interview to more objectively assess the occurrence and dependence of stressors.
Further, we did not examine or observe behaviors related to physiological reactivity, which would allow us to make more direct claims about the behaviors related to RSA reactivity that are contributing to interpersonal stress generation. In particular, future studies would benefit from conducting in-lab interaction tasks following a variety of induced stressors to identify these behaviors and possible social communication signals (e.g., facial microexpressions) to better understand this relationship. Further, there were several potential limitations in our measurement of RSA, including our instruction to participants to “breathe normally” during the baseline period, which may have had the unintended effect of altering breathing in this condition. It will therefore be important to provide a longer baseline period with uninstructed breathing with the goal of capturing a “true” baseline reading of resting RSA. In addition, it is possible that the speaking portions of the TSST stressor tasks (speech and calculation tasks) may have biased our estimates of RSA. However, we regressed respiration rates on RSA levels for each phase to account for respiration in our analysis. We also did not control for all possible variables that are associated with cardiac activity (e.g., caffeine intake, exercise), which may influence our results. Finally, we only evaluated physiological levels and reactivity at one assessment in the laboratory. It would be particularly valuable to examine physiological processes with ambulatory assessment (Schwerdtfeger, & Friedrich-Mai, 2009), which would yield rich information about the relationship between RSA reactivity and naturally-occurring stressors. Relatedly, we only tested a model in which RSA patterns predict interpersonal stressors; however, it should be noted that there is likely a transactional relationship in which stress and adversity (e.g., trauma; poverty) contribute to the development of atypical RSA patterns (Evans et al., 2013; McLaughlin et al., 2014), and these atypical patterns subsequently contribute to maladaptive interpersonal processes as demonstrated in the present study. Thus, it is possible that current stressors and RSA processes work synergistically to increase depression risk, and these dynamic and complex relationships should be further explored in future research.
Despite these limitations, the present study has important clinical implications for future research and practice. For one, our preliminary findings indicate that individuals with past subthreshold or clinical depression may have similar physiological responses to stress, as well as subsequent risk for daily stress generation and depressive symptoms. Thus, future research should consider examining depression using a more dimensional approach, and examine individual differences within depression to better understand individual risk. Second, our findings highlight the importance of resting RSA and RSA reactivity in depression risk. In particular, our findings indicate that resting levels of RSA may have a direct impact on affective mood states, and thus, may be a better physiological marker of self- and affective dysregulation, whereas RSA reactivity may have an indirect effect on depression through its influence on interpersonal functioning. Specifically, individuals with less RSA withdrawal may not appropriately cope or regulate their stress response, inadvertently contributing to stress in their interpersonal relationships. In this sense, RSA reactivity may be a better marker of stress-regulation, particularly related to interpersonal relationships. Thus, our findings suggest that resting RSA and RSA reactivity may contribute risk for depression via distinct processes. Importantly, future research should examine whether this risk is unique to depression or whether atypical RSA patterns serve as transdiagnostic risk factors for disorder through interpersonal stress generation, which is a mutable risk factor for disorder. Future research is needed to further evaluate these processes in the prospective development of depression onset and recurrence, as well as to integrate our findings with other ANS, immune system, and neurobiological indices of depression vulnerability. Finally, given research demonstrating the impact of early adversity on RSA patterns in depression (e.g., McLaughlin et al., 2014), it will be crucial to examine environmental influences on the development of atypical RSA patterns and consider the transactional relationships between stress and RSA reactivity across development.
Acknowledgments
This work was supported by grants to Jessica L. Hamilton from the National Institute of Mental Health (F31MH106184), the American Psychological Association, and the Society for a Science of Clinical Psychology. Lauren B. Alloy was supported by NIMH Grant MH101168.
Footnotes
Analyses were conducted with all RSA indices separately and simultaneously (as well as with and without covariates). However, the pattern of results was similar across all analyses, so only the analyses with all RSA indices and covariates are presented.
References
- aan het Rot M, Hogenelst K, Schoevers RA. Mood disorders in everyday life: a systematic review of experience sampling and ecological momentary assessment studies. Clinical Psychology Review. 2012;32:510–523. doi: 10.1016/j.cpr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, … Lapkin JB. The Temple-Wisconsin Cognitive Vulnerability to Depression Project: lifetime history of axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology. 2000;109:403–418. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Hughes M, Iacoviello B, … Hogan ME. Behavioral Approach System (BAS) and Behavioral Inhibition System (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. 4. Washington, D.C: 2000. [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability and pain: associations of two interrelated homeostatic processes. Biological Psychology. 2008;77:174–182. doi: 10.1016/j.biopsycho.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny D. The moderator– mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Developmental Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology. 2003;24:479–497. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Burcusa SL, Iacono WG. Risk for recurrence in depression. Clinical Psychological Review. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–69. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosomatic Medicine. 2014;76:66–73. doi: 10.1097/PSY.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AS, Allen JJ. Sex differences in cardiac vagal control in a depressed sample: implications for differential cardiovascular mortality. Biological Psychology. 2007;75:32–36. doi: 10.1016/j.biopsycho.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Wagner BM, Slavin LA, Vannatta K. A prospective study of life events, social support, and psychological symptomatology during the transition from high school to college. American Journal of Community Psychology. 1986;14:241–257. doi: 10.1007/BF00911173. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Cribbet MR. Links between adolescent sympathetic and parasympathetic nervous system functioning and interpersonal behavior over time. International Journal of Psychophysiology. 2013;88:339–348. doi: 10.1016/j.ijpsycho.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Hicks AM, Otter-Henderson KD. Individual differences in vagal regulation moderate associations between daily affect and daily couple interactions. Personality and Social Psychology Bulletin. 2011;37:731–744. doi: 10.1177/0146167211400620. [DOI] [PubMed] [Google Scholar]
- Eberhart NK, Hammen CL. Interpersonal predictors of stress generation. Personality and Social Psychology Bulletin. 2009;35:544–556. doi: 10.1177/0146167208329857. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Tulen JHM, Franken IHA, Huizink AC. Determinants of Physiological and Perceived Physiological Stress Reactivity in Children and Adolescents. PLOS ONE. 2013;8:e61724. doi: 10.1371/journal.pone.0061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults’ stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Francis-Raniere E, Alloy LB, Abramson LY. Depressive personality styles and bipolar spectrum disorders: Prospective tests of the event congruency hypothesis. Bipolar Disorders. 2006;8:382–399. doi: 10.1111/j.1399-5618.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Geisler FC, Kubiak T, Siewert K, Weber H. Cardiac vagal tone is associated with social engagement and self-regulation. Biological Psychology. 2013;93:279–286. doi: 10.1016/j.biopsycho.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Goldberg D. The heterogeneity of “major depression”. World Psychiatry. 2011;10:226–228. doi: 10.1002/j.2051-5545.2011.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) Journal of Clinical Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S. Role of neuroticism in daily stress and coping. Journal of Personality and Social Psychology. 1999;77:1087–1100. doi: 10.1037//0022-3514.77.5.1087. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Alloy LB. Atypical reactivity of of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clinical Psychology Review. 2016;50:67–79. doi: 10.1016/j.cpr.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Stange JP, Shapero BG, Connolly SL, Abramson LY, Alloy LB. Cognitive vulnerabilities as predictors of stress generation in early adolescence: pathway to depressive symptoms. Journal of Abnormal Child Psychology. 2013;41:1027–1039. doi: 10.1007/s10802-013-9742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Interpersonal dysfunction in depressed women: Impairments independent of depressive symptoms. Journal of Affective Disorders. 2002;72:145–156. doi: 10.1016/s0165-0327(01)00455-4. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. International Journal of Psychophysiology. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- Hughes BM, Howard S, James JE, Higgins NM. Individual differences in adaptation of cardiovascular response to stress. Biological Psychology. 2011;86:129–136. doi: 10.1016/j.biopsycho.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and pshysiological responses to the Trier Social Stress Test. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, … Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. Journal of Affective Disorders. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Beachaine TP, Thayer JF, Kaess M. Depression and resting heart rate variability in children and adolescents: A systematic review and meta-analysis. Clinical Psychology Review. doi: 10.1016/j.cpr.2016.04.013. In Press. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Archives of General Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Liu RT. Stress generation: future directions and clinical implications. Clinical Psychology Review. 2013;33:406–416. doi: 10.1016/j.cpr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review. 2010;30:582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Alves S, Sheridan M. Vagal Regulation and Internalizing Psychopathology Among Adolescents Exposed to Childhood Adversity. Developmental Psychobiology. 2014;56:1036–1051. doi: 10.1002/dev.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Georgiades K. The social encironment and depression: The roles of life stress. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3. New York: The Guilford Press; 2014. pp. 296–314. [Google Scholar]
- Muthén LK, Muthén BO. Mplus statistical software, Version 5. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- Olino TM, Yu L, Klein DN, Rohde P, Seeley JR, Pilkonis PA, Lewinsohn PM. Measuring depression using item response theory: an examination of three measures of depressive symptomatology. International Journal of Methods and Psychiatric Research. 2012;21:76–85. doi: 10.1002/mpr.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Yu L, McMakin DL, Forbes EE, Seeley JR, Lewinsohn PM, Pilkonis PA. Comparisons across depression assessment instruments in adolescence and young adulthood: an item response theory study using two linking methods. Journal of Abnormal Child Psychology. 2013;41:1267–1277. doi: 10.1007/s10802-013-9756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaite V, Hindash AC, Bylsma LM, Small BJ, Salomon K, Rottenberg J. Respiratory sinus arrhythmia reactivity to a sad film predicts depression symptom improvement and sympatmatic trajectory. International Journal of psychophysiology. 2016;99:108–113. doi: 10.1016/j.ijpsycho.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neuroscience Biobehavoral Review. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology & Behavior. 2003a;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Annals of the NY Academy of Sciences. 2003b;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Safford SM, Alloy LB, Abramson LY, Crossfield AG. Negative cognitive style as a predictor of negative life events in depression-prone individuals: a test of the stress generation hypothesis. Journal of Affective Disorders. 2007;99:147–154. doi: 10.1016/j.jad.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger A, Friedrich-Mai P. Social interaction moderates the relationship between depressive mood and heart rate variability: evidence from an ambulatory monitoring study. Health Psychology. 2009;28:501–509. doi: 10.1037/a0014664. [DOI] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Moyer A. The relationship between stress and depression in first onsets versus recurrences: a meta-analytic review. Journal of Abnormal Psychology. 2008;117:206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Computer Methods and Programs in Biomedicine. 2013;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience Biobehavioral Review. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. Heart period variability and depressive symptoms: gender differences. Biological Psychiatry. 1998;44:304–306. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Uliaszek AA, Zinbarg RE, Mineka S, Craske MG, Sutton JM, Griffith JW, … Hammen C. The role of neuroticism and extraversion in the stress-anxiety and stress-depression relationships. Anxiety Stress & Coping. 2010;23:363–381. doi: 10.1080/10615800903377264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lang ND, Ferdinand RF, Verhulst FC. Predictors of future depression in early and late adolescence. Journal of Affective Disorders. 2007;97:137–144. doi: 10.1016/j.jad.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Vazquez L, Blood JD, Wu J, Chaplin TM, Hommer RE, Rutherford JV, … Crowley MJ. High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. Journal of Affective Disorders. 2016;196:243–247. doi: 10.1016/j.jad.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, Kovacs M. Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biological Psychology. 2013;94:272–281. doi: 10.1016/j.biopsycho.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. The utility of combining RSA indices in depression prediction. Journal of Abnormal Psychology. 2013;122:314–321. doi: 10.1037/a0032385. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Developmental Psychopathology. 2014;26:1337–1352. doi: 10.1017/S0954579414001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Murrary G, Johnson SL, Findling RL. The 7 Up 7 Down Inventory: A 14-item measure of manic and depressive tendencies carved from the General Behavior Inventory. Psychological Assessment. 2013;25:1377–1383. doi: 10.1037/a0033975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W. The Inventory to Diagnose Depression (IDD): a self-report scale to diagnose major depressive disorder. Journal of Consulting and Clinical Psychology. 1987;55:55–59. doi: 10.1037//0022-006x.55.1.55. [DOI] [PubMed] [Google Scholar]